








生物技术通报 ›› 2023, Vol. 39 ›› Issue (5): 152-159.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1301
梁成刚1,2( ), 汪燕1, 李天3, 大杉立2, 青木直大2(
), 汪燕1, 李天3, 大杉立2, 青木直大2( )
)
收稿日期:2022-10-23
出版日期:2023-05-26
发布日期:2023-06-08
通讯作者:
梁成刚,男,博士,副教授,研究方向:作物生理与分子调控;E-mail:201503001@gznu.edu.cn;基金资助:
LIANG Cheng-gang1,2( ), WANG Yan1, LI Tian3, OHSUGI Ryu2, AOKI Naohiro2(
), WANG Yan1, LI Tian3, OHSUGI Ryu2, AOKI Naohiro2( )
)
Received:2022-10-23
Published:2023-05-26
Online:2023-06-08
摘要:
探讨SP1在水稻“源-库”系统中的调控作用,为解析稻穗形成的机理和青贮饲料稻分子育种提供科学参考。以sp1与WT为材料,进行氮处理和生长分析,表型鉴定、碳水化合物测定和RT-qPCR检测。结果发现,氮处理10 d时,sp1与WT无表型差异,但在高、中氮条件下,sp1相对生长速率显著提高;24 d时sp1叶鞘、根、植株干重提高,叶鞘氮含量降低、碳/氮比值提高,说明充裕的氮能促进sp1生长,SP1可能影响叶鞘碳-氮平衡。抽穗期sp1株高显著降低,分蘖数显著提高,稻穗变小并伴随高位分蘖发生。孕穗期sp1茎、鞘中碳水化合物含量与WT差异较小,但抽穗期茎中淀粉、蔗糖和叶鞘中淀粉、蔗糖、葡萄糖和果糖含量明显提高。SP1在叶鞘基部高表达,推测突变SP1能抑制蔗糖由茎鞘向穗的运输,导致高位分蘖发生和稻穗变小。SP1可应用于青贮饲料稻分子育种。
梁成刚, 汪燕, 李天, 大杉立, 青木直大. SP1调控碳水化合物分配对穗形态的影响[J]. 生物技术通报, 2023, 39(5): 152-159.
LIANG Cheng-gang, WANG Yan, LI Tian, OHSUGI Ryu, AOKI Naohiro. Effect of SP1 on Panicle Architecture by Regulating Carbohydrate Remobilization[J]. Biotechnology Bulletin, 2023, 39(5): 152-159.
| 基因Gene | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| SP1 | GTTCGAACCGCACGTCTAGT | GGGGACTCATATACATCCACCC |
| UBI | GGAGCTGCTGCTGTTCTTGG | CACAATGAAACGGGACACGA |
表1 RT-qPCR分析使用的基因引物
Table 1 The primers of genes for RT-qPCR analysis
| 基因Gene | 正向引物 Forward primer(5'-3') | 反向引物 Reverse primer(5'-3') |
|---|---|---|
| SP1 | GTTCGAACCGCACGTCTAGT | GGGGACTCATATACATCCACCC |
| UBI | GGAGCTGCTGCTGTTCTTGG | CACAATGAAACGGGACACGA |

图1 氮处理10 d时植株表型(A)与氮处理10-20 d相对生长速率(B) HN:高氮处理;MN:中氮处理;LN:低氮处理。*与**分别表示差异达到显著(P<0.05)或极显著(P<0.01)水平,下同。标尺为10 cm
Fig. 1 Plant phenotype at 10 d after nitrogen treatment (A), and the relative growth rate from 10 d to 20 d after nitrogen treatment(B) HN: High nitrogen treatment; MN: middle nitrogen treatment; LN: low nitrogen treatment. * and ** indicate significant(P<0.05)or extremely significant(P<0.01)difference. The same below. Scale bar=10 cm
| 材料 Material | 氮处理 Nitrogen treatment | 根重 Root weight/mg | 叶鞘重 Leaf sheath weight/mg | 叶重 Leaf weight/mg | 植株干重 Plant dry weight/mg |
|---|---|---|---|---|---|
| WT | HN-10 d | 33.94±0.54 | 38.66±1.44 | 43.56±0.76 | 116.16±2.12 |
| sp1 | HN-10 d | 35.66±1.17 | 43.96±0.83* | 44.90±0.82 | 124.52±1.08** |
| WT | MN-10 d | 38.18±1.37 | 41.78±1.05 | 40.98±1.56 | 120.94±2.54 |
| sp1 | MN-10 d | 38.14±0.78 | 45.52±0.91* | 40.32±1.55 | 123.98±2.05 |
| WT | LN-10 d | 37.76±1.09 | 37.64±1.16 | 32.06±1.33 | 107.46±1.23 |
| sp1 | LN-10 d | 42.84±1.22 | 39.74±0.61 | 34.58±0.54* | 117.16±1.33** |
| WT | HN-24 d | 106.84±3.70 | 210.38±8.05 | 218.10±14.36 | 535.32±12.39 |
| sp1 | HN-24 d | 145.24±11.94* | 294.80±27.57* | 241.12±19.97 | 681.16±31.42** |
| WT | MN-24 d | 152.26±11.37 | 213.24±13.75 | 164.36±3.92 | 529.86±13.56 |
| sp1 | MN-24 d | 194.00±19.20* | 297.76±33.23* | 212.24±18.34** | 737.86±37.61** |
| WT | LN-24 d | 126.44±5.50 | 170.76±5.10 | 104.60±1.64 | 401.80±7.08 |
| sp1 | LN-24 d | 144.42±2.64* | 193.42±4.76* | 99.26±7.14 | 437.10±5.15** |
表2 不同氮浓度下的水稻农艺性状
Table 2 Agronomic traits of rice under different nitrogen conditions
| 材料 Material | 氮处理 Nitrogen treatment | 根重 Root weight/mg | 叶鞘重 Leaf sheath weight/mg | 叶重 Leaf weight/mg | 植株干重 Plant dry weight/mg |
|---|---|---|---|---|---|
| WT | HN-10 d | 33.94±0.54 | 38.66±1.44 | 43.56±0.76 | 116.16±2.12 |
| sp1 | HN-10 d | 35.66±1.17 | 43.96±0.83* | 44.90±0.82 | 124.52±1.08** |
| WT | MN-10 d | 38.18±1.37 | 41.78±1.05 | 40.98±1.56 | 120.94±2.54 |
| sp1 | MN-10 d | 38.14±0.78 | 45.52±0.91* | 40.32±1.55 | 123.98±2.05 |
| WT | LN-10 d | 37.76±1.09 | 37.64±1.16 | 32.06±1.33 | 107.46±1.23 |
| sp1 | LN-10 d | 42.84±1.22 | 39.74±0.61 | 34.58±0.54* | 117.16±1.33** |
| WT | HN-24 d | 106.84±3.70 | 210.38±8.05 | 218.10±14.36 | 535.32±12.39 |
| sp1 | HN-24 d | 145.24±11.94* | 294.80±27.57* | 241.12±19.97 | 681.16±31.42** |
| WT | MN-24 d | 152.26±11.37 | 213.24±13.75 | 164.36±3.92 | 529.86±13.56 |
| sp1 | MN-24 d | 194.00±19.20* | 297.76±33.23* | 212.24±18.34** | 737.86±37.61** |
| WT | LN-24 d | 126.44±5.50 | 170.76±5.10 | 104.60±1.64 | 401.80±7.08 |
| sp1 | LN-24 d | 144.42±2.64* | 193.42±4.76* | 99.26±7.14 | 437.10±5.15** |
| 处理/材料 Treatment/ Material | 氮含量Nitrogen content/% | 碳含量Carbon content/% | 碳/氮比Carbon/Nitrogen ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | |
| HN-WT | 1.68±0.05 | 2.07±0.01 | 3.79±0.04 | 45.26±1.00 | 40.99±0.20 | 43.54±0.39 | 27.10±1.24 | 19.77±0.20 | 11.48±0.10 |
| HN-sp1 | 1.68±0.02 | 1.90±0.04* | 3.71±0.03 | 43.80±0.12 | 40.82±0.10 | 43.15±0.03 | 26.09±0.35 | 21.54±0.42* | 11.63±0.10 |
| MN-WT | 1.12±0.04 | 1.32±0.04 | 3.17±0.06 | 45.00±0.29 | 41.33±0.06 | 43.38±0.07 | 40.41±1.37 | 31.48±0.20 | 13.70±0.10 |
| MN-sp1 | 1.07±0.02 | 1.28±0.02 | 2.97±0.07 | 43.59±0.35 | 40.86±0.10* | 42.89±0.12 | 40.65±0.87 | 31.87±0.42 | 14.48±0.10 |
| LN-WT | 0.86±0.03 | 1.08±0.00 | 2.67±0.06 | 47.31±0.38 | 43.09±0.32 | 42.95±0.16 | 55.63±2.47 | 39.94±0.44 | 16.14±0.38 |
| LN-sp1 | 0.79±0.04 | 0.89±0.01** | 2.46±0.06 | 48.32±0.46 | 44.20±0.21 | 43.51±0.33 | 61.76±2.88 | 49.52±0.63** | 17.75±0.36 |
表3 不同氮浓度处理24 d植株各器官中的碳氮百分含量
Table 3 Carbon and nitrogen content in rice plant organs at 24 d under nitrogen treatment
| 处理/材料 Treatment/ Material | 氮含量Nitrogen content/% | 碳含量Carbon content/% | 碳/氮比Carbon/Nitrogen ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | 根 Root | 叶鞘 Leaf sheath | 叶片 Leaf blade | |
| HN-WT | 1.68±0.05 | 2.07±0.01 | 3.79±0.04 | 45.26±1.00 | 40.99±0.20 | 43.54±0.39 | 27.10±1.24 | 19.77±0.20 | 11.48±0.10 |
| HN-sp1 | 1.68±0.02 | 1.90±0.04* | 3.71±0.03 | 43.80±0.12 | 40.82±0.10 | 43.15±0.03 | 26.09±0.35 | 21.54±0.42* | 11.63±0.10 |
| MN-WT | 1.12±0.04 | 1.32±0.04 | 3.17±0.06 | 45.00±0.29 | 41.33±0.06 | 43.38±0.07 | 40.41±1.37 | 31.48±0.20 | 13.70±0.10 |
| MN-sp1 | 1.07±0.02 | 1.28±0.02 | 2.97±0.07 | 43.59±0.35 | 40.86±0.10* | 42.89±0.12 | 40.65±0.87 | 31.87±0.42 | 14.48±0.10 |
| LN-WT | 0.86±0.03 | 1.08±0.00 | 2.67±0.06 | 47.31±0.38 | 43.09±0.32 | 42.95±0.16 | 55.63±2.47 | 39.94±0.44 | 16.14±0.38 |
| LN-sp1 | 0.79±0.04 | 0.89±0.01** | 2.46±0.06 | 48.32±0.46 | 44.20±0.21 | 43.51±0.33 | 61.76±2.88 | 49.52±0.63** | 17.75±0.36 |
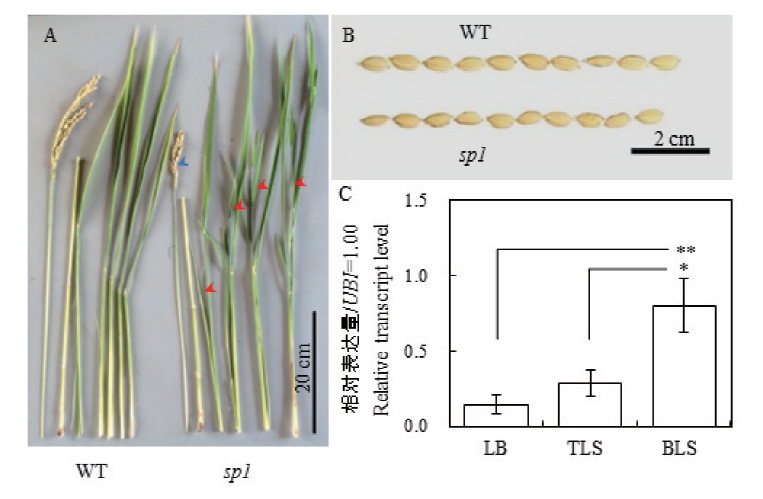
图2 水稻sp1的表型特征与SP1的RT-qRCR分析 A:水稻野生型与sp1植株的表型。红色箭头指向sp1高位分蘖,蓝色箭头指向sp1稻穗;B:水稻野生型与sp1的谷粒性状;C:SP1的表达量分析。LB:叶片,TLS:叶鞘上部,BLS:叶鞘基部
Fig. 2 Phenotype of rice sp1 and RT-qRCR analysis of SP1 gene A: Plant of WT and sp1. Red arrows point to high-node tillerings of sp1. Blue arrow points to panicle of sp1. B: Cereals of WT and sp1. C: RT-qRCR analysis of SP1 gene. LB: Leaf blade. TLS: Top of leaf sheath. BLS: Base of leaf sheath
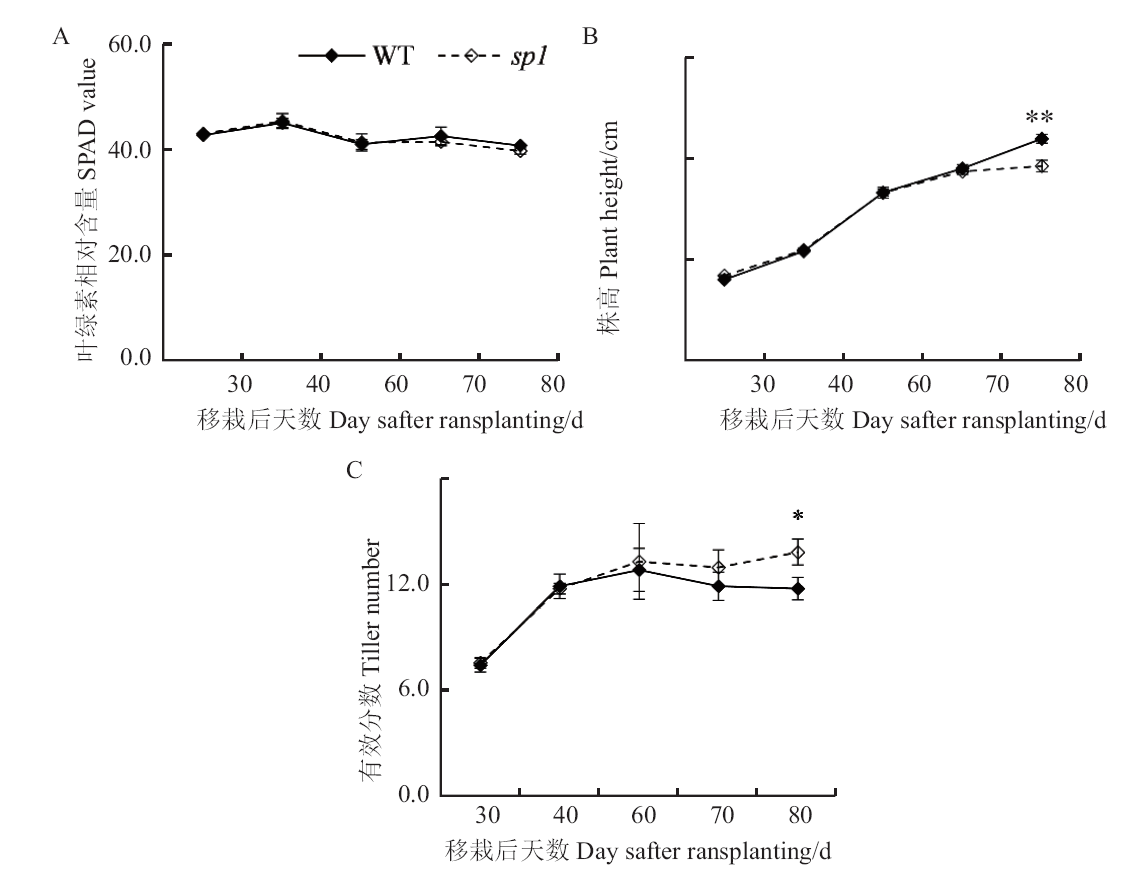
图3 移栽后sp1顶端全展叶叶绿素相对含量SPAD值(A)、株高(B)与有效分蘖(C)的动态变化
Fig. 3 Dynamic changes of SPAD value in the top comple-tely-expanded leaf(A), plant height(B)and effective tiller number(C)of sp1 after transplanting
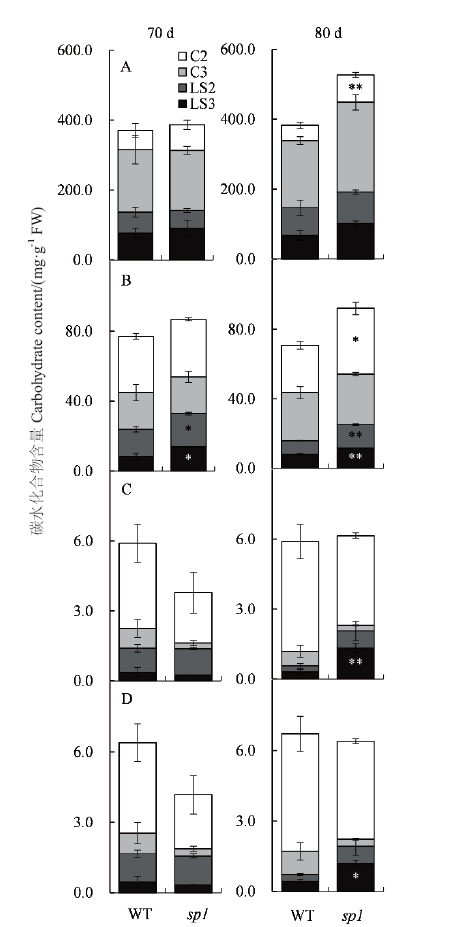
图4 移栽后70 和80 d水稻sp1顶端组织的淀粉(A)、蔗糖(B)、葡萄糖(C)和果糖(D)含量 C2:穗下第2-3节间茎;C3:穗下第3-4节间茎;LS2:倒2叶鞘;LS3:倒3叶鞘
Fig. 4 Contents of starch(A), sucrose(B), glucose,(C)and fructose(D)in the upper tissues of sp1 collected at 70 d and 80 d after transplanting C2: The internode culm between 2nd and 3rd below panicle. C3: The internode culm between 3rd and 4th below panicle. LS2: The second top leaf sheath. LS3: The third top leaf sheath
| [1] |
代明笠, 邱先进, 陈凯, 等. 水稻不同源库相关基因聚合的产量效应分析[J]. 核农学报, 2020, 34(6): 1129-1137.
doi: 10.11869/j.issn.100-8551.2020.06.1129 |
|
Dai ML, Qiu XJ, Chen K, et al. Analysis of pyramiding effect of sink-source related genes on grain yield in rice[J]. J Nucl Agric Sci, 2020, 34(6): 1129-1137.
doi: 10.11869/j.issn.100-8551.2020.06.1129 |
|
| [2] | 赵明, 李少昆. 作物产量研究三理论及其应用与发展[J]. 北京农业大学学报, 1995(S1): 70-75. |
| Zhao M, Li SK. The application and development of three theroies in crop yield study(review)[J]. Acta Agric Univ Pekin, 1995(S1): 70-75. | |
| [3] | 丛斌, 贾红武, 李严, 等. 水稻幼穗形态发生与顶端分生组织的研究[J]. 西北植物学报, 1999, 19(3): 415-421, 573. |
| Cong B, Jia HW, Li Y, et al. Studies on morphogenesis and shoot apical meristem(SAM)of rudimentary panicle in rice[J]. Acta Bot Boreali Occidentalia Sin, 1999, 19(3): 415-421, 573. | |
| [4] |
Caselli F, Zanarello F, Kater MM, et al. Crop reproductive meristems in the genomic era: a brief overview[J]. Biochem Soc Trans, 2020, 48(3): 853-865.
doi: 10.1042/BST20190441 URL |
| [5] |
Komatsu K, Maekawa M, Ujiie S, et al. LAX and SPA: major regulators of shoot branching in rice[J]. Proc Natl Acad Sci USA, 2003, 100(20): 11765-11770.
doi: 10.1073/pnas.1932414100 pmid: 13130077 |
| [6] |
Tabuchi H, Zhang Y, Hattori S, et al. LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems[J]. Plant Cell, 2011, 23(9): 3276-3287.
doi: 10.1105/tpc.111.088765 URL |
| [7] | 刘华清, 吴为人, 段远霖, 等. 水稻小穗特征基因FZP的图位克隆[J]. 遗传学报, 2003, 30(9): 811-816. |
| Liu HQ, Wu WR, Duan YL, et al. Towards the positional cloning of a spikelet identity gene Frizzle Panicle(FZP)in rice(Oryza sativa L.)[J]. Acta Genet Sin, 2003, 30(9): 811-816. | |
| [8] |
Wang YD, Wei SS, He YB, et al. Synergistic roles of LAX1 and FZP in the development of rice sterile Lemma[J]. Crop J, 2020, 8(1): 16-25.
doi: 10.1016/j.cj.2019.06.006 URL |
| [9] |
Qi WW, Sun F, Wang QJ, et al. Rice ethylene-response AP2/ERF factor OsEATB restricts internode elongation by down-regulating a gibberellin biosynthetic gene[J]. Plant Physiol, 2011, 157(1): 216-228.
doi: 10.1104/pp.111.179945 URL |
| [10] | Wu K, Wang SS, Song WZ, et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice[J]. Science, 2020, 367(6478): eaaz2046. |
| [11] |
Ikeda K, Ito M, Nagasawa N, et al. Rice ABERRANT PANICLE ORGANIZATION 1, encoding an F-Box protein, regulates meristem fate[J]. Plant J, 2007, 51(6): 1030-1040.
doi: 10.1111/j.1365-313X.2007.03200.x pmid: 17666027 |
| [12] |
Ikeda-Kawakatsu K, Maekawa M, Izawa T, et al. ABERRANT PANICLE ORGANIZATION 2/RFL, the rice ortholog of Arabidopsis LEAFY, suppresses the transition from inflorescence meristem to floral meristem through interaction with APO1[J]. Plant J, 2012, 69(1): 168-180.
doi: 10.1111/tpj.2011.69.issue-1 URL |
| [13] |
Zhang DP, Zhang MY, Wang YZ, et al. RGB1 regulates rice panicle architecture and grain filling through monitoring cytokinin level in inflorescence meristem and grain abscisic acid level during filling stage[J]. Rice Sci, 2021, 28(4): 317-321.
doi: 10.1016/j.rsci.2021.05.002 URL |
| [14] |
Zhang ZY, Sun XM, Ma XQ, et al. GNP6, a novel allele of MOC1, regulates panicle and tiller development in rice[J]. Crop J, 2021, 9(1): 57-67.
doi: 10.1016/j.cj.2020.04.011 URL |
| [15] |
Zhu ZC, Luo S, Lei B, et al. Locus TUTOU2 determines the panicle apical abortion phenotype of rice(Oryza sativa L.) in tutou2 mutant[J]. J Integr Agric, 2022, 21(3): 621-630.
doi: 10.1016/S2095-3119(20)63447-5 URL |
| [16] |
Ookawa T, Hobo T, Yano M, et al. New approach for rice improvement using a pleiotropic QTL gene for lodging resistance and yield[J]. Nat Commun, 2010, 1: 132.
doi: 10.1038/ncomms1132 |
| [17] |
Ashikari M, Sakakibara H, Lin SY, et al. Cytokinin oxidase regulates rice grain production[J]. Science, 2005, 309(5735): 741-745.
doi: 10.1126/science.1113373 pmid: 15976269 |
| [18] |
Tu B, Tao Z, Wang SG, et al. Loss of Gn1a/OsCKX2 confers heavy-panicle rice with excellent lodging resistance[J]. J Integr Plant Biol, 2022, 64(1): 23-38.
doi: 10.1111/jipb.v64.1 URL |
| [19] |
Huang XZ, Qian Q, Liu ZB, et al. Natural variation at the DEP1 locus enhances grain yield in rice[J]. Nat Genet, 2009, 41(4): 494-497.
doi: 10.1038/ng.352 pmid: 19305410 |
| [20] |
Jiao YQ, Wang YH, Xue DW, et al. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice[J]. Nat Genet, 2010, 42(6): 541-544.
doi: 10.1038/ng.591 pmid: 20495565 |
| [21] |
Wang J, Yu H, Xiong GS, et al. Tissue-specific ubiquitination by IPA1 INTERACTING PROTEIN1 modulates IPA1 protein levels to regulate plant architecture in rice[J]. Plant Cell, 2017, 29(4): 697-707.
doi: 10.1105/tpc.16.00879 URL |
| [22] |
Wang SS, Wu K, Qian Q, et al. Non-canonical regulation of SPL transcription factors by a human OTUB1-like deubiquitinase defines a new plant type rice associated with higher grain yield[J]. Cell Res, 2017, 27(9): 1142-1156.
doi: 10.1038/cr.2017.98 pmid: 28776570 |
| [23] |
Zhang L, Zou YT, Bian Z, et al. Fine mapping and candidate gene prediction of the quantitative trait locus qPL8 for panicle length in rice[J]. Phyton, 2021, 90(3): 789-802.
doi: 10.32604/phyton.2021.014880 URL |
| [24] |
Zhang L, Wang JJ, Wang JM, et al. Quantitative trait locus analysis and fine mapping of the qPL6 locus for panicle length in rice[J]. Theor Appl Genet, 2015, 128(6): 1151-1161.
doi: 10.1007/s00122-015-2496-y pmid: 25821195 |
| [25] |
Li SB, Qian Q, Fu ZM, et al. Short panicle1 encodes a putative PTR family transporter and determines rice panicle size[J]. Plant J, 2009, 58(4): 592-605.
doi: 10.1111/tpj.2009.58.issue-4 URL |
| [26] | 何宗顺, 李雪梅, 吴昌银. 水稻穗大小决定基因PS1的遗传分析及克隆[J]. 分子植物育种, 2012, 10(4): 380-387. |
| He ZS, Li XM, Wu CY. Genetic analysis and mapping of PS1 gene which may determine panicle size in rice[J]. Mol Plant Breed, 2012, 10(4): 380-387. | |
| [27] |
Jeong J, Suh S, Guan CH, et al. A nodule-specific dicarboxylate transporter from alder is a member of the peptide transporter family[J]. Plant Physiol, 2004, 134(3): 969-978.
pmid: 15001700 |
| [28] |
Léran S, Varala K, Boyer JC, et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants[J]. Trends Plant Sci, 2014, 19(1): 5-9.
doi: 10.1016/j.tplants.2013.08.008 pmid: 24055139 |
| [29] |
Nour-Eldin HH, Andersen TG, Burow M, et al. NRT/PTR transporters are essential for translocation of glucosinolate defence compounds to seeds[J]. Nature, 2012, 488(7412): 531-534.
doi: 10.1038/nature11285 |
| [30] | Liang CG, Hirose T, Okamura M, et al. Phenotypic analyses of rice lse2 and lse3 mutants that exhibit hyperaccumulation of starch in the leaf blades[J]. Rice(N Y), 2014, 7(1): 32. |
| [31] |
Zhang HB, Liang CG, Aoki N, et al. Introduction of a fungal NADP(H)-dependent glutamate dehydrogenase(gdhA)improves growth, grain weight and salt resistance by enhancing the nitrogen uptake efficiency in forage rice[J]. Plant Prod Sci, 2016, 19(2): 267-278.
doi: 10.1080/1343943X.2015.1133237 URL |
| [32] |
张海淼, 李洋, 刘海峰, 等. 水稻重要农艺性状调控基因及其育种利用研究进展[J]. 生物技术通报, 2020, 36(12): 155-169.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0537 |
| Zhang HM, Li Y, Liu HF, et al. Research progress on regulatory genes of important agronomic traits and breeding utilization in rice[J]. Biotechnol Bull, 2020, 36(12): 155-169. | |
| [33] |
Perez CM, Palmiano EP, Baun LC, et al. Starch metabolism in the leaf sheaths and culm of rice[J]. Plant Physiol, 1971, 47(3): 404-408.
doi: 10.1104/pp.47.3.404 pmid: 16657631 |
| [34] |
Matsushita K, Ishii T, Ideta O, et al. Yield and lodging resistance of ‘Tachiayaka’, a novel rice cultivar with short panicles for whole-crop silage[J]. Plant Prod Sci, 2014, 17(2): 202-206.
doi: 10.1626/pps.17.202 URL |
| [35] |
Hashida Y, Kadoya S, Okamura M, et al. Characterization of sugar metabolism in the stem of Tachisuzuka, a whole-crop silage rice cultivar with high sugar content in the stem[J]. Plant Prod Sci, 2018, 21(3): 233-243.
doi: 10.1080/1343943X.2018.1461016 URL |
| [36] |
Hirose T, Kadoya S, Hashida Y, et al. Mutation of the SP1 gene is responsible for the small-panicle trait in the rice cultivar Tachisuzuka, but not necessarily for high sugar content in the stem[J]. Plant Prod Sci, 2017, 20(1): 90-94.
doi: 10.1080/1343943X.2016.1260484 URL |
| [1] | 李思经;. 美国杜邦公司与先锋公司组建合资企业[J]. , 1997, 0(06): 47-48. |
| [2] | . 生物固氮[J]. , 1993, 0(02): 62-63. |
| [3] | . 农业废物利用及环境保护[J]. , 1993, 0(01): 79-85. |
| [4] | 孙国风;. 自生条件下根瘤菌固氮酶活性的表达[J]. , 1992, 0(04): 61-73. |
| [5] | . 核酸及蛋白质合成、提取、纯化[J]. , 1992, 0(01): 19-20. |
| [6] | . 发酵工程[J]. , 1991, 0(10): 44-48. |
| [7] | 孙国凤;. 向植物叶绿体导人基因成功[J]. , 1991, 0(09): 12-13. |
| [8] | . 生物防治[J]. , 1991, 0(07): 81-84. |
| [9] | 李思经;. Glytec公司转向碳水化合物研究[J]. , 1990, 0(11): 24-25. |
| [10] | . 发酵工程[J]. , 1990, 0(06): 58-64. |
| [11] | . 食品上的应用[J]. , 1990, 0(06): 104-108. |
| [12] | 孙雷心;. 重组白腐菌可更有效地破坏木质素[J]. , 1990, 0(01): 24-25. |
| [13] | . 生物防治因子[J]. , 1989, 0(11): 84-86. |
| [14] | 李思经;. 光合共生固氮细菌激励着植物研究人员[J]. , 1989, 0(07): 21-21. |
| [15] | . 食品上的应用[J]. , 1989, 0(06): 114-119. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||