








生物技术通报 ›› 2022, Vol. 38 ›› Issue (8): 216-224.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1457
赵静雅( ), 彭梦雅, 张时雨, 单艺轩, 邢小萍, 施艳, 李海洋, 杨雪, 李洪连, 陈琳琳(
), 彭梦雅, 张时雨, 单艺轩, 邢小萍, 施艳, 李海洋, 杨雪, 李洪连, 陈琳琳( )
)
收稿日期:2021-11-23
出版日期:2022-08-26
发布日期:2022-09-14
作者简介:赵静雅,女,硕士研究生,研究方向:植物病原真菌;E-mail: 基金资助:
ZHAO Jing-ya( ), PENG Meng-ya, ZHANG Shi-yu, SHAN Yi-xuan, XING Xiao-ping, SHI Yan, LI Hai-yang, YANG Xue, LI Hong-lian, CHEN Lin-lin(
), PENG Meng-ya, ZHANG Shi-yu, SHAN Yi-xuan, XING Xiao-ping, SHI Yan, LI Hai-yang, YANG Xue, LI Hong-lian, CHEN Lin-lin( )
)
Received:2021-11-23
Published:2022-08-26
Online:2022-09-14
摘要:
假禾谷镰孢引起的小麦茎基腐病已成为威胁我国小麦安全生产的重要病害,但是对假禾谷镰孢致病的机理还了解的比较少。C2H2锌指转录因子在人、植物和真菌中广泛分布,调控生长、发育和逆境胁迫等多种生理过程。本研究在假禾谷镰孢中鉴定到一个编码C2H2锌指转录因子的基因FpCzf7,利用PEG介导的原生质体转化,获得FpCzf7基因缺失的突变体(Δfpczf7)。生物学表型分析发现,与野生型(WT)和回补菌株(Δfpczf7-cp)相比,Δfpczf7在PDA培养基上的菌丝生长速率明显减慢、气生菌丝减少;在CMC液体中的分生孢子产量减少,但是分生孢子形态和萌发无差异。致病性测定结果显示,与WT和Δfpczf7-cp相比,Δfpczf7在大麦叶片、小麦胚芽鞘和小麦穂部的致病性均显著降低,DON毒素合成明显减少。综上所述,FpCzf7参与假禾谷镰孢的生长、产孢、致病性和毒素的产生。
赵静雅, 彭梦雅, 张时雨, 单艺轩, 邢小萍, 施艳, 李海洋, 杨雪, 李洪连, 陈琳琳. C2H2锌指转录因子FpCzf7参与假禾谷镰孢的生长和致病性[J]. 生物技术通报, 2022, 38(8): 216-224.
ZHAO Jing-ya, PENG Meng-ya, ZHANG Shi-yu, SHAN Yi-xuan, XING Xiao-ping, SHI Yan, LI Hai-yang, YANG Xue, LI Hong-lian, CHEN Lin-lin. Role of C2H2 Zinc Finger Transcription Factor FpCzf7 in the Growth and Pathogenicity of Fusarium pseudograminearum[J]. Biotechnology Bulletin, 2022, 38(8): 216-224.
| Primer | Sequence(5'-3') |
|---|---|
| F1 | CATCAAATGCAGGAACGGAGC |
| R1 | CAATATCATCTTCTGTCGACGGTTTCATGGGCTGC- TAGAAATC |
| F2 | ATAGAGTAGATGCCGACCGCGGGTTCCATCGTCT- TGCCGTGGTGAATTG |
| R2 | CAATTGAACGAGGAAACACG |
| HYG/F[ | GTCGACAGAAGATGATATTG |
| HYG/R[ | GAACCCGCGGTCGGCATCTACTCTAT |
| YG/F[ | GATGTAGGAGGGCGTGGATATGTCCT |
| HY/R[ | GTATTGACCGATTCCTTGCGGTCCGAA |
| F3 | GACCTACCCATGTTTACTTG |
| R3 | CTTCGAAATCTGCATCGCATG |
| G1 | CAACATCATATCGAACCCAC |
| G2 | GAGAGTGAGAAGACATGG |
| H850F[ | TTCCTCCCTTTATTTCAGATTCAA |
| H852R[ | ATGTTGGCGACCTCGTATTGG |
| H855R[ | GCTGATCTGACCAGTTGC |
| H856F[ | GTCGATGCGACGCAATCGT |
| PKNTG-F | CTATAGGGCGAATTGGGTACCGACCTACCCATGTT- TACTTG |
| PKNTG-R | GCAGGCATGCAAGCTTATCGATGGAGCTTTTGCT- GCTCTTG |
| nei-F | GACCACGGTTTCCACTACTC |
| GFP-R | GATGCCCTTCAGCTCGATGCGGTTCA |
| RTF | TTCAACACCACCAGCATCA |
| RTR | TGTGGTGCAAGTTCTCGTT |
| TEF1a-RTF | TCACCACTGAAGTCAAGTCC |
| TEF1a-RTR | ACCAGCGACGTTACCACGTC |
表1 本研究用到的引物
Table 1 Primers used in this study
| Primer | Sequence(5'-3') |
|---|---|
| F1 | CATCAAATGCAGGAACGGAGC |
| R1 | CAATATCATCTTCTGTCGACGGTTTCATGGGCTGC- TAGAAATC |
| F2 | ATAGAGTAGATGCCGACCGCGGGTTCCATCGTCT- TGCCGTGGTGAATTG |
| R2 | CAATTGAACGAGGAAACACG |
| HYG/F[ | GTCGACAGAAGATGATATTG |
| HYG/R[ | GAACCCGCGGTCGGCATCTACTCTAT |
| YG/F[ | GATGTAGGAGGGCGTGGATATGTCCT |
| HY/R[ | GTATTGACCGATTCCTTGCGGTCCGAA |
| F3 | GACCTACCCATGTTTACTTG |
| R3 | CTTCGAAATCTGCATCGCATG |
| G1 | CAACATCATATCGAACCCAC |
| G2 | GAGAGTGAGAAGACATGG |
| H850F[ | TTCCTCCCTTTATTTCAGATTCAA |
| H852R[ | ATGTTGGCGACCTCGTATTGG |
| H855R[ | GCTGATCTGACCAGTTGC |
| H856F[ | GTCGATGCGACGCAATCGT |
| PKNTG-F | CTATAGGGCGAATTGGGTACCGACCTACCCATGTT- TACTTG |
| PKNTG-R | GCAGGCATGCAAGCTTATCGATGGAGCTTTTGCT- GCTCTTG |
| nei-F | GACCACGGTTTCCACTACTC |
| GFP-R | GATGCCCTTCAGCTCGATGCGGTTCA |
| RTF | TTCAACACCACCAGCATCA |
| RTR | TGTGGTGCAAGTTCTCGTT |
| TEF1a-RTF | TCACCACTGAAGTCAAGTCC |
| TEF1a-RTR | ACCAGCGACGTTACCACGTC |
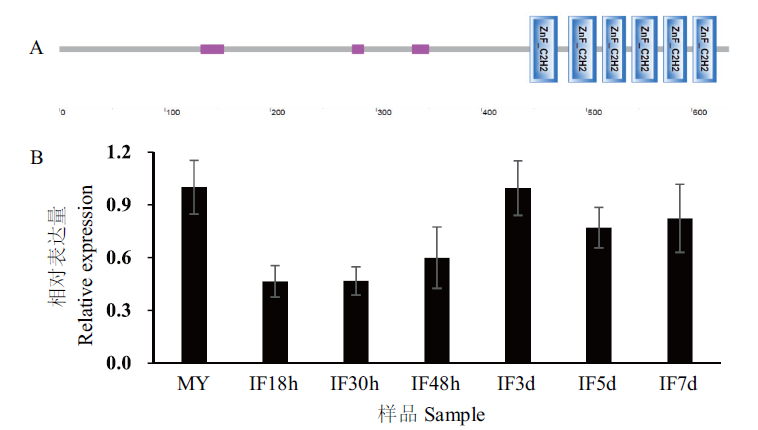
图1 FpCzf7的结构域及基因转录水平 A:FpCzf7的C2H2锌指基序;B:FpCzf7在侵染阶段的相对表达水平。MY和IF18h、IF30h、IF48h、IF3d、IF5d、IF7d分别表示假禾谷镰孢野生型菌丝阶段和侵染小麦胚芽鞘18 h、30 h、48 h、3 d、5 d、7 d
Fig. 1 Domains and expression levels of FpCzf7 in F. pseudograminearum A:The C2H2 zinc finger motifs in FpCzf7. B:The relative expression levels of FpCzf7 during infection stages. MY and IF18h,IF30h,IF48h,IF3d,IF5d,IF7d represent the wild-type mycelia and the F. pseudograminearum infected the wheat coleoptile at 18 h,30 h,48 h,3 d,5 d,and 7 d,respectively
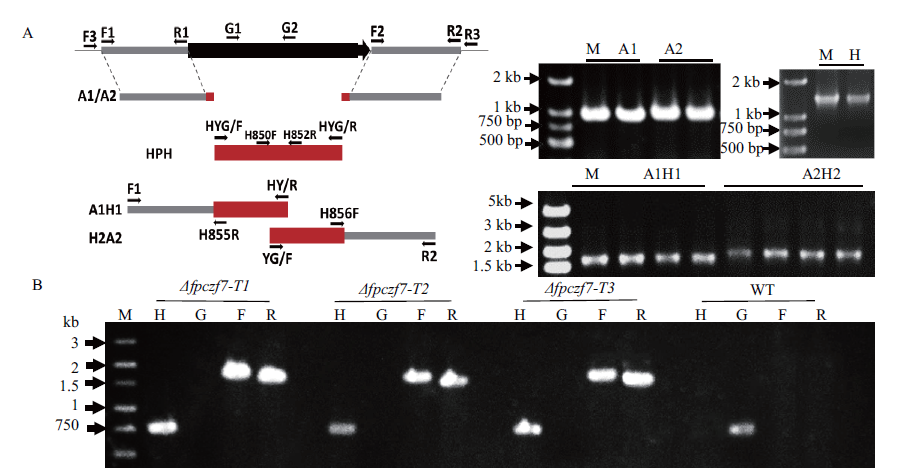
图2 假禾谷镰孢FpCzf7基因的敲除 A:FpCzf7基因缺失策略示意图和PCR扩增结果(M:DNA分子量标准;A1:964 bp的FpCzf7上游片段;A2:997 bp的下游片段;HPH:1 350 bp的潮霉素片段;A1H1:1 762 bp的FpCzf7和HPH的上游融合片段;H2A2:1 928 bp的HPH和FpCzf7下游融合片段);B:FpCzf7基因缺失突变体PCR检测(M:DNA分子量标准;H:750 bp的潮霉素(HPH)片段;G:751 bp的FpCzf7基因片段;F:1 673 bp的FpCzf7和HPH上游融合片段;R:1 568 bp的HPH和FpCzf7下游融合片段)
Fig. 2 FpCzf7 gene knockout in F. pseudograminearum A:Schematic representation of the FpCzf7 deletion strategy and PCR amplification(M:DNA size marker;A1:a 964 bp fragment of FpCzf7 upstream;A2:a 997 -bp fragment of FpCzf7 downstream;HPH:a 1 350 bp fragment of the hygromycin phosphotransferase gene;A1H1:a 1 762 bp fragment of FpCzf7 and HPH upstream cassette;H2A2:a 1 928 bp fragment of FpCzf7 and HPH downstream cassette). B:PCR assay of the FpCzf7 deletion mutants(M:DNA size marker;H:a 750 bp fragment of the HPH;G:a 751 bp fragment of the FpCzf7 gene;F:a 1 673 bp fragment of FpCzf7 and HPH upstream cassette;R:a 1 568 bp fragment of FpCzf7 and HPH downstream cassette)

图3 野生型(WT)、Δfpczf7和Δfpczf7-cp在PDA平板上生长的菌落和菌丝测定 A:生长3 d的菌落形态;B:生长3d的菌落直径(**P < 0.01,t测验)C:生长24 h的菌丝形态(标尺=50 µm)
Fig. 3 Mycelia growth and morphology assays of WT,Δfpczf7 and Δfpczf7-cp on PDA plates A:Colonies morphology for 3 d. B:Colony diameters for 3 d(**P < 0.01,t-test). C:Hyphal morphology for 24 h(Bar=50 µm)
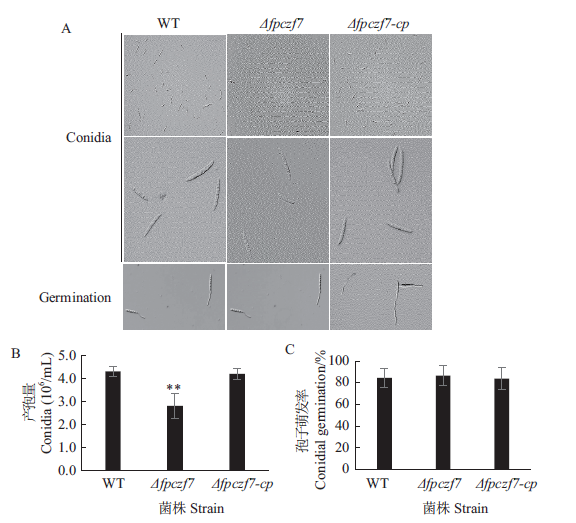
图4 分生孢子产生和萌发测定 A:野生型、Δfpczf7和Δfpczf7-cp的分生孢子及萌发的芽管;B:WT、Δfpczf7和Δfpczf7-cp的产孢量(**P < 0.01,t测验);C:WT、Δfpczf7和Δfpczf7-cp分生孢子的萌发
Fig. 4 Conidia production and germination assays A:Conidia and germinated tubes of WT,Δfpczf7 and Δfpczf7-cp. B:Production of conidia of WT,Δfpczf7 and Δfpczf7-cp(**P < 0.01,t-test). C:Conidia germination rates of WT,Δfpczf7 and Δfpczf7-cp
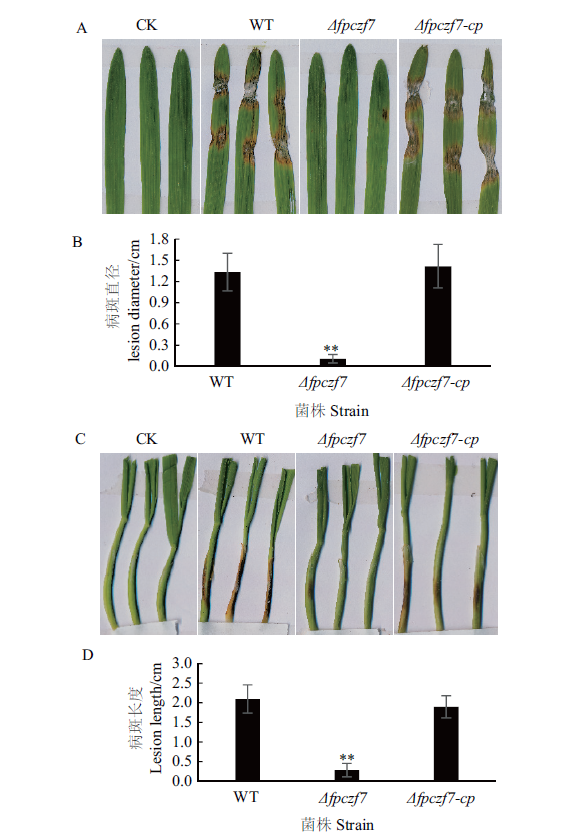
图 5 大麦叶片和小麦胚芽鞘接种 A:野生型、Δfpczf7和Δfpczf7-cp菌丝块接种大麦叶片后3 d;B:大麦叶片病斑直径(**P < 0.01,t测验);C:野生型、Δfpczf7和Δfpczf7-cp菌丝块接种小麦胚芽鞘后3 d;D:小麦胚芽鞘病斑长度(**P < 0.01,t测验)
Fig. 5 Inoculation on barley leaves and wheat coleoptiles A:Barley leaves inoculated with mycelial blocks of WT,Δfpczf7 and Δfpczf7-cp at 3 d. B:The lesion diameters on barley leaves(**P < 0.01,t-test). C:Wheat coleoptiles were inoculated with mycelial blocks of WT,Δfpczf7 and Δfpczf7-cp at 3 d. D:Lesion lengths on wheat coleoptiles(**P < 0.01,t-test)

图 6 小麦盆栽和小麦穗接种实验 A:野生型、Δfpczf7和Δfpczf7-cp接种小麦10 d;B:小麦茎基腐病病情指数(**P < 0.01,t测验);C:野生型、Δfpczf7和Δfpczf7-cp分生孢子悬浮液接种小麦穗部20 d
Fig. 6 Experiments of pot-culture and wheat heads inoculation A:Wheat seedlings inoculated by WT,Δfpczf7 and Δfpczf7-cp for 10 d. B:The disease severity index of Fusarium crown rot.(**P < 0.01,t-test). C:Wheat heads were inoculated with conidial suspension of WT,Δfpczf7 and Δfpczf7-cp strains for 20 d
| [1] |
Kazan K, Gardiner DM. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops:recent progress and future prospects[J]. Mol Plant Pathol, 2018, 19(7):1547-1562.
doi: 10.1111/mpp.12639 URL |
| [2] |
Li HL, Yuan HX, Fu B, et al. First report of Fusarium pseudogram-inearum causing crown rot of wheat in Henan, China[J]. Plant Dis, 2012, 96(7):1065.
doi: 10.1094/PDIS-01-12-0007-PDN pmid: 30727237 |
| [3] |
Zhou HF, He XL, Wang S, et al. Diversity of the Fusarium pathogens associated with crown rot in the Huanghuai wheat-growing region of China[J]. Environ Microbiol, 2019, 21(8):2740-2754.
doi: 10.1111/1462-2920.14602 URL |
| [4] |
Deng YY, Li W, Zhang P, et al. Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in Eastern China[J]. Plant Pathol, 2020, 69(2):240-248.
doi: 10.1111/ppa.13122 |
| [5] |
Obanor F, Neate S, Simpfendorfer S, et al. Fusarium graminearum and Fusarium pseudograminearumcaused the 2010 head blight epidemics in Australia[J]. Plant Pathol, 2013, 62(1):79-91.
doi: 10.1111/j.1365-3059.2012.02615.x URL |
| [6] |
Weirauch MT, Hughes TR. A catalogue of eukaryotic transcription factor types, their evolutionary origin, and species distribution[J]. Subcell Biochem, 2011, 52:25-73.
doi: 10.1007/978-90-481-9069-0_3 pmid: 21557078 |
| [7] |
Englbrecht CC, Schoof H, Böhm S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome[J]. BMC Genomics, 2004, 5(1):39.
pmid: 15236668 |
| [8] |
Han GL, Lu CX, Guo JR, et al. C2H2 zinc finger proteins:master regulators of abiotic stress responses in plants[J]. Front Plant Sci, 2020, 11:115.
doi: 10.3389/fpls.2020.00115 URL |
| [9] |
Cao HJ, Huang PY, Zhang LL, et al. Characterization of 47 Cys2-His2 zinc finger proteins required for the development and pathogenicity of the rice blast fungus Magnaporthe oryzae[J]. New Phytol, 2016, 211(3):1035-1051.
doi: 10.1111/nph.13948 URL |
| [10] |
Son H, Seo YS, Min K, et al. A phenome-based functional analysis of transcription factors in the cereal head blight fungus, Fusarium graminearum[J]. PLoS Pathog, 2011, 7(10):e1002310.
doi: 10.1371/journal.ppat.1002310 URL |
| [11] | 岳晓凤, 阙亚伟, 王政逸. 基于RNA-Seq的稻瘟病菌Δznf1突变体的表达谱分析[J]. 中国农业科学, 2016, 49(17):3347-3358, 3476. |
| Yue XF, Que YW, Wang ZY. Analysis of RNA-seq-based expression profiles of Δznf1 mutants in Magnaporthe oryzae[J]. Sci Agric Sin, 2016, 49(17):3347-3358, 3476. | |
| [12] |
Jung B, Park J, Son H, et al. A putative transcription factor pcs1 positively regulates both conidiation and sexual reproduction in the cereal pathogen Fusarium graminearum[J]. Plant Pathol J, 2014, 30(3):236-244.
doi: 10.5423/PPJ.OA.04.2014.0037 URL |
| [13] |
Yun YZ, Zhou X, Yang S, et al. Fusarium oxysporum f. sp. lycopersici C2H2 transcription factor FolCzf1 is required for conidiation, fusaric acid production, and early host infection[J]. Curr Genet, 2019, 65(3):773-783.
doi: 10.1007/s00294-019-00931-9 URL |
| [14] |
Malapi-Wight M, Kim JE, Shim WB. The N-Terminus region of the putative C2H2 transcription factor Ada1 harbors a species-specific activation motif that regulates asexual reproduction in Fusarium verticillioides[J]. Fungal Genet Biol, 2014, 62:25-33.
doi: 10.1016/j.fgb.2013.10.008 pmid: 24161731 |
| [15] |
Chen LL, Zhao JY, Xia HQ, et al. FpCzf14 is a putative C2H2 transcription factor regulating conidiation in Fusarium pseudograminearum[J]. Phytopathol Res, 2020, 2:33.
doi: 10.1186/s42483-020-00074-7 URL |
| [16] | 王光辉. 禾谷镰刀菌AMT1基因的功能研究[D]. 杨凌: 西北农林科技大学, 2010. |
| Wang SD. Functional characterization of AMT1 genes in Fusarium graminearum[D]. Yangling: Northwest A & F University, 2010. | |
| [17] |
Wang LM, Zhang YF, Du ZL, et al. FpPDE1 function of Fsarium pseudograminearum on pathogenesis in wheat[J]. J Integr Agric, 2017, 16(11):2504-2512.
doi: 10.1016/S2095-3119(17)61689-7 URL |
| [18] |
Poole GJ, Smiley RW, Paulitz TC, et al. Identification of quantitative trait loci(QTL)for resistance to Fusarium crown rot(Fusarium pseudograminearum)in multiple assay environments in the Pacific Northwestern US[J]. Theor Appl Genet, 2012, 125(1):91-107.
doi: 10.1007/s00122-012-1818-6 pmid: 22366812 |
| [19] |
Klug A. The discovery of zinc fingers and their applications in gene regulation and genome manipulation[J]. Annu Rev Biochem, 2010, 79:213-231.
doi: 10.1146/annurev-biochem-010909-095056 URL |
| [20] |
Estruch F, Carlson M. Two homologous zinc finger genes identified by multicopy suppression in a SNF1 protein kinase mutant of Saccharomyces cerevisiae[J]. Mol Cell Biol, 1993, 13(7):3872-3881.
doi: 10.1128/mcb.13.7.3872-3881.1993 pmid: 8321194 |
| [21] |
Nicholls S, Straffon M, Enjalbert B, et al. Msn2- and Msn4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen Candida albicans[J]. Eukaryot Cell, 2004, 3(5):1111-1123.
pmid: 15470239 |
| [22] |
Aoki T, O’Donnell K. Morphological and molecular characterization of Fusarium pseudograminearum sp. nov., formerly recognized as the Group 1 population of F. graminearum[J]. Mycologia, 1999, 91(4):597-609.
doi: 10.1080/00275514.1999.12061058 URL |
| [23] |
Gardiner DM, Benfield AH, Stiller J, et al. A high-resolution genetic map of the cereal crown rot pathogen Fusarium pseudograminearum provides a near-complete genome assembly[J]. Mol Plant Pathol, 2018, 19(1):217-226.
doi: 10.1111/mpp.12519 pmid: 27888554 |
| [1] | 陈福暖, 黄瑜, 蔡佳, 王忠良, 简纪常, 王蓓. ABC转运蛋白结构及其在细菌致病性中的研究进展[J]. 生物技术通报, 2022, 38(6): 43-52. |
| [2] | 田李, 李俊娇, 戴小枫, 张丹丹, 陈捷胤. 从功能基因到生物学性状:大丽轮枝菌致病性形成的分子基础[J]. 生物技术通报, 2022, 38(1): 51-69. |
| [3] | 刘沙玉, 曹健, 李蒙, 柳志强, 李晓宇. 橡胶树胶孢炭疽菌Zn2Cys6型转录因子CgAswA的生物学功能[J]. 生物技术通报, 2021, 37(9): 161-170. |
| [4] | 张文泽, 张艳丽, 门彦明, 张玉娇, 孙志昕, 李文慧, 鲁国东, 齐尧尧. 稻瘟病菌精氨酸甲基转移酶基因MoHMT1的功能分析[J]. 生物技术通报, 2019, 35(12): 38-44. |
| [5] | 李杨, 李河, 周国英, 刘君昂. 油茶新炭疽病原Colletotrichum camelliae鉴定及致病性测定[J]. 生物技术通报, 2016, 32(6): 96-102. |
| [6] | 卫昱君, 王紫婷, 徐瑗聪, 许文涛. 致病性大肠杆菌现状分析及检测技术研究进展[J]. 生物技术通报, 2016, 32(11): 80-92. |
| [7] | 夏淑春 ,王学武, 张茹琴 ,鄢洪海. 玉米弯孢叶斑病菌REMI白化突变体的筛选与致病性测定[J]. 生物技术通报, 2014, 0(12): 111-116. |
| [8] | 王彤彤, 汪晓飞, 李心安, 田国宁, 刘立涛, 王广文, 李宁, 王方昆, 李红梅, 赵孝民, 肖一红. 高致病性猪繁殖与呼吸综合征病毒TA-12株ORF6基因的克隆、鉴定及真核表达[J]. 生物技术通报, 2013, 0(11): 159-163. |
| [9] | 麦合木提江·米吉提;王旭丽;张桦;曾洪梅;Sehroon Khan;邱德文;. FgβNAC基因对禾谷镰刀菌生长发育及致病性的影响[J]. , 2012, 0(11): 177-184. |
| [10] | 刘永宏;赵丽;刘俊峰;王凤龙;刘月焕;. 高致病性猪繁殖与呼吸综合征病毒BJBLZ株序列剖析[J]. , 2011, 0(10): 185-190. |
| [11] | 李国旺;苗志国;陈俊杰;. 高致病性猪繁殖与呼吸综合征病毒ORF7基因的克隆与原核表达[J]. , 2011, 0(02): 141-145. |
| [12] | 秦春圃;. 野油菜黄单胞菌两个γPON基因的突变和功能分析[J]. , 2007, 0(03): 170-170. |
| [13] | 吴彦彬;李亚丹;李小俊;边艳青;. 拟杆菌的研究及应用[J]. , 2007, 0(01): 66-69. |
| [14] | 曾千春;叶华智;朱祯;周开达. 稻瘟病分子生物学研究进展[J]. , 2000, 0(03): 1-7. |
| [15] | 李思经;. 植物抗病机理[J]. , 1997, 0(05): 51-52. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||