








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 272-281.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0423
• 研究报告 • 上一篇
陈琳琳( ), 苏增青, 姬小雅, 刘新月, 刘家瑶, 张时雨, 邢小萍, 李洪连(
), 苏增青, 姬小雅, 刘新月, 刘家瑶, 张时雨, 邢小萍, 李洪连( )
)
收稿日期:2025-04-22
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
李洪连,男,博士,教授,研究方向 :作物土传病害防控;E-mail: honglianli@sina.com作者简介:陈琳琳,女,博士,副教授,研究方向 :植物病理学;E-mail: llchensky@163.com
基金资助:
CHEN Lin-lin( ), SU Zeng-qing, JI Xiao-ya, LIU Xin-yue, LIU Jia-yao, ZHANG Shi-yu, XING Xiao-ping, LI Hong-lian(
), SU Zeng-qing, JI Xiao-ya, LIU Xin-yue, LIU Jia-yao, ZHANG Shi-yu, XING Xiao-ping, LI Hong-lian( )
)
Received:2025-04-22
Published:2025-11-26
Online:2025-12-09
摘要:
目的 探究类半胱氨酸蛋白酶FpMca3在假禾谷镰孢致病过程中的生物学功能,为解析病原菌致病的分子机制提供依据,并为病害防控提供潜在的药物靶标。 方法 利用PCR和RT-PCR扩增假禾谷镰孢中的FpMcas基因,通过RT-qPCR分析FpMca3在病原菌侵染阶段的表达。借助生物信息手段分析FpMca3的结构域、系统进化关系和序列相似度。利用split-PCR方法构建FpMca3的敲除盒,并通过PEG介导的原生质体转化方法转化假禾谷镰孢野生型菌株。利用潮霉素抗性筛选和PCR检测获得FpMca3缺失突变体(Δfpmca3)。构建pKNTG-FpMca3重组载体,导入Δfpmca3突变体中,获得FpMca3回补的菌株(Δfpmca3-C)。在PDA培养基上测定假禾谷镰孢不同菌株的生长速率,在CMC培养液中测定分生孢子的产生,利用无菌水刺激分生孢子萌发测定萌发率。通过小麦胚芽鞘和大麦叶片接种以及盆栽试验测定假禾谷镰孢不同菌株的致病力。利用原核表达和蛋白纯化检测FpMca3蛋白的完整性。 结果 根据在假禾谷镰孢基因组中鉴定到的4个包含Peptidase_C14结构域的候选metacaspase蛋白,在假禾谷镰孢基因组中仅扩增到FpMca1、FpMca2和FpMca3的基因和开放阅读框序列。蛋白序列分析发现FpMca1和FpMca2是真菌中保守的Ⅰ型metacaspase,FpMca3与细菌的metacaspase结构域类似,是真菌中一种新型半胱氨酸蛋白酶。FpMca3在假禾谷镰孢侵染阶段诱导表达,对其基因缺失和回补菌株的表型测定发现,假禾谷镰孢野生型、Δfpmca3和Δfpmca3-C菌株的菌丝生长、分生孢子产生和萌发无显著差异,而与野生型和回补菌株相比,Δfpmca3对大麦叶片和小麦胚芽鞘的致病力显著降低,引起的小麦茎基腐病症状明显减轻。原核表达获得的FpMca3具有两条蛋白带,暗示其具有自切酶活性。 结论 类半胱氨酸蛋白酶FpMca3参与假禾谷镰孢的致病力,且具有自切酶活性。
陈琳琳, 苏增青, 姬小雅, 刘新月, 刘家瑶, 张时雨, 邢小萍, 李洪连. 类半胱氨酸蛋白酶FpMca3参与假禾谷镰孢的致病力[J]. 生物技术通报, 2025, 41(11): 272-281.
CHEN Lin-lin, SU Zeng-qing, JI Xiao-ya, LIU Xin-yue, LIU Jia-yao, ZHANG Shi-yu, XING Xiao-ping, LI Hong-lian. Metacaspase-like FpMca3 Involved in Pathogenicity of Fusarium pseudograminearum[J]. Biotechnology Bulletin, 2025, 41(11): 272-281.
| Primer | Primer sequence (5′-3′) |
|---|---|
| FpMca1-F | ATGTCGCAATACCCAGGC |
| FpMca1-R | CTAATACTTGGTCAATGGC |
| FpMca2-F | ATGTCCTACTTTCCAGGTC |
| FpMca2-R | CATAACAAACAAGAGGTCGG |
| FpMca3-F | ATGTCCAACAAGGCCGTC |
| FpMca3-R | TCACACATTCGTAGGTACC |
| FpMca4-F | ATGACAAACCAACCGACTCATC |
| FpMca4-R | CTAAAGTACCGGTATCGAGGT |
| F1 | ACAAGCGAATGCGAGTCGGTCAG |
| R1 | TTGACCTCCACTAGCTCCAGCCAAGCCGATTGGTCTGACCATGTAAT |
| F2 | ATAGAGTAGATGCCGACCGCGGGTTCAATTAATTGCAGAATTCTC |
| R2 | TACTTCCAAAGTATATCACTGC |
| UP-F | GAGTGTGAGAGGATGTTGTC |
| H855R | GCTGATCTGACCAGTTGC |
| H856F | GTCGATGCGACGCAATCGT |
| Down-R | GCTGAATATCCTTTGCTGTTG |
| H850F | TTCCTCCCTTTATTTCAGATTCAA |
| H852R | ATGTTGGCGACCTCGTATTGG |
| G-F | CTAGCAATATCACCCTCATC |
| G-R | GGAGACTTTGGCAGTGTTC |
| Com-F | CTATAGGGCGAATTGGGTACCGAATTGATCTGTATACTGTAG |
| Com-R | GCAGGCATGCAAGCTTATCGATCACATTCGTAGGTACCATTTC |
| RT-F | GAGCTGGTCAACAATGGA |
| RT-R | CTTGTAGCCCCTTGAGTTC |
| FpTEF1-RTF | TCACCACTGAAGTCAAGTCC |
| FpTEF1-RTR | ACCAGCGACGTTACCACGTC |
表1 研究所用引物序列
Table 1 Sequences of primers used in this study
| Primer | Primer sequence (5′-3′) |
|---|---|
| FpMca1-F | ATGTCGCAATACCCAGGC |
| FpMca1-R | CTAATACTTGGTCAATGGC |
| FpMca2-F | ATGTCCTACTTTCCAGGTC |
| FpMca2-R | CATAACAAACAAGAGGTCGG |
| FpMca3-F | ATGTCCAACAAGGCCGTC |
| FpMca3-R | TCACACATTCGTAGGTACC |
| FpMca4-F | ATGACAAACCAACCGACTCATC |
| FpMca4-R | CTAAAGTACCGGTATCGAGGT |
| F1 | ACAAGCGAATGCGAGTCGGTCAG |
| R1 | TTGACCTCCACTAGCTCCAGCCAAGCCGATTGGTCTGACCATGTAAT |
| F2 | ATAGAGTAGATGCCGACCGCGGGTTCAATTAATTGCAGAATTCTC |
| R2 | TACTTCCAAAGTATATCACTGC |
| UP-F | GAGTGTGAGAGGATGTTGTC |
| H855R | GCTGATCTGACCAGTTGC |
| H856F | GTCGATGCGACGCAATCGT |
| Down-R | GCTGAATATCCTTTGCTGTTG |
| H850F | TTCCTCCCTTTATTTCAGATTCAA |
| H852R | ATGTTGGCGACCTCGTATTGG |
| G-F | CTAGCAATATCACCCTCATC |
| G-R | GGAGACTTTGGCAGTGTTC |
| Com-F | CTATAGGGCGAATTGGGTACCGAATTGATCTGTATACTGTAG |
| Com-R | GCAGGCATGCAAGCTTATCGATCACATTCGTAGGTACCATTTC |
| RT-F | GAGCTGGTCAACAATGGA |
| RT-R | CTTGTAGCCCCTTGAGTTC |
| FpTEF1-RTF | TCACCACTGAAGTCAAGTCC |
| FpTEF1-RTR | ACCAGCGACGTTACCACGTC |
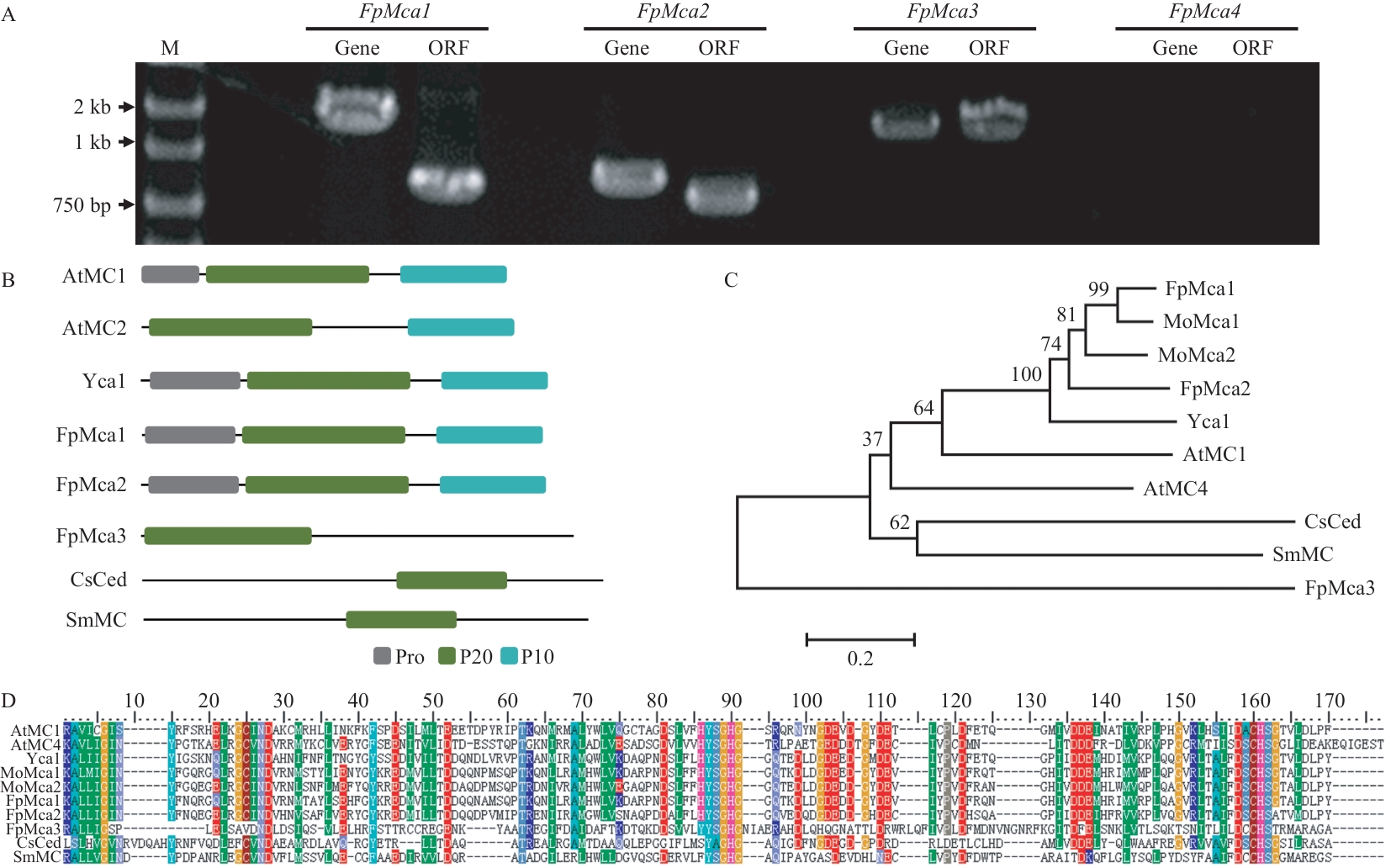
图1 FpMcas的克隆与分析A:假禾谷镰孢FpMca1-4的克隆;B:FpMcas的蛋白结构域;C:FpMcas的进化树;D:P20亚基的序列分析
Fig. 1 Cloning and analysis of FpMcasA: Cloning of FpMca1-4 in F. pseudograminearum. B: Protein domains of FpMcas. C: Phylogenetic tree of FpMcas. D: Sequence analysis of the P20 subunit
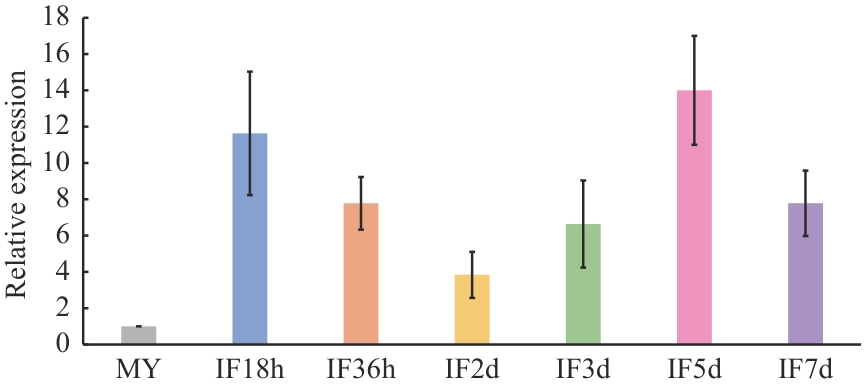
图2 FpMca3在假禾谷镰孢侵染阶段的表达分析FpMca3在菌丝体(MY)中的表达水平定为1,误差棒指4次重复试验的标准差。IF18h、IF36h、IF2d、IF3d、IF5d和IF7d分别表示假禾谷镰孢野生型菌丝接种小麦(矮抗58)胚芽鞘后18 h、36 h、2 d、3 d、5 d和7 d
Fig. 2 Expression analysis of FpMca3 during the infection stages of F. pseudograminearumThe expression of FpMca3 in mycelia was arbitrarily set as 1. Bars denote standard errors from four repeated experiments. IF18h, IF36h, IF2d, IF3d, IF5d, and IF7d represent 18 h, 36 h, 2 d, 3 d, 5 d, and 7 d after inoculation of wheat (Aikang 58) coleoptiles with the wild-type mycelium of F. pseudograminearum, respectively
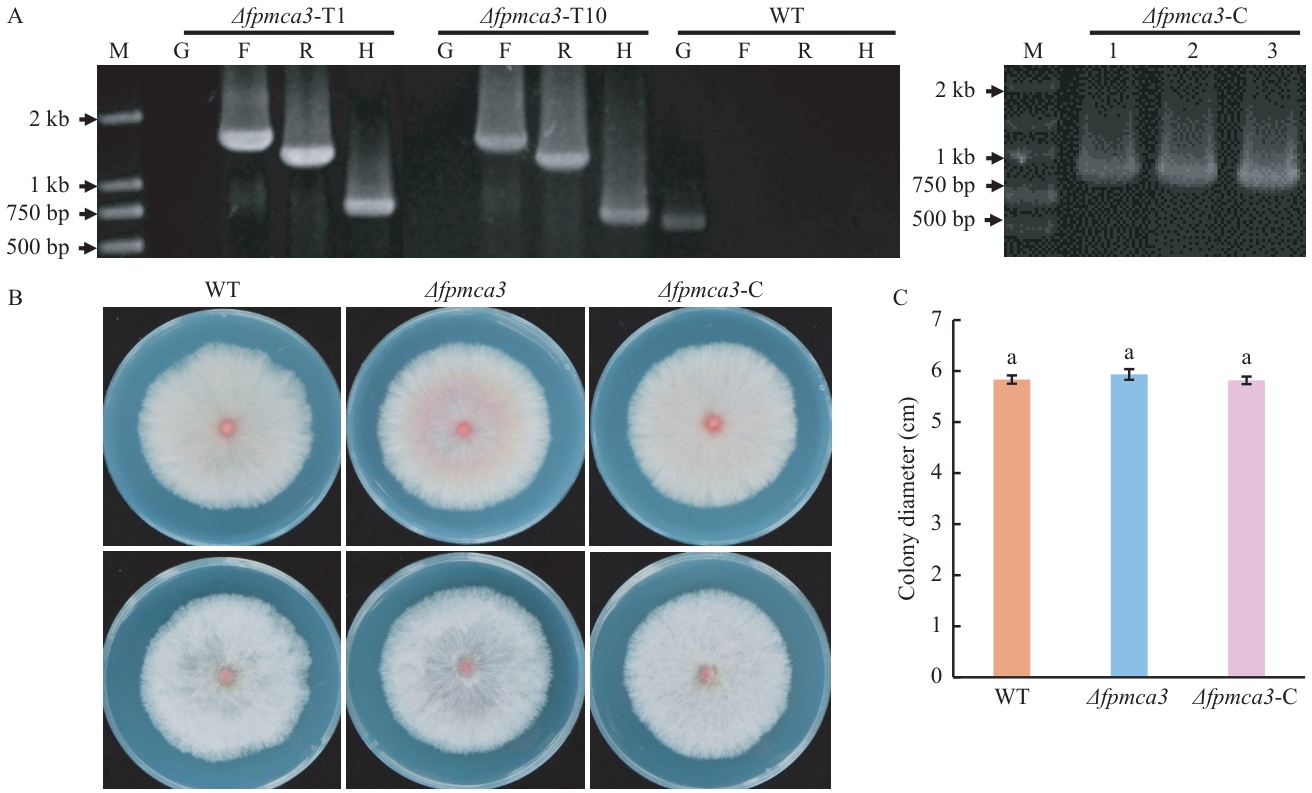
图3 FpMca3缺失突变体的获得及生长测定A:FpMca3缺失突变体和回补菌株的PCR检测;B:FpMca3缺失突变体的生长测定,上排是菌落背面,下排是菌落正面;C:菌落直径测定,小写字母表示在P<0.05水平上的数据差异
Fig. 3 Generation and growth phenotype determination of FpMca3-knockout mutantA: PCR verification of the FpMca3-knockout mutants and FpMca3-complemented strains. B: Growth assay of the FpMca3-knockout mutant. The upper row shows the reverse side of the colonies, while the lower row displays the front side. C: Measurement of colony diameter. Lowercase letters indicate data difference at the P<0.05 level
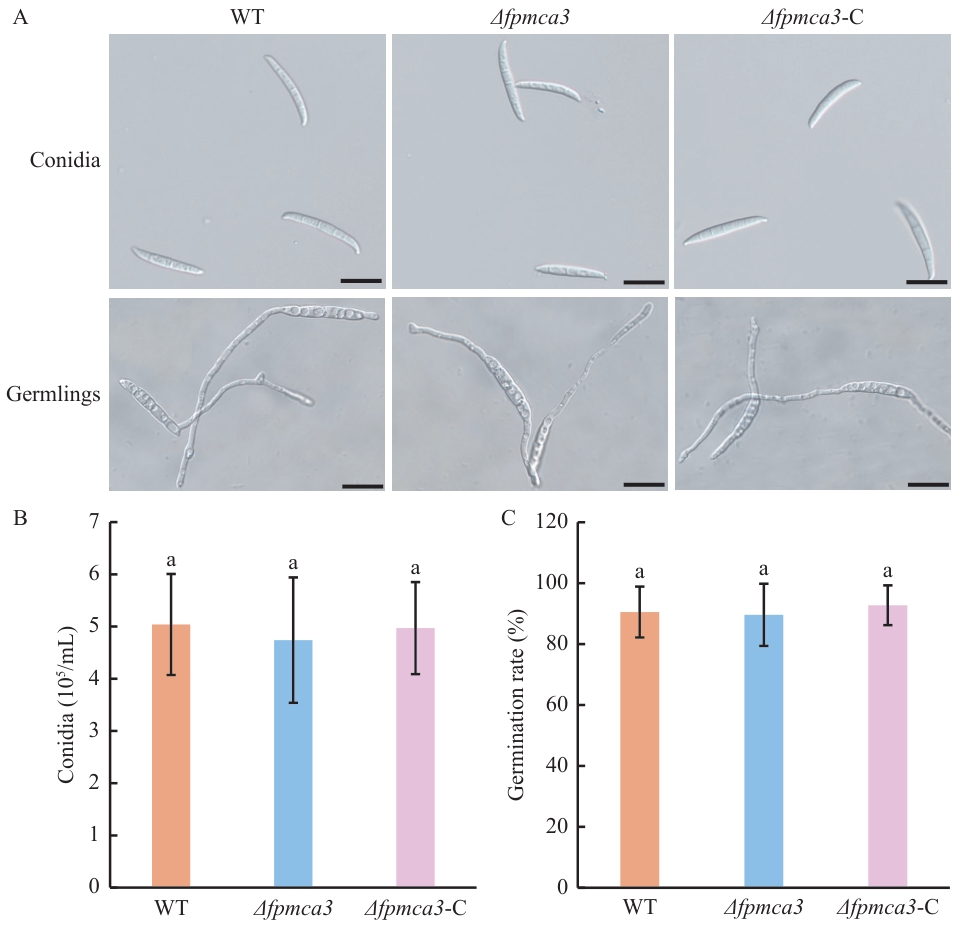
图4 分生孢子产生和萌发测定A:假禾谷镰孢野生型、Δfpmca3和Δfpmca3-C菌株分生孢子及其萌发的形态观察;B:野生型、Δfpmca3和Δfpmca3-C菌株的产孢量;C:野生型、Δfpmca3和Δfpmca3-C菌株分生孢子萌发速率;小写字母表示在P<0.05水平上的数据差异
Fig. 4 Conidial production and germination assaysA: Morphology of conidia and germination of the wild-type, Δfpmca3 and Δfpmca3-C F. pseudograminearum. B: The conidia quantity of the wild-type, Δfpmca3 and Δfpmca3-C strains. C: Germination rates of the wild-type, Δfpmca3 and Δfpmca3-C strains. Lowercase letters indicate data difference at the P<0.05 level
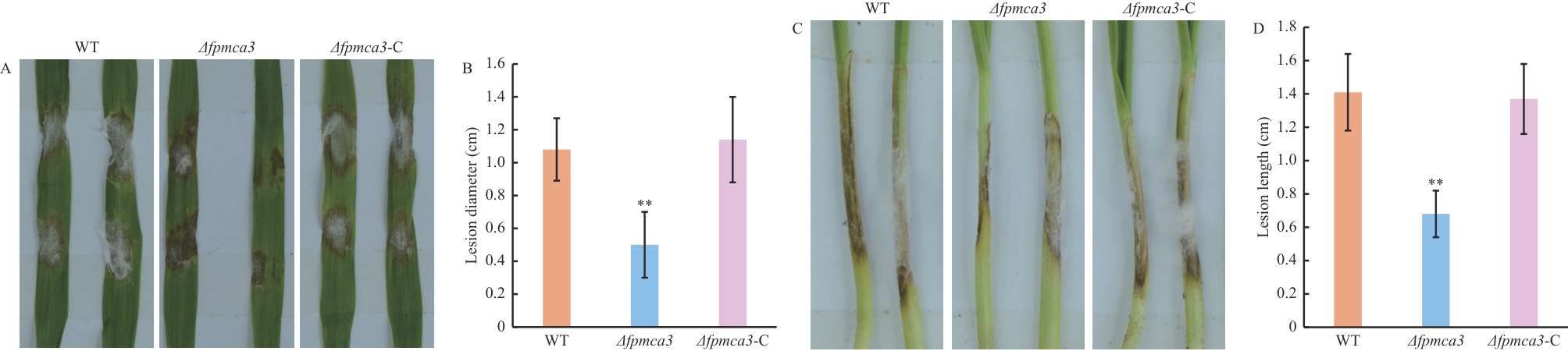
图5 假禾谷镰孢对大麦叶片和小麦胚芽鞘的致病力测定A:假禾谷镰孢野生型、Δfpmca3和Δfpmca3-C菌株接种大麦叶片引起的症状;B:接种4 d时大麦叶片上坏死病斑的直径;C:野生型、Δfpmca3和Δfpmca3-C菌株接种小麦胚芽鞘引起的症状;D:接种4 d时小麦胚芽鞘上坏死病斑的直径。** P<0.01
Fig. 5 Pathogenicity assay of F. pseudograminearum on barley leaves and wheat coleoptilesA: Phenotypes of lesions on barley leaves inoculated with the wild-type, Δfpmca3 and Δfpmca3-C F. pseudograminearum. B: Diameters of lesions on barley leaves measured at 4 d after inoculation. C: Phenotypes of lesions on wheat coleoptiles inoculated with the wild-type, Δfpmca3 and Δfpmca3-C strains. D: Diameters of lesions on wheat coleoptiles measured at 4 d after inoculation. ** P<0.01

图6 盆栽试验测定假禾谷镰孢的致病力A:假禾谷镰孢接种小麦的生长状态;B:小麦幼苗茎基腐病的病情指数,** P<0.01
Fig. 6 Pathogenicity assay of F. pseudograminearum in pot trialsA: Wheat growth induced by F. pseudograminearum infection. B: Crown rot disease index (DSI) in wheat seedlings infected with different F. pseudograminearum strains. ** P<0.01
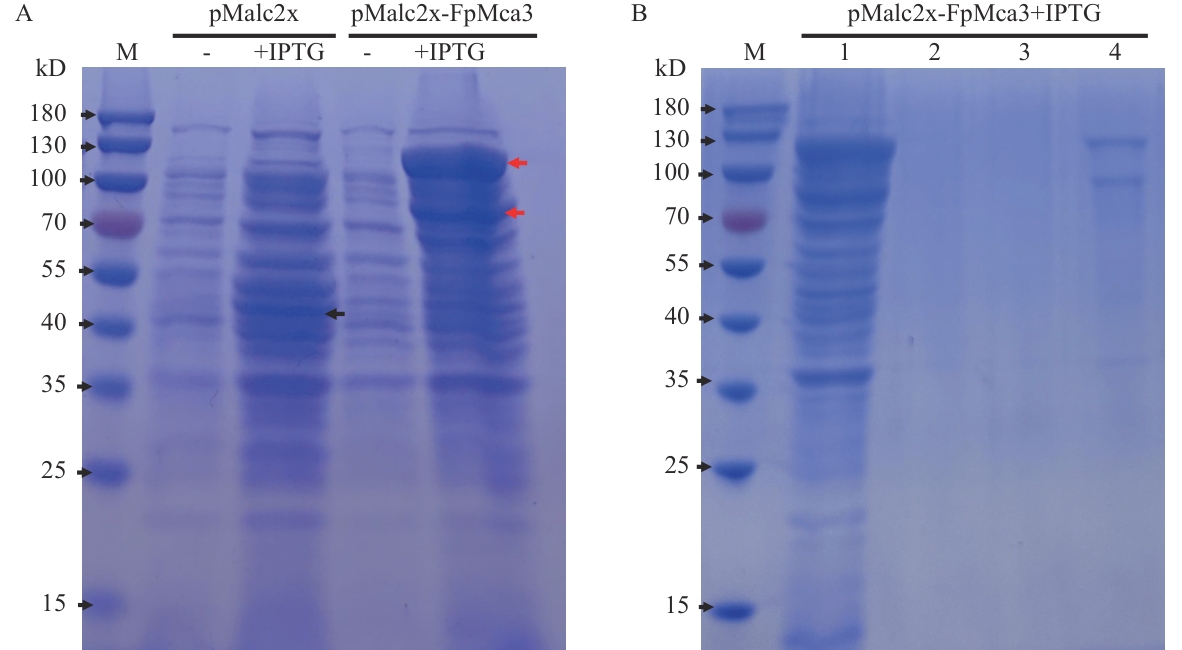
图7 FpMca3原核表达蛋白检测A:IPTG诱导FpMca3的原核表达。黑色箭头指示pMalc2x空载体对照中诱导表达的MBP蛋白,红色箭头指示的是重组质粒中诱导表达的FpMca3-MBP融合蛋白;B:FpMca3-MBP融合蛋白的纯化。1:未纯化的蛋白;2:结合后的上清液(CK1);3:MBP缓冲液清洗后的上清液(CK2);4:PBS缓冲液洗脱后的蛋白
Fig. 7 Detection of prokaryotically expressed FpMca3 proteinA: IPTG-induced expression of FpMca3 in prokaryotic cells. The black arrow marks the position of MBP protein expressed by the pMalc2x empty vector control. The red arrows mark the FpMca3-MBP fusion protein expressed in the recombinant strain. B: Purification of FpMca3-MBP. 1: Unpurified protein. 2: Supernatant (post-binding, CK1). 3: Supernatant after washing with MBP buffer (CK2). 4: Protein after eluted with PBS
| [1] | Choi CJ, Berges JA. New types of metacaspases in phytoplankton reveal diverse origins of cell death proteases [J]. Cell Death Dis, 2013, 4(2): e490. |
| [2] | Fagundes D, Bohn B, Cabreira C, et al. Caspases in plants: metacaspase gene family in plant stress responses [J]. Funct Integr Genomics, 2015, 15(6): 639-649. |
| [3] | 姚绍嫦, 潘东进, 高程海. 海洋浮游植物metacaspase蛋白酶研究进展 [J]. 海洋科学, 2019, 43(8): 108-116. |
| Yao SC, Pan DJ, Gao CH. Research progress of metacaspase proteases in marine phytoplankton [J]. Mar Sci, 2019, 43(8): 108-116. | |
| [4] | Tsiatsiani L, Van Breusegem F, Gallois P, et al. Metacaspases [J]. Cell Death Differ, 2011, 18(8): 1279-1288. |
| [5] | Ghorbani N, Yaghubi R, Davoodi J, et al. How does caspases regulation play role in cell decisions? apoptosis and beyond [J]. Mol Cell Biochem, 2024, 479(7): 1599-1613. |
| [6] | Hill SM, Nyström T. The dual role of a yeast metacaspase: What doesn't kill you makes you stronger [J]. Bioessays, 2015, 37(5): 525-531. |
| [7] | Hill SM, Hao XX, Liu BD, et al. Life-span extension by a metacaspase in the yeast Saccharomyces cerevisiae [J]. Science, 2014, 344(6190): 1389-1392. |
| [8] | Mukherjee D, Gupta S, Saran N, et al. Induction of apoptosis-like cell death and clearance of stress-induced intracellular protein aggregates: dual roles for Ustilago maydis metacaspase Mca1 [J]. Mol Microbiol, 2017, 106(5): 815-831. |
| [9] | Coll NS, Vercammen D, Smidler A, et al. Arabidopsis type I metacaspases control cell death [J]. Science, 2010, 330(6009): 1393-1397. |
| [10] | Ruiz-Solaní N, Salguero-Linares J, Armengot L, et al. Arabidopsis metacaspase MC1 localizes in stress granules, clears protein aggregates, and delays senescence [J]. Plant Cell, 2023, 35(9): 3325-3344. |
| [11] | Pitsili E, Rodriguez-Trevino R, Ruiz-Solani N, et al. A phloem-localized Arabidopsis metacaspase (AtMC3) improves drought tolerance [J]. New Phytol, 2023, 239(4): 1281-1299. |
| [12] | Hander T, Fernández-Fernández ÁD, Kumpf RP, et al. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides [J]. Science, 2019, 363(6433): eaar7486. |
| [13] | Zhu P, Yu XH, Wang C, et al. Structural basis for Ca2+-dependent activation of a plant metacaspase [J]. Nat Commun, 2020, 11(1): 2249. |
| [14] | Madeo F, Herker E, Maldener C, et al. A caspase-related protease regulates apoptosis in yeast [J]. Mol Cell, 2002, 9(4): 911-917. |
| [15] | Shrestha A, Brunette S, Stanford WL, et al. The metacaspase Yca1 maintains proteostasis through multiple interactions with the ubiquitin system [J]. Cell Discov, 2019, 5: 6. |
| [16] | Eyssen LE, Coetzer THT. Expression, purification and characterisation of Trypanosoma congolense metacaspase 5 (TcoMCA5) - a potential drug target for animal African trypanosomiasis [J]. Protein Expr Purif, 2019, 164: 105465. |
| [17] | Kumar B, Mohammad T, Amaduddin, et al. Targeting metacaspase-3 from Plasmodium falciparum towards antimalarial therapy: a combined approach of in-silico and in-vitro investigation [J]. J Biomol Struct Dyn, 2021, 39(2): 421-430. |
| [18] | Fernandez J, Lopez V, Kinch L, et al. Role of two metacaspases in development and pathogenicity of the rice blast fungus Magnaporthe oryzae [J]. mBio, 2021, 12(1): e03471-20. |
| [19] | Zhou HF, He XL, Wang S, et al. Diversity of the Fusarium pathogens associated with crown rot in the Huanghuai wheat-growing region of China [J]. Environ Microbiol, 2019, 21(8): 2740-2754. |
| [20] | 赵静雅, 凡卓, 彭梦雅, 等. FpStuA参与假禾谷镰孢的产孢和致病性 [J]. 植物病理学报, 2022, 52(1): 37-46. |
| Zhao JY, Fan Z, Peng MY, et al. FpStuA is involved in conidiation and pathogenicity of Fusarium pseudograminearum [J]. Acta Phytopathol Sin, 2022, 52(1): 37-46. | |
| [21] | Wang LM, Zhang YF, Du ZL, et al. FpPDE1 function of Fsarium pseudograminearum on pathogenesis in wheat [J]. J Integr Agric, 2017, 16(11): 2504-2512. |
| [22] | Chen LL, Geng XJ, Ma YM, et al. Identification of basic helix-loop-helix transcription factors reveals candidate genes involved in pathogenicity of Fusarium pseudograminearum [J]. Can J Plant Pathol, 2019, 41(2): 200-208. |
| [23] | Yang X, Pan YB, Singh PK, et al. Investigation and genome-wide association study for Fusarium crown rot resistance in Chinese common wheat [J]. BMC Plant Biol, 2019, 19(1): 153. |
| [24] | Chen LL, Ma YM, Peng MY, et al. Analysis of apoptosis-related genes reveals that apoptosis functions in conidiation and pathogenesis of Fusarium pseudograminearum [J]. mSphere, 2021, 6(1): e01140-20. |
| [25] | Murray GM, Brennan JP. Estimating disease losses to the Australian wheat industry [J]. Australas Plant Pathol, 2009, 38(6): 558-570. |
| [26] | Bragard C, Baptista P, Chatzivassiliou E, et al. Pest categorisation of Fusarium pseudograminearum [J]. EFSA J, 2022, 20(6): e07399. |
| [27] | Kazan K, Gardiner DM. Fusarium crown rot caused by Fusarium pseudograminearum in cereal crops: recent progress and future prospects [J]. Mol Plant Pathol, 2018, 19(7): 1547-1562. |
| [28] | Li HL, Yuan HX, Fu B, et al. First report of Fusarium pseudograminearum causing crown rot of wheat in Henan, China [J]. Plant Dis, 2012, 96(7): 1065. |
| [29] | 栾冬冬, 贾吉玉, 王光州, 等. 中国小麦茎基腐病的发生现状及防治策略[J]. 麦类作物学报 2022, 42(4): 512-520. |
| Luan DD, Jia JY, Wang GZ, et al. Occurrence status and control strategies of wheat crown rot in China [J]. J Triticeae Crops, 2022, 42(4): 512-520. | |
| [30] | 宋嘉庆, 潘鑫, 闫书味, 等. 假禾谷镰孢菌的分离及其对小麦茎基部和穗部的致病力分析 [J]. 麦类作物学报, 2022, 42(12): 1575-1581. |
| Song JQ, Pan X, Yan SW, et al. Analysis of the pathogenicity of Fusarium pseudograminearum on wheat stem bases and panicles [J]. J Triticeae Crops, 2022, 42(12): 1575-1581. | |
| [31] | Aoki T, O'Donnell K. Morphological and molecular characterization of Fusarium pseudograminearum sp. nov., formerly recognized as the group 1 population of F. graminearum [J]. Mycologia, 1999, 91(4): 597-609. |
| [32] | Tunali B, Obanor F, Erginbaş G, et al. Fitness of three Fusarium pathogens of wheat [J]. FEMS Microbiol Ecol, 2012, 81(3): 596-609. |
| [33] | Powell JJ, Carere J, Fitzgerald TL, et al. The Fusarium crown rot pathogen Fusarium pseudograminearum triggers a suite of transcriptional and metabolic changes in bread wheat (Triticum aestivum L.) [J]. Ann Bot, 2017, 119(5): 853-867. |
| [34] | Gardiner DM, McDonald MC, Covarelli L, et al. Comparative pathogenomics reveals horizontally acquired novel virulence genes in fungi infecting cereal hosts [J]. PLoS Pathog, 2012, 8(9): e1002952. |
| [1] | 潘国强, 吴思源, 刘璐, 郭惠明, 程红梅, 苏晓峰. 大丽轮枝菌(Verticillim dahliae)突变体库的构建与分析[J]. 生物技术通报, 2023, 39(5): 112-119. |
| [2] | 杜清洁, 周璐瑶, 杨思震, 张嘉欣, 陈春林, 李娟起, 李猛, 赵士文, 肖怀娟, 王吉庆. 过表达CaCP1提高转基因烟草对盐胁迫的敏感性[J]. 生物技术通报, 2023, 39(2): 172-182. |
| [3] | 吴莉丹, 冉雪琴, 牛熙, 黄世会, 李升, 王嘉福. 猪源致病性大肠杆菌基因组比较与毒力因子分析[J]. 生物技术通报, 2023, 39(12): 287-299. |
| [4] | 尹国英, 刘畅, 常永春, 羽王洁, 王兵, 张盼, 郭玉双. 烟草半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对PVY的响应[J]. 生物技术通报, 2023, 39(10): 184-196. |
| [5] | 赵静雅, 彭梦雅, 张时雨, 单艺轩, 邢小萍, 施艳, 李海洋, 杨雪, 李洪连, 陈琳琳. C2H2锌指转录因子FpCzf7参与假禾谷镰孢的生长和致病性[J]. 生物技术通报, 2022, 38(8): 216-224. |
| [6] | 苏雨, 李宗芸, 韩永华. 植物液泡加工酶研究进展[J]. 生物技术通报, 2021, 37(6): 181-191. |
| [7] | 莫黎杰, 刘夏瞳, 李慧, 陆海. 植物半胱氨酸蛋白酶在植物生长发育中的功能研究[J]. 生物技术通报, 2021, 37(6): 202-212. |
| [8] | 雒丽丽, 张昊, 杨美欣, 王云飞, 许景升, 徐进, 姚强, 冯洁. 黄淮与东北麦区小麦赤霉菌温度相关的致病力分化研究[J]. 生物技术通报, 2021, 37(4): 47-55. |
| [9] | 张梦恬, 裴娟 ,李国 ,赵辉 ,陈建权 ,祝建波, 王爱英. 新疆石河子地区棉花黄萎病菌分离鉴定及其致病力分析[J]. 生物技术通报, 2018, 34(6): 73-78. |
| [10] | 郭云峰, 安邦. 橡胶树胶孢炭疽菌NADPH氧化酶功能研究[J]. 生物技术通报, 2018, 34(10): 165-171. |
| [11] | 龙翔宇, 梁启福, 戚继艳, 方永军, 唐朝荣. 橡胶树半胱氨酸蛋白酶抑制剂HbCYS2的克隆与表达分析[J]. 生物技术通报, 2017, 33(3): 86-92. |
| [12] | 简桂良;卢美光;赵磊;王深正;. 继代对棉花黄萎病落叶型菌系致病力的影响[J]. , 2009, 0(S1): 165-168. |
| [13] | 汪开治;. 几种具有重要的药物学用途的海洋微生物酶抑制剂[J]. , 2006, 0(03): 104-106. |
| [14] | 李思经;. 政府承包商寻求研究AIDS与癌症的关系[J]. , 1990, 0(11): 18-19. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||