








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 97-105.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1607
张小妮( ), 翁伊纯, 范奕浩, 王晓娟, 赵佳宇, 张云龙(
), 翁伊纯, 范奕浩, 王晓娟, 赵佳宇, 张云龙( )
)
收稿日期:2021-12-30
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:张小妮,女,硕士研究生,研究方向:靶向荧光蛋白示踪技术;E-mail:基金资助:
ZHANG Xiao-ni( ), WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long(
), WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long( )
)
Received:2021-12-30
Published:2022-10-26
Online:2022-11-11
摘要:
线粒体氧化应激(mitochondrial oxidative stress,Mito-OS)是一种线粒体内活性氧产生与抗氧化系统失衡状态,活性氧簇(reactive oxygen species,ROS)诱发氧化应激会造成线粒体损伤,被认为是促发衰老和疾病的一个重要因素。目前,氧化应激细胞或动物模型的评价方法主要基于细胞形态、动物表型或特征代谢产物产生情况等指标,无法实时监测动态变化。本研究建立了一种靶向线粒体的氧化应激荧光蛋白监测系统,命名为Mito-OS-Timer,可实时监测线粒体氧化应激动态变化。主要基于荧光蛋白DsRed1-E5红绿荧光转变速率与氧浓度变化呈正相关机理,将DsRed1-E5基因与定位线粒体内膜的ATP合酶亚基(ATP5PB片段)进行基因融合,构建了pMito-OS-Timer重组质粒以及HEK293T稳定表达细胞株,0-300 μmol/L H2O2和0-5 μmol/L 鱼藤酮分别诱导处理后,结果显示细胞模型线粒体内红绿荧光转变速率与线粒体氧化应激程度呈现明显正相关。另外,利用Mito-OS-Timer检测pLVX-shFLCN沉默folliculin(FLCN)基因诱导氧化应激程度增强。此系统为研究线粒体氧化应激提供了一种新的可视化方法。
张小妮, 翁伊纯, 范奕浩, 王晓娟, 赵佳宇, 张云龙. Mito-OS-Timer:一种靶向监测线粒体氧化应激的荧光秒表[J]. 生物技术通报, 2022, 38(10): 97-105.
ZHANG Xiao-ni, WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long. Mito-OS-Timer:A Targeted Fluorescent Stopwatch for Monitoring Mitochondrial Oxidative Stress[J]. Biotechnology Bulletin, 2022, 38(10): 97-105.
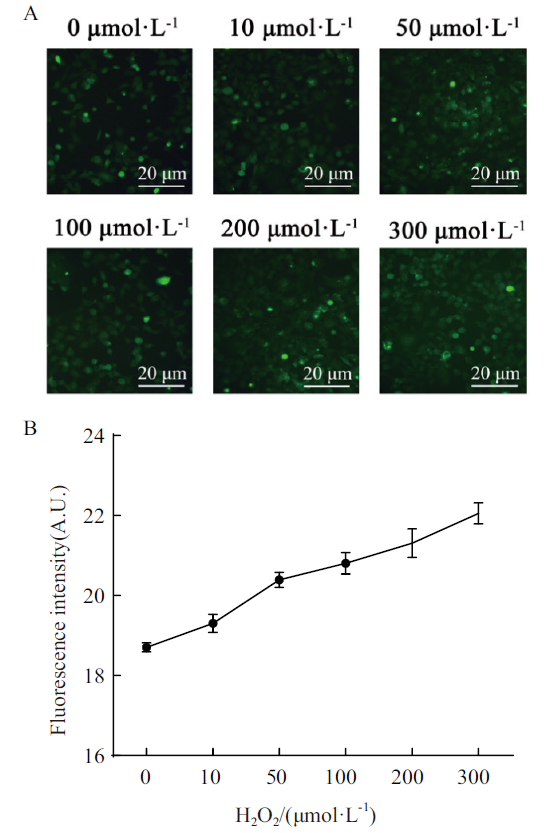
图1 H2O2诱导氧化应激细胞模型的评价 A:DCFH-DA检测不同浓度H2O2处理HEK293T细胞氧化应激状态;B:Image J软件分析图A平均荧光强度与H2O2浓度相关性
Fig. 1 Evaluation of H2O2-induced oxidative stress cell model A:Oxidative stress state of HEK293T cells treated with different concentrations of H2O2 was detected by DCFH-DA. B:Image J software for analyzing correlation between mean fluorescence intensity and H2O2 concentration in Fig. A
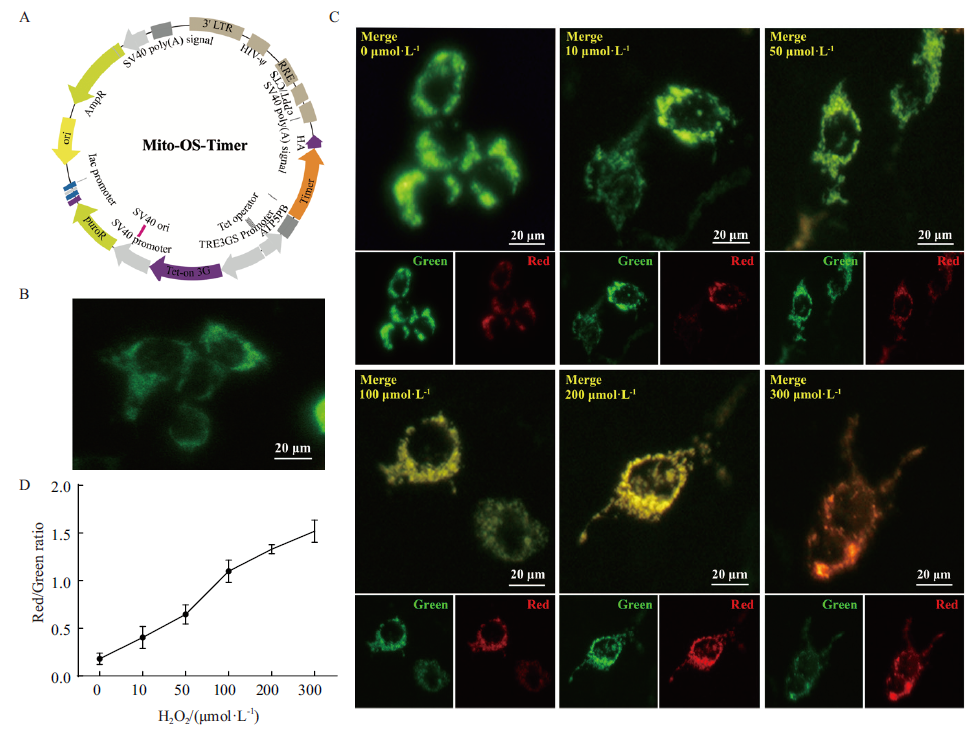
图2 Mito-OS-Timer系统靶向监测H2O2诱导细胞模型的线粒体氧化应激动态变化 A:Mito-OS-Timer,即pLVX-Mito-OS-Timer重组表达质粒图谱;B:评价Mito-OS-Timer质粒转染HEK293T细胞表达情况。Doxycyclin诱导表达,倒置荧光显微镜检测荧光蛋白表达情况,Bar:20 μm;C:Mito-OS-Timer靶向监测H2O2诱导细胞模型的线粒体氧化应激变化情况。6组H2O2处理浓度分别为0、10、50、100、200、300 μmol/L,避光处理24 h(Green:绿色荧光;Red:红色荧光;Merge:红绿荧光叠加;Bar:20 μm);D:Image J软件分析图C中 H2O2诱导浓度与细胞内红绿荧光比变化相关性
Fig. 2 Mito-OS-Timer targets to monitor the dynamic changes of mitochondrial oxidative stress induced by H2O2 in cell models A:Mito-OS-Timer,namely pLVX-Mito-OS-Timer recombinant expression plasmid map. B:Evaluate the expression of HEK293T cells transfected with Mito-OS-Timer plasmid. The fluorescence protein expression was detected by inverted fluorescence microscope after induction by Doxycyclin. Bar:20 μm. C:Mito-OS-Timer targets to monitor the changes of mitochondrial oxidative stress induced by H2O2 in cell models. The concentrations of H2O2 in the 6 groups were 0,10,50,100,200 and 300 μmol/L,respectively. Light avoidance treatment for 24 h(Green:Green fluorescence. Red:Red fluorescence. Merge:Red-green fluorescence superposition. Bar:20 μm). D:Image J software for analyzing correlation between H2O2-induced concentration and changes in intracellular red-green fluorescence ratio in Fig. C
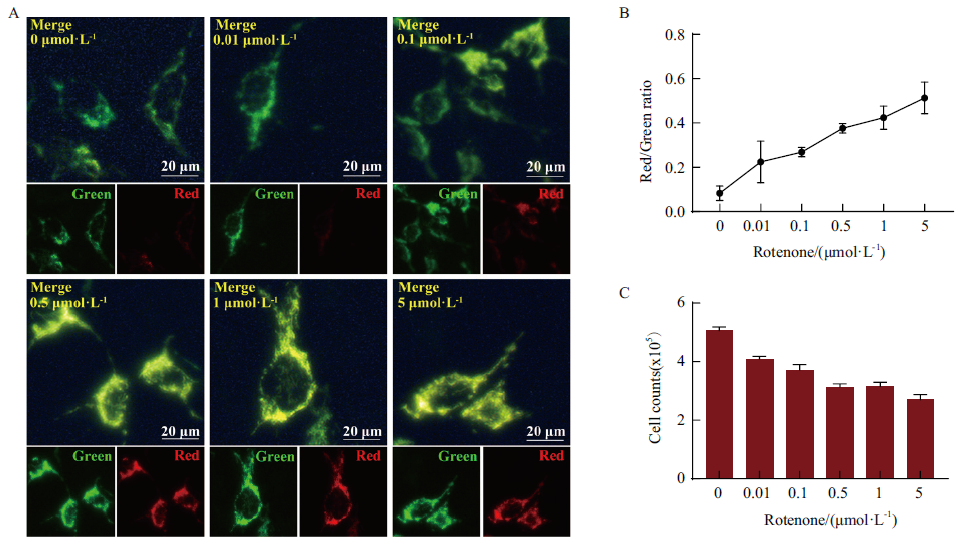
图3 鱼藤酮诱导线粒体氧化应激细胞模型评价Mito-OS-Timer系统 A:基于鱼藤酮诱导氧化应激细胞模型评价Mito-OS-Timer靶向监测效果。采用0、0.01、0.1、0.5、1和5 μmol/L不同浓度的鱼藤酮处理经Doxycyclin诱导表达的HEK293T细胞24 h(Green:绿色荧光;Red:红色荧光;Merge:红绿荧光叠加;Bar:20 μm);B:Image J软件分析图A中鱼藤酮诱导浓度与细胞内红绿荧光比变化相关性;C:不同浓度的鱼藤酮处理HEK293T细胞后的细胞计数统计结果
Fig. 3 Evaluation of Mito-OS-Timer system in rotenone-induced mitochondrial oxidation cell model A:Evaluation of targeted monitoring effect of Mito-OS-Timer based on rotenone-induced oxidative stress cell model Doxycyclin-induced HEK293T cells were treated with rotenone at different concentrations of 0,0.01,0.1,0.5,1 and 5 μmol/L for 24 h(Green:green fluorescence;Red:red fluorescence;Merge:red-green fluorescence superposition;Bar:20 μm);B:Image J software analysis of correlation between rotenone induced concentration and intracellular red-green fluorescence ratio in Fig. A;C:Statistical results of cell count of HEK293T cells treated with different concentrations of rotenone

图4 Mito-OS-Timer监测鱼藤酮诱导线粒体氧化应激细胞模型的流式细胞术检测结果 A:流式细胞术检测不同浓度鱼藤酮处理细胞的荧光表达情况。采用0、0.01、0.1、0.5、1和5 μmol/L鱼藤酮处理HEK293T细胞24 h后,流式细胞仪检测荧光变化强度;B:Image J软件分析图A鱼藤酮诱导浓度与细胞内红绿荧光比变化相关性
Fig. 4 Flow cytometry results of rotenone-induced mitochondrial oxidation in rotenone-induced cell models monitored by Mito-OS-Timer A:Fluorescence expression of rotenone treated cells was detected by flow cytometry. HEK293T cells were treated with 0,0.01,0.1,0.5,1 and 5 μmol/L rotenone for 24 h,and the fluorescence intensity was detected by flow cytometry;B:Image J software analysis the correlation between rotenone induced concentration in Fig. A and changes in intracellular red-green fluorescence ratio

图5 Mito-OS-Timer检测FLCN基因表达与线粒体氧化应激动态变化关系 A:pLVX-shFLCN重组质粒图谱;B:1%琼脂糖凝胶电泳检测图谱(M:DNA Marker;1:经EcoR I /Xho I消化后的FLCN基因片段,1 740 bp;2:经EcoR I /Xho I消化后的pLVX-shRNA载体片段,7 881 bp;3:pLVX-shFLCN质粒DNA,9621 bp);C:蛋白质印迹反应检测转染细胞内FLCN基因表达情况(WT:wide type,正常HEK293T细胞内FLCN基因表达情况;OE:overexpression,转染pcDNA-3HA-FLCN的HEK293T细胞内FLCN过表达情况);D:Mito-OS-Timer检测FLCN基因过表达(OE)、FLCN沉默(RNAi)和0.1 μmol/L鱼藤酮诱导(OS)细胞的线粒体氧化应激动态变化图谱(Green:绿色荧光;Red:红色荧光;Merge:红绿荧光叠加;Bar:20 μm);E:流式细胞术检测Mito-OS-Timer监测细胞情况,细胞同图D处理细胞;F:定量分析流式细胞术结果,即各类细胞内红绿荧光比变化情况
Fig. 5 Relationship between FLCN gene expression and mitochondrial oxidative stress dynamic changes detected by Mito-OS-Timer A:pLVX-shFLCN recombinant plasmid map;B:1% agarose gel electrophoresis detection map(M:DNA Marker;1:FLCN gene fragment digested by EcoR I /Xho I,1 740 bp;2:pLVX-shRNA vector fragment digested by EcoR I /Xho I,7 881 bp;3:pLVX-shFLCN plasmid DNA,9621 bp);C:FLCN gene expression in transfected cells was detected by western blot(WT:Wide type,FLCN gene expression in normal HEK293T cells;OE:overexpression,FLCN overexpression in HEK293T cells transfected with pcDNA-3HA-FLCN);D:Mito-OS-Timer was used to detect dynamic changes of mitochondrial oxidative stress in OE,FLCN silencing(RNAi)and 0.1 μmol/L rotenone-induced(OS)cells(Green:green fluorescence;Red:red fluorescence;Merge:red-green fluorescence superposition;Bar:20 μm);E:Flow cytometry was used to detect Mito-OS-Timer to monitor the cells;F:Quantitative analysis of flow cytometry results for the changes of red-green fluorescence ratio in various cells
| [1] |
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species(ROS)and ROS-induced ROS release[J]. Physiol Rev, 2014, 94(3):909-950.
doi: 10.1152/physrev.00026.2013 URL |
| [2] | Brillo V, Chieregato L, Leanza L, et al. Mitochondrial dynamics, ROS, and cell signaling:a blended overview[J]. Life(Basel), 2021, 11(4):332-351. |
| [3] |
Saotome M, Katoh H, Yaguchi Y, et al. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury[J]. Am J Physiol Heart Circ Physiol, 2009, 296(4):H1125-H1132.
doi: 10.1152/ajpheart.00436.2008 URL |
| [4] |
Tatsuta T, Langer T. Quality control of mitochondria:protection against neurodegeneration and ageing[J]. EMBO J, 2008, 27(2):306-314.
doi: 10.1038/sj.emboj.7601972 URL |
| [5] |
Pereira CV, Lebiedzinska M, Wieckowski MR, et al. Regulation and protection of mitochondrial physiology by sirtuins[J]. Mitochondrion, 2012, 12(1):66-76.
doi: 10.1016/j.mito.2011.07.003 pmid: 21787885 |
| [6] |
Gao H, Liu YH, Zheng M, et al. Characterization of murine mammary stem/progenitor cells in a D-galactose-induced aging model[J]. Aging, 2021, 13(8):11762-11773.
doi: 10.18632/aging.202870 URL |
| [7] |
McDonald S, Rubin P, Chang AY, et al. Pulmonary changes induced by combined mouse beta-interferon(rMuIFN-beta)and irradiation in normal mice—toxic versus protective effects[J]. Radiother Oncol J Eur Soc Ther Radiol Oncol, 1993, 26(3):212-218.
doi: 10.1016/0167-8140(93)90262-7 URL |
| [8] |
Choudhary I, Vo T, Paudel K, et al. Vesicular and extravesicular protein analyses from the airspaces of ozone-exposed mice revealed signatures associated with mucoinflammatory lung disease[J]. Sci Rep, 2021, 11(1):23203-23225.
doi: 10.1038/s41598-021-02256-5 pmid: 34853335 |
| [9] |
Miura T, Shimizu M, Uehara S, et al. Different hepatic concentrations of bromobenzene, 1, 2-dibromobenzene, and 1, 4-dibromobenzene in humanized-liver mice predicted using simplified physiologically based pharmacokinetic models as putative markers of toxicological potential[J]. Chem Res Toxicol, 2020, 33(12):3048-3053.
doi: 10.1021/acs.chemrestox.0c00387 URL |
| [10] |
Mu HM, Liu HW, Zhang JY, et al. Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through ENOS activation and inhibition of ENOS uncoupling[J]. J Cell Mol Med, 2019, 23(3):2174-2183.
doi: 10.1111/jcmm.14130 URL |
| [11] |
Matsuura Y, Yamashita A, Zhao Y, et al. Altered glucose metabolism and hypoxic response in alloxan-induced diabetic atherosclerosis in rabbits[J]. PLoS One, 2017, 12(4):e0175976.
doi: 10.1371/journal.pone.0175976 URL |
| [12] |
Malińska D, Więckowski MR, Michalska B, et al. Mitochondria as a possible target for nicotine action[J]. J Bioenerg Biomembr, 2019, 51(4):259-276.
doi: 10.1007/s10863-019-09800-z pmid: 31197632 |
| [13] |
MacDougall G, Anderton RS, Mastaglia FL, et al. Proteomic analysis of cortical neuronal cultures treated with poly-arginine peptide-18(R18)and exposed to glutamic acid excitotoxicity[J]. Mol Brain, 2019, 12(1):66-81.
doi: 10.1186/s13041-019-0486-8 pmid: 31315638 |
| [14] | Sánchez-Rodríguez MA, Mendoza-Núñez VM. Oxidative stress indexes for diagnosis of health or disease in humans[J]. Oxidative Med Cell Longev, 2019, 2019:4128152. |
| [15] |
Terskikh A, Fradkov A, Ermakova G, et al. “Fluorescent timer”:protein that changes color with time[J]. Science, 2000, 290(5496):1585-1588.
doi: 10.1126/science.290.5496.1585 pmid: 11090358 |
| [16] |
Wilson RJ, Drake JC, Cui D, et al. Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy[J]. Mitochondrion, 2019, 44:20-26.
doi: S1567-7249(17)30196-4 pmid: 29274400 |
| [17] |
Hernandez G, Thornton C, Stotland A, et al. MitoTimer:a novel tool for monitoring mitochondrial turnover[J]. Autophagy, 2013, 9(11):1852-1861.
doi: 10.4161/auto.26501 pmid: 24128932 |
| [18] |
Schmidt LS, Warren MB, Nickerson ML, et al. Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11. 2[J]. Am J Hum Genet, 2001, 69(4):876-882.
pmid: 11533913 |
| [19] | Sies H, Jones D. Oxidative stress[M]// Fink G. Encyclopedia of Stress. Amsterdam:Elsevier, 2007:45-48. |
| [20] |
Zhang BB, Li M, Zou Y, et al. NFκB/Orai1 facilitates endoplasmic Reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease[J]. Front Cell Dev Biol, 2019, 7:202-214.
doi: 10.3389/fcell.2019.00202 URL |
| [21] |
Margolin W. Green fluorescent protein as a reporter for macromolecular localization in bacterial cells[J]. Methods, 2000, 20(1):62-72.
pmid: 10610805 |
| [22] |
Cunningham JT, Rodgers JT, Arlow DH, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex[J]. Nature, 2007, 450(7170):736-740.
doi: 10.1038/nature06322 URL |
| [23] |
Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling[J]. Proc Natl Acad Sci USA, 2006, 103(42):15552-15557.
doi: 10.1073/pnas.0603781103 URL |
| [24] |
Hasumi H, Baba M, Hasumi Y, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN[J]. J Natl Cancer Inst, 2012, 104(22):1750-1764.
doi: 10.1093/jnci/djs418 pmid: 23150719 |
| [1] | 刘宏博, 郑鹏, 印泽, 张同存. 应用CRISPR/Cas9系统下调长链非编码RNA HOTAIR[J]. 生物技术通报, 2017, 33(11): 180-187. |
| [2] | . 淋巴因子和干扰素[J]. , 1986, 0(02): 113-114. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||