








生物技术通报 ›› 2021, Vol. 37 ›› Issue (12): 169-179.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0256
收稿日期:2021-03-05
出版日期:2021-12-26
发布日期:2022-01-19
作者简介:杨悦,女,硕士研究生,研究方向:水产生物蛋白质分子生物学;E-mail: 基金资助:
YANG Yue( ), TAO Yan(
), TAO Yan( ), XIE Jing, QIAN Yun-fang
), XIE Jing, QIAN Yun-fang
Received:2021-03-05
Published:2021-12-26
Online:2022-01-19
摘要:
C型溶菌酶(Chicken-type lysozyme)作为草鱼(Ctenopharyngodon idella)内源性免疫系统中的重要蛋白质类免疫因子,能在草鱼抵抗病原微生物侵染的过程中发挥重要作用,亦是开发绿色饲料添加剂或生物类抗菌剂的佳选。本研究通过逆转录PCR(reverse transcription PCR,RT-PCR)克隆草鱼C型溶菌酶的编码基因“CilyC”,再经二次PCR在其5'和3'端添加各种必需位点。将“CilyC”与表达载体“pPICZαA”连接后转入工程菌毕赤酵母(Pichia pastoris)X-33中,获得重组菌株“X-33/pPICZαA-CilyC”。经含高浓度博莱霉素的培养基筛选得到高拷贝重组菌株后,对其最适蛋白质表达条件进行优化和筛选。通过镍离子亲和层析法纯化重组菌株的表达产物,并对纯化产物进行Western blot分析和LC-MS/MS质谱鉴定。此外,经平板涂布法和最小抑菌浓度(MIC)法考察重组菌株表达产物的抑菌活性。结果表明:X-33/pPICZαA-CilyC在29℃、250 r/min、1%甲醇浓度、96 h的发酵培养条件下,能产13.7 mg/L的重组蛋白;该重组蛋白经结构鉴定为预期分子量14.5 kD的CilyC蛋白。抑菌试验结果显示:重组CilyC具有明显的抗革兰氏阳性的金黄色葡萄球菌(Staphylococcus aureus)、枯草芽孢杆菌(Bacillus subtilisi)、单增李斯特菌(Listeria monocytogenes)和蜡样芽孢杆菌(Bacillus cereus)以及革兰氏阴性的铜绿假单胞菌(Pseudomonas aeruginosa)和沙门氏菌(Salmonella)的生物学活性。本研究构建的重组毕赤酵母菌株“X-33/pPICZαA-CilyC”能有效合成草鱼C型溶菌酶,为鱼类来源C型溶菌酶的大规模制备奠定了良好基础。
杨悦, 陶妍, 谢晶, 钱韻芳. 基于重组毕赤酵母的草鱼C型溶菌酶生物合成及其抑菌活性[J]. 生物技术通报, 2021, 37(12): 169-179.
YANG Yue, TAO Yan, XIE Jing, QIAN Yun-fang. Biosynthesis of Ctenopharyngodon idella C-type Lysozyme Based on Recombinant Pichia pastoris and Its Antibacterial Activity[J]. Biotechnology Bulletin, 2021, 37(12): 169-179.
| Primer name | Primer sequence(5'-3') | Size/bp |
|---|---|---|
| CF | TCTTCAGATAGCAGAAATTGACAGT | 25 |
| CR | TAGGTAATTAAAGATCCCTCAGGCAA | 26 |
| CP1 | CATCATCATCATCATCATCGCACAATGGG | 29 |
| CP2 | TCTAGATTATCAGTGCGATTCGCA | 24 |
| CP3 | CTCGAGAAAAGACATCATCATCATCATCATC | 31 |
| 3'AOX1 | GCAAATGGCATTCTGACATCC | 21 |
| 5'AOX1 | GACTGGTTCCAATTGACAAGC | 21 |
表1 引物序列
Table 1 Primer sequences
| Primer name | Primer sequence(5'-3') | Size/bp |
|---|---|---|
| CF | TCTTCAGATAGCAGAAATTGACAGT | 25 |
| CR | TAGGTAATTAAAGATCCCTCAGGCAA | 26 |
| CP1 | CATCATCATCATCATCATCGCACAATGGG | 29 |
| CP2 | TCTAGATTATCAGTGCGATTCGCA | 24 |
| CP3 | CTCGAGAAAAGACATCATCATCATCATCATC | 31 |
| 3'AOX1 | GCAAATGGCATTCTGACATCC | 21 |
| 5'AOX1 | GACTGGTTCCAATTGACAAGC | 21 |
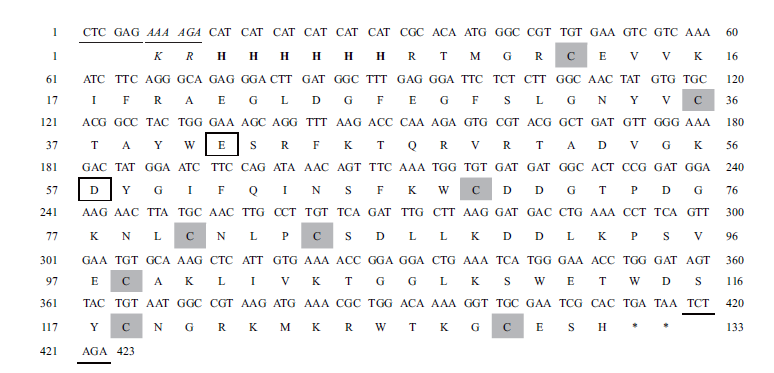
图1 目的基因CilyC的核苷酸及其推断的氨基酸序列 粗体表示6×His标签;斜体表示Kex2信号肽酶切割位点;下划线表示Xho I和Xba I限制性内切酶位点;阴影表示半胱氨酸残基;方框表示活性位点残基
Fig. 1 Nucleotide and deduced amino acid sequences of the target gene CilyC Bold letters refer to 6×His;italics refer to Kex2 signal peptidase cleavage site. Underlines refer to Xho I and Xba I restriction endonuclease sites. Shaded letters refer to conserved cysteine residues. Boxes refer to active site residues
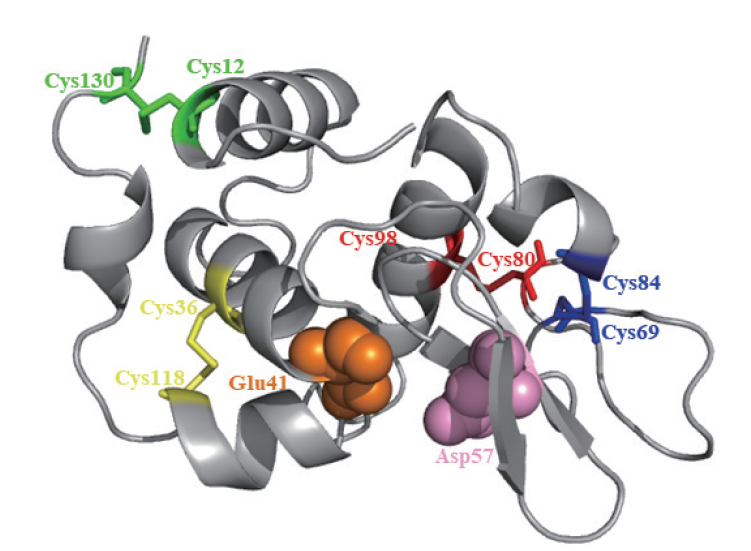
图2 CilyC蛋白的三维结构 标注不同数字的Cys表示半胱氨酸残基;红色、绿色、黄色和蓝色表示4对二硫键;橙色和粉色球体表示2个活性位点残基
Fig.2 Three-dimensional structure of the CilyC protein Different numbers of Cys refer to cysteine residues. Red,green,yellow and blue refer to 4 pairs of disulfide bonds. Orange and pink refer to residues of the active site

图3 重组表达载体pPICZαA-CilyC的菌落PCR(A)和双酶切鉴定(B) M:DNA分子量标准;1-9:菌落PCR产物;10:双酶切产物
Fig. 3 Identification of recombinant expression vector pPICZαA-CilyC by colony PCR(A)and restriction endonuclease digestion(B) M:DNA ladder. 1-9:Colony PCR products. 10:Products digested by restriction endonucleases
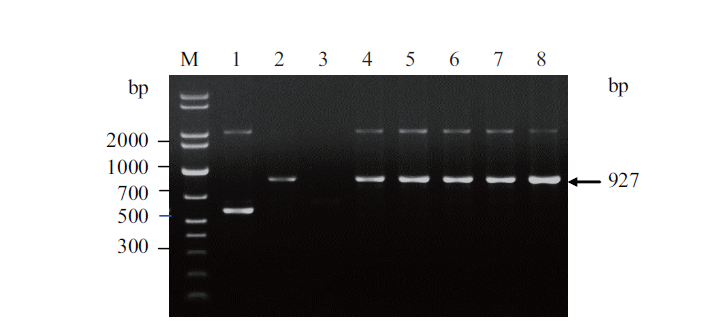
图4 重组毕赤酵母菌株基因组DNA的PCR鉴定 M:DNA分子量标准;1-8:PCR 产物
Fig. 4 PCR identification for the genome DNA of recom-binant P. pastoris strain M:DNA ladder. 1-8:PCR products
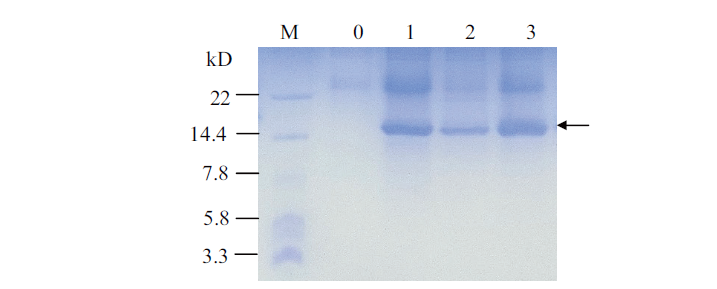
图5 重组菌株预表达产物的Tricine-SDS-PAGE分析 M:超低蛋白质分子量标准;0:X-33/pPICZαA的表达产物;1-3:X-33/pPICZαA-CilyC的表达产物
Fig. 5 Tricine-SDS-PAGE analysis for pre-expressed products of the recombinant strains M:Ultra-low molecular weight protein marker. 0:Expression product of the X-33/pPICZαA. 1-3:Expression products of X-33/pPICZαA-CilyC
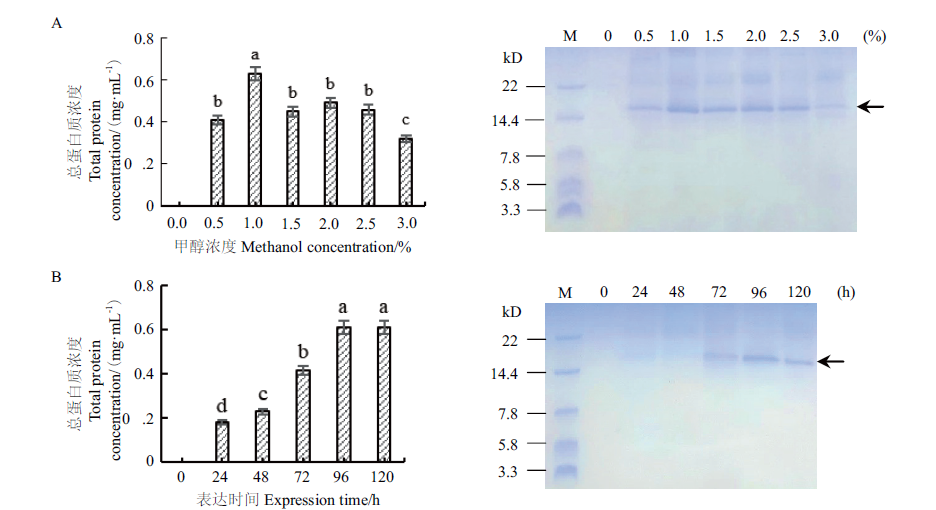
图6 甲醇浓度(A)和表达时间(B)对重组菌株表达产物的影响 相同字母项之间无显著差异,不同字母项之间有显著差异(P < 0.05);数据为平均值±标准差,n=3;M:超低蛋白质分子量标准
Fig. 6 Effects of methanol concentration(A)and expression time(B)on the expression products of the recombinant strain There is no significant difference among the same letter items,and there is a significant difference among different letter items(P <0.05). Data are in format of mean ±SD,n=3. M:Ultra-low molecular weight protein marker
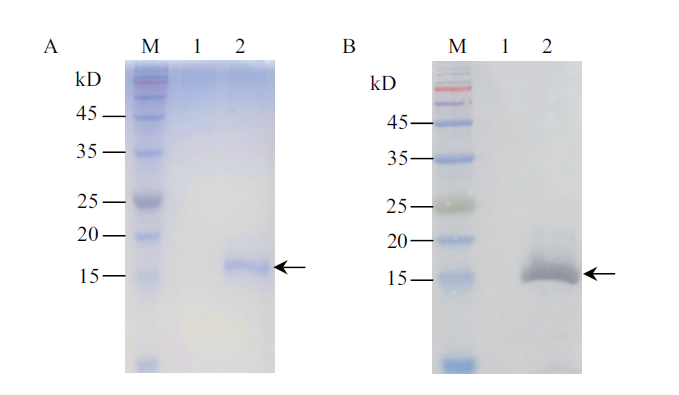
图7 纯化蛋白质的Tricine-SDS-PAGE(A)和Western blot(B)分析 M:彩虹预染蛋白质分子量标准;1:X-33/pPICZαA的表达产物;2:纯化蛋白质
Fig. 7 Tricine-SDS-PAGE(A)and Western blot(B)analysis for the purified protein M:Rainbow pre-stained protein marker. 1:Expression product of the X-33/pPICZαA. 2:Purified protein
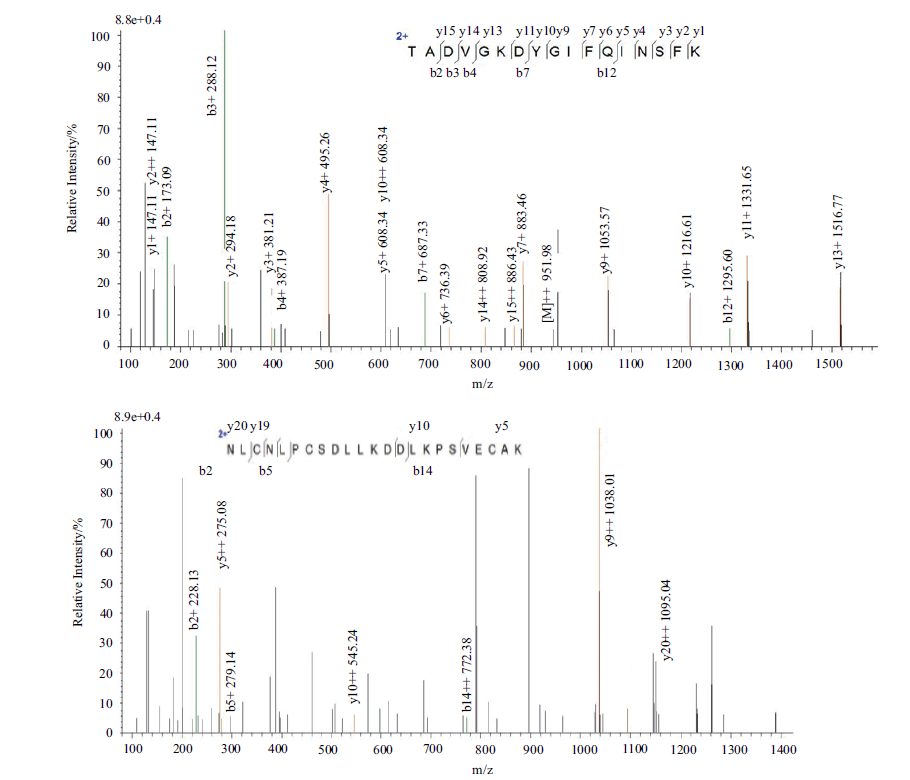
图8 纯化蛋白质的LC-MS/MS质谱分析 大写英文字母表示氨基酸残基
Fig. 8 LC-MS/MS analysis for the purified protein Capital letters refer to amino acid sequences of the peptide fragments

图9 重组菌株培养液上清对革兰氏阳性和阴性细菌的抑菌活性 A:用X-33/pPICZαA的培养液上清处理的细菌;B:用X-33/pPICZαA-CilyC的培养液上清处理的细菌
Fig. 9 Antibacterial activities of the recombinant strain culture supernatant against Gram-positive and Gram-negative bacteria A:Bacteria treated with the culture supernatant of X-33/pPICZαA. B:Bbacteria treated with the culture supernatant of X-33/pPICZαA-CilyC
| 受试菌株 Test strain | MIC/(µg·mL-1) |
|---|---|
| 革兰氏阳性细菌Gram-positive | |
| 金黄色葡萄球菌Staphylococcus aureus | 60 |
| 枯草芽孢杆菌Bacillus subtilis | 80 |
| 单增李斯特菌Listeria monocytogenes | 80 |
| 蜡样芽孢杆菌Bacillus cereus | 80 |
| 革兰氏阴性细菌Gram-negative | |
| 沙门氏菌Salmonella | 80 |
| 铜绿假单胞菌Pseudomonas aeruginosa | 120 |
表2 重组CilyC对受试菌株的最小抑菌浓度
Table 2 Minimum inhibitory concentration(MIC)of the recombinant CilyC against different test bacteria
| 受试菌株 Test strain | MIC/(µg·mL-1) |
|---|---|
| 革兰氏阳性细菌Gram-positive | |
| 金黄色葡萄球菌Staphylococcus aureus | 60 |
| 枯草芽孢杆菌Bacillus subtilis | 80 |
| 单增李斯特菌Listeria monocytogenes | 80 |
| 蜡样芽孢杆菌Bacillus cereus | 80 |
| 革兰氏阴性细菌Gram-negative | |
| 沙门氏菌Salmonella | 80 |
| 铜绿假单胞菌Pseudomonas aeruginosa | 120 |
| [1] |
Xie JW, Cheng CH, Ma HL, et al. Molecular characterization, expression and antimicrobial activities of a c-type lysozyme from the mud crab, Scylla paramamosain[J]. Dev Comp Immunol, 2019, 98:54-64.
doi: 10.1016/j.dci.2019.04.002 URL |
| [2] |
Gao FY, Qu L, Yu SG, et al. Identification and expression analysis of three c-type lysozymes in Oreochromis aureus[J]. Fish Shellfish Immunol, 2012, 32(5):779-788.
doi: 10.1016/j.fsi.2012.01.031 URL |
| [3] |
Yu LP, Sun BG, Li J, et al. Characterization of a c-type lysozyme of Scophthalmus maximus:expression, activity, and antibacterial effect[J]. Fish Shellfish Immunol, 2013, 34(1):46-54.
doi: 10.1016/j.fsi.2012.10.007 URL |
| [4] |
Wu TT, Jiang QQ, Wu D, et al. What is new in lysozyme research and its application in food industry? A review[J]. Food Chem, 2019, 274:698-709.
doi: 10.1016/j.foodchem.2018.09.017 URL |
| [5] | Hester PY. Enrichments in cages[M]// Egg Innovations and Strategies for Improvements. Amsterdam:Elsevier, 2017:77-88. |
| [6] |
Wei S, Huang Y, Cai J, et al. Molecular cloning and characterization of c-type lysozyme gene in orange-spotted grouper, Epinephelus coioides[J]. Fish Shellfish Immunol, 2012, 33(2):186-196.
doi: 10.1016/j.fsi.2012.03.027 URL |
| [7] |
Li SW, Wang D, Liu HB, et al. Expression and antimicrobial activity of c-type lysozyme in Taimen(Hucho taimen, Pallas)[J]. Dev Comp Immunol, 2016, 63:156-162.
doi: 10.1016/j.dci.2016.06.003 URL |
| [8] |
Yang D, Wang Q, Cao R, et al. Molecular characterization, expression and antimicrobial activities of two c-type lysozymes from Manila clam Venerupis philippinarum[J]. Dev Comp Immunol, 2017, 73:109-118.
doi: 10.1016/j.dci.2017.03.018 URL |
| [9] | 杨杰, 丛丽娜, 夏涛, 等. 日本对虾c型溶菌酶的高效重组表达及产物分析[J]. 生物技术通报, 2012(6):99-105. |
| Yang J, Cong LN, Xia T, et al. High level recombinant expression of c-type lysozyme from Marsupenaeus japonicus and product analysis[J]. Biotechnol Bull, 2012(6):99-105. | |
| [10] | 瞿兰, 叶星, 田园园, 等. 罗非鱼3种C型溶菌酶重组蛋白的制备及与几种鱼虾溶菌酶溶菌谱的比较[J]. 生物技术通报, 2012(11):161-166. |
| Qu L, Ye X, Tian YY, et al. Preparation of three C-type recombinant lysozymes of Oreochromis aureus and comparation of their bacteriolytic spectrum with several other lysozymes[J]. Biotechnol Bull, 2012(11):161-166. | |
| [11] | Neshani A, Eydgahi MRA, Zare H, et al. Extended-spectrum antimicrobial activity of the low cost produced tilapia piscidin 4(TP4)marine antimicrobial peptide[J]. Journal of Research in Medical and Dental Science, 2018, 6(5):327-334. |
| [12] |
Meng DM, Zhao JF, Ling X, et al. Recombinant expression, purification and antimicrobial activity of a novel antimicrobial peptide PaDef in Pichia pastoris[J]. Protein Expr Purif, 2017, 130:90-99.
doi: 10.1016/j.pep.2016.10.003 URL |
| [13] |
冯亚东, 陶妍, 李雯, 等. 斑点叉尾鮰C型溶菌酶在毕赤酵母中的表达及其抑菌活性[J]. 生物技术通报, 2017, 33(7):195-202.
doi: 10.13560/j.cnki.biotech.bull.1985.2017-0094 |
| Feng YD, Tao Y, Li W, et al. Expression of channel catfish C-type lysozyme in Pichia pastoris and its bacteriostatic activity[J]. Biotechnol Bull, 2017, 33(7):195-202. | |
| [14] |
王强厚, 陶妍, 崔旭, 等. 三疣梭子蟹C型溶菌酶在毕赤酵母中的表达及其抑菌活性[J]. 生物技术通报, 2018, 34(10):135-142.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0241 |
| Wang QH, Tao Y, Cui X, et al. Expression of swimming crab C-type lysozyme in Pichia pastoris and its bacteriostatic activity[J]. Biotechnol Bull, 2018, 34(10):135-142. | |
| [15] | 颜倩倩, 陶妍, 李雯, 等. 鲤鱼c型溶菌酶在毕赤酵母中的重组表达及其抑菌活性[J]. 农业生物技术学报, 2019, 27(11):1912-1922. |
| Yan QQ, Tao Y, Li W, et al. Recombinant expression of common carp(Cyprinus carpio)c-type lysozyme in Pichia pastoris and its antibacterial activity[J]. J Agric Biotechnol, 2019, 27(11):1912-1922. | |
| [16] | 农业农村部渔业渔政管理局, 2018中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2018:5-8. |
| Ministry of Agriculture and Rural Affairs of PRC, Fisheries and Fisheries Administration. China Fishery Statistical Yearbook[M]. Beijing: China Agriculture Press, 2018:5-8. | |
| [17] | 农业部渔业渔政管理局, 2014中国渔业统计年鉴[M]. 北京: 中国农业出版社, 2014. |
| Ministry of Agriculture and Rural Affairs of PRC, Fisheries and Fisheries Administration. China Fishery Statistical Yearbook[M]. Beijing: China Agriculture Press, 2020:4-25. | |
| [18] |
Ye X, Zhang LL, Tian YY, et al. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyn-godon idellus[J]. Dev Comp Immunol, 2010, 34(5):501-509.
doi: 10.1016/j.dci.2009.12.009 URL |
| [19] | 陈佩. 维氏气单胞菌对草鱼溶菌酶表达的影响及其免疫效果的研究[D]. 长沙:湖南师范大学, 2020. |
| Chen P. Study on the expression change of lysozymes and its immune effect in grass carp(Ctenopharyngodon idella)after infected with Aeromonas veronii[D]. Changsha:Hunan Normal University, 2020. | |
| [20] | 金虹, 李海帅, 帖金凤, 等. 应用微量稀释法测定消毒剂最小抑菌浓度方法的建立[J]. 中国消毒学杂志, 2018, 35(11):801-804, 808. |
| Jin H, Li HS, Tie JF, et al. Establishment of a broth microdilution MIC testing method for disinfectants[J]. Chin J Disinfect, 2018, 35(11):801-804, 808. | |
| [21] |
Leśnierowski G, Yang T. Lysozyme and its modified forms:a critical appraisal of selected properties and potential[J]. Trends Food Sci Technol, 2021, 107:333-342.
doi: 10.1016/j.tifs.2020.11.004 URL |
| [22] |
Lu XM, Jin XB, Zhu JY, et al. Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli[J]. Appl Microbiol Biotechnol, 2010, 87(6):2169-2176.
doi: 10.1007/s00253-010-2606-3 URL |
| [23] |
Zhao H, Tang J, Cao L, et al. Characterization of bioactive recombinant antimicrobial peptide parasin I fused with human lysozyme expressed in the yeast Pichia pastoris system[J]. Enzyme Microb Technol, 2015, 77:61-67.
doi: 10.1016/j.enzmictec.2015.06.001 URL |
| [24] |
Wang T, Xu Y, Liu W, et al. Expression of Apostichopus japonicus lysozyme in the methylotrophic yeast Pichia pastoris[J]. Protein Expr Purif, 2011, 77(1):20-25.
doi: 10.1016/j.pep.2011.01.002 URL |
| [25] |
Zhou XY, Yu Y, Tao JJ, et al. Production of LYZL6, a novel human c-type lysozyme, in recombinant Pichia pastoris employing high cell density fed-batch fermentation[J]. J Biosci Bioeng, 2014, 118(4):420-425.
doi: 10.1016/j.jbiosc.2014.03.009 URL |
| [1] | 王子琰, 王建, 张伦, 桂水清, 卢雪梅. 家蝇抗菌肽MDC对鼠伤寒沙门氏菌的抑菌稳定性研究[J]. 生物技术通报, 2022, 38(3): 149-156. |
| [2] | 杨瑞先, 刘萍, 王祖华, 阮宝硕, 汪智达. 牡丹根腐病原菌拮抗细菌抑菌活性物质分析[J]. 生物技术通报, 2022, 38(2): 57-66. |
| [3] | 王小河, 辜夕容, 祁顺菊, 李杰, 崔瑶, 李得霞, 杨莉荟. 巴山榧树枝和叶提取物的抗氧化能力、抑菌活性与挥发性成分[J]. 生物技术通报, 2021, 37(8): 152-161. |
| [4] | 蔡国磊, 陆小凯, 娄水珠, 杨海英, 杜刚. 芽孢杆菌LM基于全基因组的分类鉴定及抑菌原理的研究[J]. 生物技术通报, 2021, 37(8): 176-185. |
| [5] | 潘镜宇, 陈佳乐, 钱玙呈, 刘鑫, 杨昊宁, 刘立, 魏步云, 赵洪新. 深海出芽短梗霉(Aureobasidium sp. 3A00493)菌株特征与胞外多糖特性分析[J]. 生物技术通报, 2021, 37(12): 71-81. |
| [6] | 赵震, 王莎莎, 吕星星, 陶妍, 谢晶, 钱韻芳. 重组毕赤酵母产青蛤Mytimacin抗菌肽的表达研究[J]. 生物技术通报, 2020, 36(5): 150-158. |
| [7] | 严冬, 曾为林, 罗旭璐, 陈肖学, 刘惠民, 赵平. 樟叶越桔嫩枝1株内生真菌的鉴定及抑菌活性测定[J]. 生物技术通报, 2020, 36(11): 30-38. |
| [8] | 郭晓平, 刘兴飞, 李晓楠, 吕雪茹, 郤少梅, 田园. 泰山黄精内生细菌的抗菌活性研究[J]. 生物技术通报, 2020, 36(11): 48-54. |
| [9] | 蔡娟, 刘流, 王灵军, 曹建平, 郑明辉, 刘晖. 基于转录组测序的冈田绕眼果蝇aoattacin基因筛选及在昆虫细胞中表达、抑菌活性检测[J]. 生物技术通报, 2019, 35(9): 118-124. |
| [10] | 让凤菊, 任艳利, 张维, 欧阳艳. 伊犁野核桃内抑菌抗氧化内生真菌的分离、筛选和鉴定[J]. 生物技术通报, 2019, 35(9): 218-223. |
| [11] | 张亚莉, 陶妍, 谢晶, 钱韻芳. 厚壳贻贝Mytilin-1成熟肽在毕赤酵母中的重组表达及其抑菌活性[J]. 生物技术通报, 2019, 35(7): 54-60. |
| [12] | 董聪, 高庆华, 王玥, 罗同阳. 基于密码子优化的FAD依赖葡萄糖脱氢酶在毕赤酵母中的高效表达及酶学性质[J]. 生物技术通报, 2019, 35(7): 114-120. |
| [13] | 刘进兰, 杨雪, 李双双, 张玉明, 柳峰松, 唐婷, 李红权. 家蝇抗菌肽Domesticin在毕赤酵母中的表达及抑菌活性检测[J]. 生物技术通报, 2019, 35(2): 109-115. |
| [14] | 潘洁明 ,张荣意 ,邓加艾, 谭志琼. 原始热带雨林鹦歌岭土壤抗MRSA放线菌的分离与筛选[J]. 生物技术通报, 2018, 34(6): 128-133. |
| [15] | 刘凌燕, 陈志宇, 曾还雄, 林培彬, 金小宝. 美洲大蠊肠道内生微杆菌的分离鉴定及其抑菌活性研究[J]. 生物技术通报, 2018, 34(6): 172-177. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||