








生物技术通报 ›› 2023, Vol. 39 ›› Issue (1): 305-314.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0408
王祥锟1( ), 宋学宏2, 刘金龙3, 郭培红4, 庄晓峰3, 韦良孟1, 周凡1, 张树宇1, 高攀攀1, 魏凯1(
), 宋学宏2, 刘金龙3, 郭培红4, 庄晓峰3, 韦良孟1, 周凡1, 张树宇1, 高攀攀1, 魏凯1( )
)
收稿日期:2022-04-03
出版日期:2023-01-26
发布日期:2023-02-02
作者简介:王祥锟,男,硕士研究生,研究方向:动物疫病防控;E-mail: 基金资助:
WANG Xiang-kun1( ), SONG Xue-hong2, LIU Jin-long3, GUO Pei-hong4, ZHUANG Xiao-feng3, WEI Liang-meng1, ZHOU Fan1, ZHANG Shu-yu1, GAO Pan-pan1, WEI Kai1(
), SONG Xue-hong2, LIU Jin-long3, GUO Pei-hong4, ZHUANG Xiao-feng3, WEI Liang-meng1, ZHOU Fan1, ZHANG Shu-yu1, GAO Pan-pan1, WEI Kai1( )
)
Received:2022-04-03
Published:2023-01-26
Online:2023-02-02
摘要:
旨为推动新型冠状病毒(2019-nCoV)亚单位疫苗的开发并探索筛选适用于该疫苗的高效免疫增强剂,本试验分别诱导表达2019-nCoV- S和2019-nCoV- N两个蛋白,经纯化后测定目的蛋白含量,并分别加入不同浓度的松花粉多糖(PPPS)(200、400、800 mg/mL)作为佐剂制备基因工程亚单位疫苗,黄芪多糖佐剂作为对照。选取100只SPF级BALB/c小鼠随机分为10组,每种亚单位疫苗使用5组,免疫疫苗后检测各组小鼠免疫指标,探讨PPPS对2019-nCoV- S和2019-nCoV- N两种亚单位疫苗的免疫增强效果。结果显示,重组表达并纯化的目的蛋白分别在55.68 kD和45.64 kD处出现单一条带,经鉴定表达正确,蛋白质量浓度分别为1.12 mg/mL和0.66 mg/mL。用制作成的含佐剂亚单位疫苗免疫小鼠后发现400 mg/mL PPPS对两种疫苗的免疫效果均有显著的提升效果,并且效果优于黄芪多糖佐剂。综上所述,两种重组蛋白均能诱导较高的抗体水平,PPPS可以作为2019-nCoV亚单位疫苗的候选佐剂。
王祥锟, 宋学宏, 刘金龙, 郭培红, 庄晓峰, 韦良孟, 周凡, 张树宇, 高攀攀, 魏凯. 新型冠状病毒亚单位疫苗研制及其高效免疫增强剂的筛选[J]. 生物技术通报, 2023, 39(1): 305-314.
WANG Xiang-kun, SONG Xue-hong, LIU Jin-long, GUO Pei-hong, ZHUANG Xiao-feng, WEI Liang-meng, ZHOU Fan, ZHANG Shu-yu, GAO Pan-pan, WEI Kai. Novel Coronavirus Subunit Vaccine and Screening of Its Efficient Immune Enhancer[J]. Biotechnology Bulletin, 2023, 39(1): 305-314.
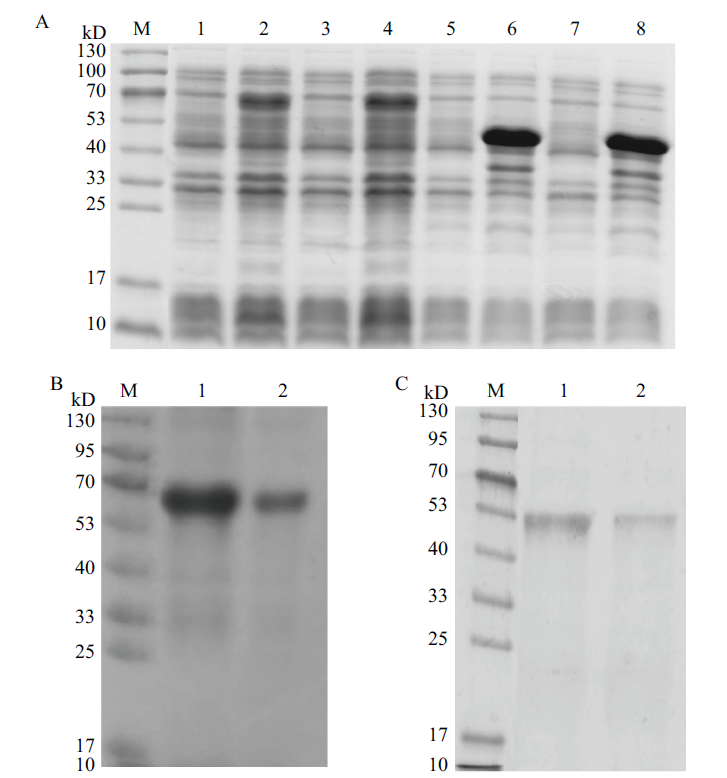
图1 2019-nCoV- S和2019-nCoV- N重组蛋白的诱导表达及纯化 M,Marker;A:1,pET28a(+)-2019-nCoV- S/ BL21菌株1诱导前菌体总蛋白;2,pET28a(+)-2019-nCoV- S/ BL21菌株1诱导后菌体总蛋白;3,pET28a(+)-2019-nCoV- S/ BL21菌株2诱导前菌体总蛋白;4,pET28a(+)-2019-nCoV- S/ BL21菌株2诱导后菌体总蛋白;5,pET28a(+)-2019-nCoV- N/ BL21菌株1诱导前菌体总蛋白;6,pET28a(+)-2019-nCoV- N/ BL21菌株1诱导后菌体总蛋白;7,pET28a(+)-2019-nCoV- N/ BL21菌株2诱导前菌体总蛋白;8,pET28a(+)-2019-nCoV- N/ BL21菌株2诱导后菌体总蛋白。B:1,纯化后的2019-nCoV- S重组蛋白,上样20 μL;2,纯化后的2019-nCoV- S重组蛋白,上样10 μL。C:1,纯化后的2019-nCoV- N重组蛋白,上样20 μL;2,纯化后的2019-nCoV- N重组蛋白,上样10 μL
Fig. 1 Induced expressions and purifications of recombinant protein 2019-nCoV-S and 2019-nCoV-N M, Marker. A: 1, Total cell protein of pET28a(+)-2019-nCoV-S/BL21 strain 1 before induction. 2, Total cell protein pET28a(+)-2019-nCoV-S/BL21 strain 1 after induction. 3, Total cell protein of pET28a(+)-2019-nCoV-S / BL21 strain 2 before induction. 4, Total cell protein of pET28a(+)-2019-nCoV-S/BL21 strain after induction. 2. 5, Total cell protein of pET28a(+)-2019-nCoV-N/BL21 strain 1 before induction; 6, Total cell protein of pET28a(+)-2019-nCoV-N/ L21 strain 1 after induction. 7, Total cell protein of pET28a(+)-2019-nCoV-N / BL21 strain 2 before induction. 8,Total cell protein of pET28a(+)-2019-nCoV-N /BL21 strain 2 after induction. B: 1, The purified recombinant protein 2019-nCoV-S was sampled at 20 μL. 2, The purified recombinant protein 2019-nCoV-S was sampled at 10 μL. C: 1, The purified recombinant protein 2019-nCoV-N was sampled at 20 μL. 2, The purified recombinant protein 2019-nCoV-N was sampled at 10 μL

图3 疫苗免疫后特异性IgG抗体的测定结果 A:免疫2019-nCoV- S亚单位疫苗后血清中IgG含量的变化;B:免疫2019-nCoV- N亚单位疫苗后血清中IgG含量的变化。肩注相同小写字母表示差异不显著(P > 0.05),不同小写字母表示差异显著(P < 0.05),不同大写字母表示差异极显著(P < 0.01),下同
Fig. 3 Determination of specific IgG antibody after immu-nization with vaccine A: Changes of 2019-nCoV-S subunit vaccine on the content of IgG in serum. B: Changes of 2019-nCoV-N subunit vaccine on the content of IgG in serum. Shoulder injection of the same lowercase letters indicates no significant difference(P > 0.05), different lowercase letters indicate significant differences(P < 0.05), and different uppercase letters indicate extremely significant differences(P < 0.01), the same below
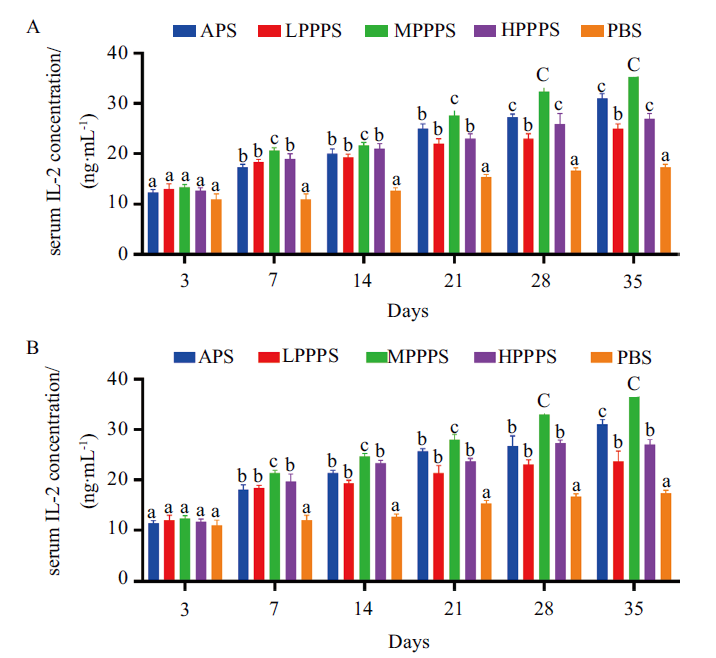
图4 疫苗免疫后血清中IL-2的含量变化 A:免疫2019-nCoV- S亚单位疫苗后血清中IL-2含量的变化;B:免疫2019-nCoV- N亚单位疫苗对血清中IL-2含量的变化
Fig. 4 Changes of IL-2 content in serum after immuniza-tion with vaccine A: Changes of IL-2 content in serum after immunization with 2019-nCoV-S subunit vaccine. B: Changes of IL-2 content in serum after immunization with 2019-nCoV-N subunit vaccine.

图5 疫苗免疫后外周血淋巴细胞转换率的变化 A:免疫2019-nCoV- S亚单位疫苗后外周血淋巴细胞转换率的变化;B:免疫2019-nCoV- N亚单位疫苗后后外周血淋巴细胞转换率的变化
Fig. 5 Changes of peripheral blood lymphocyte conversion rate after immunization with vaccine A: Changes of peripheral blood lymphocyte conversion rate after immunization with 2019-nCoV-S subunit vaccine. B: Changes of peripheral blood lymphocyte conversion rate after immunization with 2019-nCoV-N subunit vaccine.
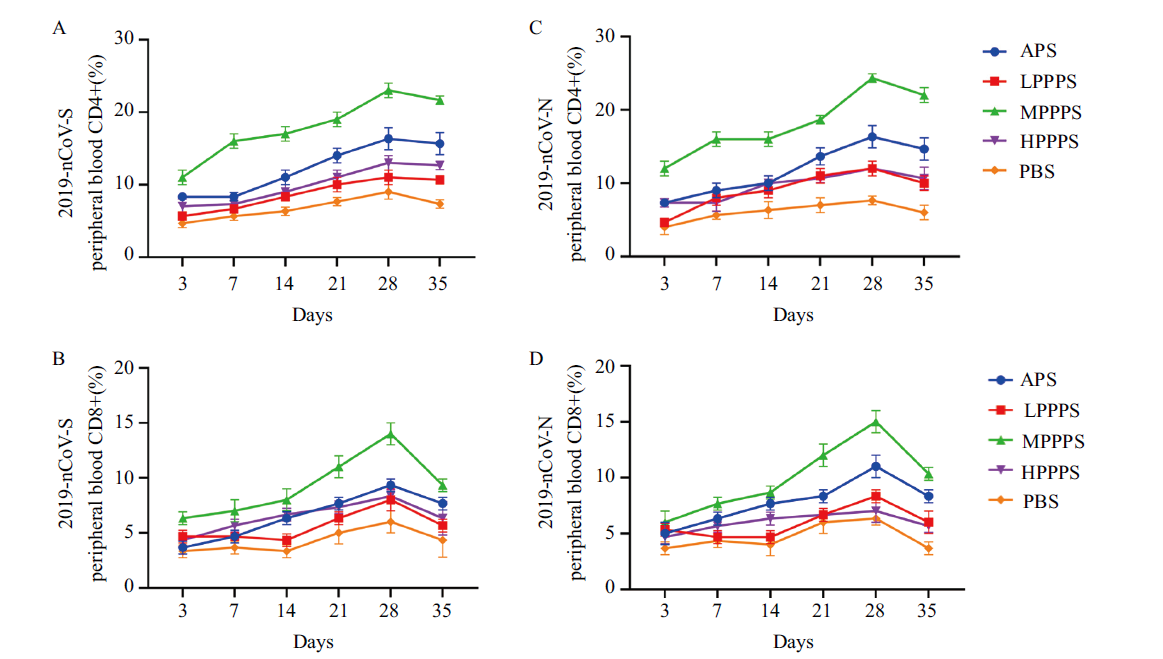
图6 疫苗免疫后外周血CD4+T和CD8+T细胞含量的测定结果 A:免疫2019-nCoV- S亚单位疫苗后血液CD4+T细胞含量的变化;B:免疫2019-nCoV- S亚单位疫苗后血液CD8+T细胞含量的变化;C:免疫2019-nCoV- N亚单位疫苗后血液CD4+T细胞含量的变化;D:免疫2019-nCoV- N亚单位疫苗后血液CD8+T细胞含量的变化
Fig. 6 Determination of CD4+T and CD8+T cell content in peripheral blood after immunization with vaccine A: Changes of CD4+T cell content in blood after immunization with 2019 n-CoV-S subunit vaccine. B: Changes of CD8+T cell content in blood after immunization with 2019 n-CoV-S subunit vaccine. C: Changes of CD4+T cell content in blood after immunization with 2019 n-CoV-N subunit vaccine. D: Changes of CD8+T cell content in blood after immunization with 2019 n-CoV-N subunit vaccine.
| [1] |
Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19[J]. Nat Rev Microbiol, 2021, 19(3): 141-154.
doi: 10.1038/s41579-020-00459-7 pmid: 33024307 |
| [2] |
Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis[J]. J Microbiol Immunol Infect, 2021, 54(2): 159-163.
doi: 10.1016/j.jmii.2020.03.022 URL |
| [3] |
Bhat EA, Khan J, Sajjad N, et al. SARS-CoV-2: insight in genome structure, pathogenesis and viral receptor binding analysis - An updated review[J]. Int Immunopharmacol, 2021, 95: 107493.
doi: 10.1016/j.intimp.2021.107493 URL |
| [4] | 陈嘉源, 施劲松, 丘栋安, 等. 2019新型冠状病毒基因组的生物信息学分析[J]. 生物信息学, 2020, 18(2): 96-102. |
| Chen JY, Shi JS, Qiu DA, et al. Bioinformatics analysis of the 2019 novel coronavirus genome[J]. Chin J Bioinform, 2020, 18(2): 96-102. | |
| [5] |
Sharma A, Ahmad Farouk I, Lal SK. COVID-19: a review on the novel coronavirus disease evolution, transmission, detection, control and prevention[J]. Viruses, 2021, 13(2): 202.
doi: 10.3390/v13020202 URL |
| [6] |
Khalaj-Hedayati A, Chua CLL, Smooker P, et al. Nanoparticles in influenza subunit vaccine development: Immunogenicity enhancement[J]. Influenza Other Respir Viruses, 2020, 14(1): 92-101.
doi: 10.1111/irv.12697 URL |
| [7] |
Awadasseid A, Wu YL, Tanaka Y, et al. Effective drugs used to combat SARS-CoV-2 infection and the current status of vaccines[J]. Biomed Pharmacother, 2021, 137: 111330.
doi: 10.1016/j.biopha.2021.111330 pmid: 33550043 |
| [8] | 刘娜, 朱琳, 王玉建, 等. 泰山松花粉多糖对新城疫-禽流感二联灭活苗的免疫增强作用[J]. 中国兽医学报, 2018, 38(11): 2068-2072. |
| Liu N, Zhu L, Wang YJ, et al. Effects of polysaccharides from Taishan pine pollen on the immunization of Newcastle disease-avian influenza[J]. Chin J Vet Sci, 2018, 38(11): 2068-2072. | |
| [9] | 朱福杰. 泰山松花粉多糖对禽波氏杆菌DNA疫苗的免疫增强作用[D]. 泰安: 山东农业大学, 2015. |
| Zhu FJ. Immune-enhancing effects of Taishan Pinus massoniana pollen polysaccharides on DNA vaccine expressing Bordetella avium OmpA[D]. Tai'an: Shandong Agricultural University, 2015. | |
| [10] |
Cui GL, Zhong SX, Yang SF, et al. Effects of Taishan Pinus massoniana pollen polysaccharide on the subunit vaccine of Proteus mirabilis in birds[J]. Int J Biol Macromol, 2013, 56: 94-98.
doi: 10.1016/j.ijbiomac.2013.02.006 URL |
| [11] |
Wei K, Sun ZH, Yan ZG, et al. Effects of Taishan Pinus massoniana pollen polysaccharide on immune response of rabbit haemorrhagic disease tissue inactivated vaccine and on production performance of Rex rabbits[J]. Vaccine, 2011, 29(14): 2530-2536.
doi: 10.1016/j.vaccine.2011.01.068 URL |
| [12] |
Li B, Wei K, Yang SF, et al. Immunomodulatory effects of Taishan Pinus massoniana pollen polysaccharide and Propolis on immunosuppressed chickens[J]. Microb Pathog, 2015, 78: 7-13.
doi: 10.1016/j.micpath.2014.11.010 URL |
| [13] | Zhou J, Wei K, Wang C, et al. Oral immunisation with Taishan Pinus massoniana pollen polysaccharide adjuvant with recombinant Lactococcus lactis-expressing Proteus mirabilis ompA confers optimal protection in mice[J]. Allergol Immunopathol(Madr), 2017, 45(5): 496-505. |
| [14] |
Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study[J]. Lancet, 2020, 395(10225): 689-697.
doi: S0140-6736(20)30260-9 pmid: 32014114 |
| [15] | Du LY, He YX, Jiang SB, et al. Development of subunit vaccines against severe acute respiratory syndrome[J]. Drugs Today(Barc), 2008, 44(1): 63-73. |
| [16] |
Deng MP, Hu ZH, Wang HL, et al. Developments of subunit and VLP vaccines against influenza A virus[J]. Virol Sin, 2012, 27(3): 145-153.
doi: 10.1007/s12250-012-3241-1 URL |
| [17] |
Li YJ, Xu FX, Zheng MM, et al. Maca polysaccharides: a review of compositions, isolation, therapeutics and prospects[J]. Int J Biol Macromol, 2018, 111: 894-902.
doi: S0141-8130(17)33431-1 pmid: 29341924 |
| [18] |
Mbow ML, de Gregorio E, Valiante NM, et al. New adjuvants for human vaccines[J]. Curr Opin Immunol, 2010, 22(3): 411-416.
doi: 10.1016/j.coi.2010.04.004 pmid: 20466528 |
| [19] |
Sun BN, Yu S, Zhao DY, et al. Polysaccharides as vaccine adjuvants[J]. Vaccine, 2018, 36(35): 5226-5234.
doi: S0264-410X(18)31009-0 pmid: 30057282 |
| [20] | 董雯雯. 禽波氏杆菌ompA-IgY Fc融合蛋白表达及松花粉多糖对鸡体免疫应答的调节作用[D]. 泰安: 山东农业大学, 2017. |
| Dong WW. Immunomodulatory functions of recombinant Bordetella avium ompA-IgY fc and Taishan Pinus massoniana pollen polysaccharides on chickens[D]. Tai'an: Shandong Agricultural University, 2017. | |
| [21] |
Porter KR, Raviprakash K. DNA vaccine delivery and improved immunogenicity[J]. Curr Issues Mol Biol, 2017, 22: 129-138.
doi: 10.21775/cimb.022.129 pmid: 27831541 |
| [22] |
Guo LW, Liu JG, Hu YL, et al. Astragalus polysaccharide and sulfated epimedium polysaccharide synergistically resist the immunosuppression[J]. Carbohydr Polym, 2012, 90(2): 1055-1060.
doi: 10.1016/j.carbpol.2012.06.042 URL |
| [23] |
Kallon S, Li XR, Ji J, et al. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo[J]. J Anim Sci Biotechnol, 2013, 4(1): 22.
doi: 10.1186/2049-1891-4-22 URL |
| [24] |
Zhu L, Wang QJ, Wang YJ, et al. Comparison of immune effects between Brucella recombinant Omp10-Omp28-L7/L12 proteins expressed in eukaryotic and prokaryotic systems[J]. Front Vet Sci, 2020, 7: 576.
doi: 10.3389/fvets.2020.00576 URL |
| [25] |
Wang HN, Shan SF, Wang SJ, et al. Fused IgY fc and polysaccharide adjuvant enhanced the immune effect of the recombinant VP2 and VP5 subunits-A prospect for improvement of infectious bursal disease virus subunit vaccine[J]. Front Microbiol, 2017, 8: 2258.
doi: 10.3389/fmicb.2017.02258 pmid: 29184548 |
| [26] | 赵雪. 禽波氏杆菌ompA表达、松花粉多糖亚单位疫苗制备及其免疫原性分析[D]. 泰安: 山东农业大学, 2014. |
| Zhao X. Expression of Bordetella avium ompA, preparation of TPPPS-subunit vaccines and immunogenicity analysis of ompA[D]. Tai'an: Shandong Agricultural University, 2014. | |
| [27] |
Mizui M. Natural and modified IL-2 for the treatment of cancer and autoimmune diseases[J]. Clin Immunol, 2019, 206: 63-70.
doi: S1521-6616(18)30624-7 pmid: 30415086 |
| [28] |
Zander R, Schauder D, Xin G, et al. CD4+ T cell help is required for the formation of a cytolytic CD8+ T cell subset that protects against chronic infection and cancer[J]. Immunity, 2019, 51(6): 1028-1042.e4.
doi: S1074-7613(19)30453-4 pmid: 31810883 |
| [29] |
Yang Y, Wei K, Yang SF, et al. Co-adjuvant effects of plant polysaccharide and propolis on chickens inoculated with Bordetella avium inactivated vaccine[J]. Avian Pathol, 2015, 44(4): 248-253.
doi: 10.1080/03079457.2015.1040372 pmid: 25989924 |
| [1] | 刘晓玫, 王东鑫, 张春, 魏双施. AAV介导的RNAi对SARS-CoV-2 S基因表达的抑制作用[J]. 生物技术通报, 2022, 38(3): 188-193. |
| [2] | 李家俊, 郑潇, 盛杰, 徐瑶. 新型冠状病毒及其临床检测方法研究进展[J]. 生物技术通报, 2021, 37(4): 282-292. |
| [3] | 吕继洲, 吴绍强, 张舟, 邓俊花, 袁向芬, 王彩霞, 冯春燕, 林祥梅. 新型冠状病毒实时荧光双重逆转录RPA的建立及其在食品检测中的应用[J]. 生物技术通报, 2020, 36(11): 238-244. |
| [4] | 周梅仙,周业飞. 蛹虫草基质多糖对鸡传染性支气管炎灭活疫苗的免疫佐剂作用[J]. 生物技术通报, 2016, 32(12): 108-112. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||