








生物技术通报 ›› 2023, Vol. 39 ›› Issue (9): 1-11.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1506
• 综述与专论 • 下一篇
收稿日期:2022-12-08
出版日期:2023-09-26
发布日期:2023-10-24
通讯作者:
田新朋,男,博士,研究员,研究方向:海洋微生物资源与生态;E-mail: xinpengtian@scsio.ac.cn作者简介:张坤,男,博士研究生,研究方向:海洋微生物资源与生态;E-mail: zhangkunynu@163.com
基金资助:
ZHANG Kun1,3( ), YAN Chang1,3, TIAN Xin-peng1,2(
), YAN Chang1,3, TIAN Xin-peng1,2( )
)
Received:2022-12-08
Published:2023-09-26
Online:2023-10-24
摘要:
自然界中大多数微生物处于未培养状态,被称为 “微生物暗物质”。随着微生物单细胞分离方法的不断更新,利用新技术、新方法应对微生物纯培养的挑战获得了重要进展,这些新的分离及培养策略对推动微生物资源学的发展具有重要意义。尽管宏基因组学和基因组学数据相关成果日益增多,但微生物单细胞的分离与培养对于系统研究微生物的生态功能、遗传进化等仍至关重要。本文主要概述了目前使用的或正在研发的膜扩散培养法、微流控分选、荧光激活细胞分选、单细胞拉曼分选、光镊技术、显微操作技术等单细胞分离技术的原理与应用,及其在微生物单细胞分离和培养方面的优点与不足,同时展望了这些单细胞分离技术未来的发展和应用前景。
张坤, 闫畅, 田新朋. 微生物单细胞分离方法研究进展[J]. 生物技术通报, 2023, 39(9): 1-11.
ZHANG Kun, YAN Chang, TIAN Xin-peng. Research Progress in Microbial Single Cell Separation Methods[J]. Biotechnology Bulletin, 2023, 39(9): 1-11.

图1 单细胞分离方法 a:膜扩散培养法[18];b:微流控技术[24];c:单细胞拉曼分选[25];d:荧光激活细胞分选[26];e:光镊技术[27];f:显微操作技术[28]
Fig. 1 Single cell separation methods a: Membrane diffusion culture method[18]. b: Microfluidic technology[24]. c: Single cell Raman sorting[25]. d: Fluorescence activated cell sorting[26]. e: Optical tweezers technology[27]. f : Micromanipulation technology[28]
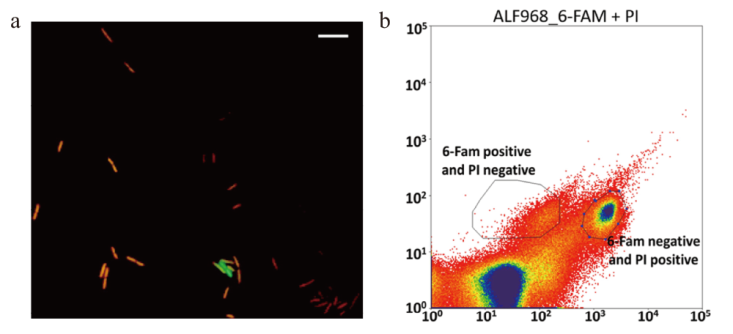
图2 基于Live-FISH技术的单细胞分离方法 a: Live-FISH标记及活细胞染色;b: 复染后的流式细胞分选
Fig. 2 Single cell separation method based on Live-FISH technique a: Live-FISH labeling and live cells staining. b: Flow cytometry after counterstain
| 分离方法 Separation method | 优点 Advantages | 不足 Disadvantages | |
|---|---|---|---|
| 高通量分离方法 | 膜扩散培养法 | 原位培养微生物,增加可培养率 | 耗时久,工作量大 |
| High throughput separation methods | 微流控技术 | 高通量,结构简单,成本低 | 通用性及特异性低 |
| 单细胞拉曼分选 | 分选效率高,无创,无标记 | 仪器要求较高,拉曼光谱比较弱 | |
| 荧光激活细胞分选 | 高通量,效率高,灵敏度高 | 成本高,仪器要求较高,损伤细胞 | |
| 靶向分离方法 | Live-FISH | 靶向分离细胞效果较强 | 耗时久,细胞破坏性大,成活率低 |
| Targeted separation methods | 反向基因组学 | 靶向分离细胞,适用性广泛 | 需要依赖抗体标记和流式分选 |
| 其他方法 | 光镊技术 | 精度高、效率高 | 会损伤细胞,成本高 |
| Other methods | 显微操作技术 | 适用范围广,可分离选定细胞 | 效率低,容易损伤细胞 |
表1 微生物单细胞分离方法的优点及不足
Table 1 Advantages and disadvantages of single cell separation methods for microorganisms
| 分离方法 Separation method | 优点 Advantages | 不足 Disadvantages | |
|---|---|---|---|
| 高通量分离方法 | 膜扩散培养法 | 原位培养微生物,增加可培养率 | 耗时久,工作量大 |
| High throughput separation methods | 微流控技术 | 高通量,结构简单,成本低 | 通用性及特异性低 |
| 单细胞拉曼分选 | 分选效率高,无创,无标记 | 仪器要求较高,拉曼光谱比较弱 | |
| 荧光激活细胞分选 | 高通量,效率高,灵敏度高 | 成本高,仪器要求较高,损伤细胞 | |
| 靶向分离方法 | Live-FISH | 靶向分离细胞效果较强 | 耗时久,细胞破坏性大,成活率低 |
| Targeted separation methods | 反向基因组学 | 靶向分离细胞,适用性广泛 | 需要依赖抗体标记和流式分选 |
| 其他方法 | 光镊技术 | 精度高、效率高 | 会损伤细胞,成本高 |
| Other methods | 显微操作技术 | 适用范围广,可分离选定细胞 | 效率低,容易损伤细胞 |
| [1] |
Rosenfeld N, Young JW, Alon U, et al. Gene regulation at the single-cell level[J]. Science, 2005, 307(5717): 1962-1965.
pmid: 15790856 |
| [2] |
Blainey PC, Quake SR. Dissecting genomic diversity, one cell at a time[J]. Nat Methods, 2014, 11(1): 19-21.
pmid: 24524132 |
| [3] | 马倩, 王丽华. 单细胞技术研究进展[C]// 第十三届福州市科协学术年会论文集. 福州: 福州市科协, 2015: 138-142. |
| Ma Q, Wang LH. Advances in single-cell technology[C]// Proceedings of the 13th Annual Conference of Fuzhou Association for Science and Technology. Fuzhou: Fuzhou Association for Science and Technology, 2015: 138-142. | |
| [4] |
Brehm-Stecher BF, Johnson EA. Single-cell microbiology: tools, technologies, and applications[J]. Microbiol Mol Biol Rev, 2004, 68(3): 538-559.
doi: 10.1128/MMBR.68.3.538-559.2004 URL |
| [5] |
Song YZ, Kaster AK, Vollmers J, et al. Single-cell genomics based on Raman sorting reveals novel carotenoid-containing bacteria in the Red Sea[J]. Microb Biotechnol, 2017, 10(1): 125-137.
doi: 10.1111/1751-7915.12420 pmid: 27748032 |
| [6] |
Liang P, Liu B, Wang Y, et al. Isolation and culture of single microbial cells by laser ejection sorting technology[J]. Appl Environ Microbiol, 2022, 88(3): e0116521.
doi: 10.1128/aem.01165-21 URL |
| [7] |
Hatzenpichler R, Krukenberg V, Spietz RL, et al. Next-generation physiology approaches to study microbiome function at single cell level[J]. Nat Rev Microbiol, 2020, 18(4): 241-256.
doi: 10.1038/s41579-020-0323-1 pmid: 32055027 |
| [8] |
Salam N, Xian WD, Asem MD, et al. From ecophysiology to cultivation methodology: filling the knowledge gap between uncultured and cultured microbes[J]. Mar Life Sci Technol, 2020, 3(2): 132-147.
doi: 10.1007/s42995-020-00064-w |
| [9] |
Xian WD, Salam N, Li MM, et al. Network-directed efficient isolation of previously uncultivated Chloroflexi and related bacteria in hot spring microbial mats[J]. NPJ Biofilms Microbiomes, 2020, 6(1): 20.
doi: 10.1038/s41522-020-0131-4 |
| [10] |
Curtis TP, Sloan WT, Scannell JW. Estimating prokaryotic diversity and its limits[J]. Proc Natl Acad Sci USA, 2002, 99(16): 10494-10499.
doi: 10.1073/pnas.142680199 pmid: 12097644 |
| [11] |
Alain K, Querellou J. Cultivating the uncultured: limits, advances and future challenges[J]. Extremophiles, 2009, 13(4): 583-594.
doi: 10.1007/s00792-009-0261-3 pmid: 19548063 |
| [12] |
Kamagata Y. Keys to cultivating uncultured microbes: elaborate enrichment strategies and resuscitation of dormant cells[J]. Microb Environ, 2015, 30(4): 289-290.
doi: 10.1264/jsme2.ME3004rh URL |
| [13] |
Lewis WH, Tahon G, Geesink P, et al. Innovations to culturing the uncultured microbial majority[J]. Nat Rev Microbiol, 2021, 19(4): 225-240.
doi: 10.1038/s41579-020-00458-8 pmid: 33093661 |
| [14] |
Batani G, Bayer K, Böge J, et al. Fluorescence in situ hybridization(FISH)and cell sorting of living bacteria[J]. Sci Rep, 2019, 9: 18618.
doi: 10.1038/s41598-019-55049-2 |
| [15] |
Cross KL, Campbell JH, Balachandran M, et al. Targeted isolation and cultivation of uncultivated bacteria by reverse genomics[J]. Nat Biotechnol, 2019, 37(11): 1314-1321.
doi: 10.1038/s41587-019-0260-6 pmid: 31570900 |
| [16] | Hesse W. Walther and Angelina Hesse-Early Contributors to Bacteriology[J]. ASM News. 1992, 58(8): 425-428. |
| [17] |
Zengler K. Central role of the cell in microbial ecology[J]. Microbiol Mol Biol Rev, 2009, 73(4): 712-729.
doi: 10.1128/MMBR.00027-09 URL |
| [18] |
Kaeberlein T, Lewis K, Epstein SS. Isolating uncultivable microorganisms in pure culture in a simulated natural environment[J]. Science, 2002, 296(5570): 1127-1129.
doi: 10.1126/science.1070633 pmid: 12004133 |
| [19] |
Chen YC, Li P, Huang PH, et al. Rare cell isolation and analysis in microfluidics[J]. Lab Chip, 2014, 14(4): 626-645.
doi: 10.1039/c3lc90136j pmid: 24406985 |
| [20] |
Ashkin A, Dziedzic JM, Bjorkholm JE, et al. Observation of a single-beam gradient force optical trap for dielectric particles[J]. Opt Lett, 1986, 11(5): 288-290.
pmid: 19730608 |
| [21] |
Yamamoto H, Sano T. Study of micromanipulation using stereoscopic microscope[J]. IEEE Trans Instrum Meas, 2002, 51(2): 182-187.
doi: 10.1109/19.997809 URL |
| [22] |
Yan SS, Qiu JX, Guo L, et al. Development overview of Raman-activated cell sorting devoted to bacterial detection at single-cell level[J]. Appl Microbiol Biotechnol, 2021, 105(4): 1315-1331.
doi: 10.1007/s00253-020-11081-1 pmid: 33481066 |
| [23] |
Herzenberg LA, De Rosa SC, Herzenberg LA. Monoclonal antibodies and the FACS: complementary tools for immunobiology and medicine[J]. Immunol Today, 2000, 21(8): 383-390.
pmid: 10916141 |
| [24] |
Clausell-Tormos J, Lieber D, Baret JC, et al. Droplet-based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms[J]. Chem Biol, 2008, 15(5): 427-437.
doi: 10.1016/j.chembiol.2008.04.004 URL |
| [25] |
Zhang PR, Ren LH, Zhang X, et al. Raman-activated cell sorting based on dielectrophoretic single-cell trap and release[J]. Anal Chem, 2015, 87(4): 2282-2289.
doi: 10.1021/ac503974e pmid: 25607599 |
| [26] |
Pereira H, Schulze PSC, Schüler LM, et al. Fluorescence activated cell-sorting principles and applications in microalgal biotechnology[J]. Algal Res, 2018, 30: 113-120.
doi: 10.1016/j.algal.2017.12.013 URL |
| [27] |
Li X, Cheah CC, Hu SY, et al. Dynamic trapping and manipulation of biological cells with optical tweezers[J]. Automatica, 2013, 49(6): 1614-1625.
doi: 10.1016/j.automatica.2013.02.067 URL |
| [28] | Fröhlich J, König H. Micromanipulation techniques for the isolation of single microorganisms[M]// Soil Biology. Berlin/Heidelberg: Springer-Verlag, 2005: 425-437. |
| [29] |
Aoi Y, Kinoshita T, Hata T, et al. Hollow-fiber membrane chamber as a device for in situ environmental cultivation[J]. Appl Environ Microbiol, 2009, 75(11): 3826-3833.
doi: 10.1128/AEM.02542-08 URL |
| [30] |
Nichols D, Cahoon N, Trakhtenberg EM, et al. Use of ichip for high-throughput in situ cultivation of “uncultivable” microbial species[J]. Appl Environ Microbiol, 2010, 76(8): 2445-2450.
doi: 10.1128/AEM.01754-09 URL |
| [31] |
Li CX, Wang GQ, Li WS, et al. New cell separation technique for the isolation and analysis of cells from biological mixtures in forensic caseworks[J]. Croat Med J, 2011, 52(3): 293-298.
doi: 10.3325/cmj.2011.52.293 URL |
| [32] |
Hu P, Zhang WH, Xin HB, et al. Single cell isolation and analysis[J]. Front Cell Dev Biol, 2016, 4: 116.
pmid: 27826548 |
| [33] |
Ishøy T, Kvist T, Westermann P, et al. An improved method for single cell isolation of prokaryotes from meso-, thermo- and hyperthermophilic environments using micromanipulation[J]. Appl Microbiol Biotechnol, 2006, 69(5): 510-514.
pmid: 16034558 |
| [34] |
Ashida N, Ishii S, Hayano S, et al. Isolation of functional single cells from environments using a micromanipulator: application to study denitrifying bacteria[J]. Appl Microbiol Biotechnol, 2010, 85(4): 1211-1217.
doi: 10.1007/s00253-009-2330-z pmid: 19936739 |
| [35] |
Zhang H, Liu KK. Optical tweezers for single cells[J]. J R Soc Interface, 2008, 5(24): 671-690.
doi: 10.1098/rsif.2008.0052 pmid: 18381254 |
| [36] | Koch MD, Shaevitz JW. Introduction to Optical Tweezers[M]// Optical Tweezers. New York: Humana Press, 2017: 3-24. |
| [37] |
Ishii S, Tago K, Senoo K. Single-cell analysis and isolation for microbiology and biotechnology: methods and applications[J]. Appl Microbiol Biotechnol, 2010, 86(5): 1281-1292.
doi: 10.1007/s00253-010-2524-4 pmid: 20309540 |
| [38] |
Zhang Z, Kimkes TEP, Heinemann M. Manipulating rod-shaped bacteria with optical tweezers[J]. Sci Rep, 2019, 9: 19086.
doi: 10.1038/s41598-019-55657-y pmid: 31836805 |
| [39] |
Dasgupta R. Optical tweezers: light for manipulating microscopic world[J]. J Phys: Conf Ser, 2012, 365: 012007.
doi: 10.1088/1742-6596/365/1/012007 URL |
| [40] |
Liu B, Liu KX, Wang N, et al. Laser tweezers Raman spectroscopy combined with deep learning to classify marine bacteria[J]. Talanta, 2022, 244: 123383.
doi: 10.1016/j.talanta.2022.123383 URL |
| [41] |
Bhagat AAS, Bow H, Hou HW, et al. Microfluidics for cell separation[J]. Med Biol Eng Comput, 2010, 48(10): 999-1014.
doi: 10.1007/s11517-010-0611-4 pmid: 20414811 |
| [42] |
Collins DJ, Neild A, DeMello A, et al. The Poisson distribution and beyond: methods for microfluidic droplet production and single cell encapsulation[J]. Lab Chip, 2015, 15(17): 3439-3459.
doi: 10.1039/c5lc00614g pmid: 26226550 |
| [43] |
Zhu HW, Lin XG, Su Y, et al. Screen-printed microfluidic dielectrophoresis chip for cell separation[J]. Biosens Bioelectron, 2015, 63: 371-378.
doi: S0956-5663(14)00573-9 pmid: 25127471 |
| [44] |
Wang KY, Zhou W, Lin ZR, et al. Sorting of tumour cells in a microfluidic device by multi-stage surface acoustic waves[J]. Sens Actuat B Chem, 2018, 258: 1174-1183.
doi: 10.1016/j.snb.2017.12.013 URL |
| [45] |
Lee H, Purdon AM, Westervelt RM. Manipulation of biological cells using a microelectromagnet matrix[J]. Appl Phys Lett, 2004, 85(6): 1063-1065.
doi: 10.1063/1.1776339 URL |
| [46] |
Dalili A, Samiei E, Hoorfar M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: microfluidic approaches[J]. Analyst, 2019, 144(1): 87-113.
doi: 10.1039/c8an01061g |
| [47] |
Pucetaite M, Ohlsson P, Persson P, et al. Shining new light into soil systems: Spectroscopy in microfluidic soil chips reveals microbial biogeochemistry[J]. Soil Biol Biochem, 2021, 153: 108078.
doi: 10.1016/j.soilbio.2020.108078 URL |
| [48] |
Nakamura IT, Ikegami M, Hasegawa N, et al. Development of an optimal protocol for molecular profiling of tumor cells in pleural effusions at single-cell level[J]. Cancer Sci, 2021, 112(5): 2006-2019.
doi: 10.1111/cas.v112.5 URL |
| [49] |
Qiao YX, Hu R, Chen DW, et al. Fluorescence-activated droplet sorting of PET degrading microorganisms[J]. J Hazard Mater, 2022, 424: 127417.
doi: 10.1016/j.jhazmat.2021.127417 URL |
| [50] |
Aynur A, Nokuzola M, Ding XT. Application of microfluidics in single-cell manipulation, omics and drug development[J]. Curr Med Chem, 2021: 28(40): 8433-8450.
doi: 10.2174/0929867328666210203205641 pmid: 33538663 |
| [51] |
Xu T, Han X, Zhu PF, et al. A milliliter to picoliter-level centrifugal microfluidic concentrator for fast pathogen detection and antimicrobial susceptibility testing[J]. Sens Actuat B Chem, 2021, 343: 130117.
doi: 10.1016/j.snb.2021.130117 URL |
| [52] |
McIlvenna D, Huang WE, Davison P, et al. Continuous cell sorting in a flow based on single cell resonance Raman spectra[J]. Lab Chip, 2016, 16(8): 1420-1429.
doi: 10.1039/c6lc00251j pmid: 26974400 |
| [53] |
Song YZ, Yin HB, Huang WE. Raman activated cell sorting[J]. Curr Opin Chem Biol, 2016, 33: 1-8.
doi: 10.1016/j.cbpa.2016.04.002 pmid: 27100046 |
| [54] |
Wang Y, Xu JB, Kong LC, et al. Raman-activated sorting of antibiotic-resistant bacteria in human gut microbiota[J]. Environ Microbiol, 2020, 22(7): 2613-2624.
doi: 10.1111/1462-2920.14962 pmid: 32114713 |
| [55] |
Su XL, Gong YH, Gou HL, et al. Rational optimization of Raman-activated cell ejection and sequencing for bacteria[J]. Anal Chem, 2020, 92(12): 8081-8089.
doi: 10.1021/acs.analchem.9b05345 pmid: 32401011 |
| [56] |
Hewitt Z, Forsyth NR, Waterfall M, et al. Fluorescence-activated single cell sorting of human embryonic stem cells[J]. Cloning Stem Cells, 2006, 8(3): 225-234.
pmid: 17009898 |
| [57] |
Czechowska K, Johnson DR, van der Meer JR. Use of flow cytometric methods for single-cell analysis in environmental microbiology[J]. Curr Opin Microbiol, 2008, 11(3): 205-212.
doi: 10.1016/j.mib.2008.04.006 pmid: 18562243 |
| [58] |
Espina L. An approach to increase the success rate of cultivation of soil bacteria based on fluorescence-activated cell sorting[J]. PLoS One, 2020, 15(8): e0237748.
doi: 10.1371/journal.pone.0237748 URL |
| [59] |
Ozawa S, Okabe S, Ishii S. Specific single-cell isolation ofEscherichia coli O157 from environmental water samples by using flow cytometry and fluorescence-activated cell sorting[J]. Foodborne Pathog Dis, 2016, 13(8): 456-461.
doi: 10.1089/fpd.2016.2125 URL |
| [60] |
Amann RI, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology[J]. J Bacteriol, 1990, 172(2): 762-770.
doi: 10.1128/jb.172.2.762-770.1990 pmid: 1688842 |
| [61] |
Yilmaz S, Haroon MF, Rabkin BA, et al. Fixation-free fluorescence in situ hybridization for targeted enrichment of microbial populations[J]. ISME J, 2010, 4(10): 1352-1356.
doi: 10.1038/ismej.2010.73 pmid: 20505753 |
| [62] | Haroon MF, Skennerton CT, Steen JA, et al. In-solution fluorescence in situ hybridization and fluorescence-activated cell sorting for single cell and population genome recovery[M]// Methods in Enzymology. Amsterdam: Elsevier, 2013: 3-19. |
| [63] |
Dam HT, Vollmers J, Sobol MS, et al. Targeted cell sorting combined with single cell genomics captures low abundant microbial dark matter with higher sensitivity than metagenomics[J]. Front Microbiol, 2020, 11: 1377.
doi: 10.3389/fmicb.2020.01377 pmid: 32793124 |
| [64] |
Hugenholtz P, Goebel BM, Pace NR. Impact of culture-independent studies on the emerging phylogenetic view of bacterial diversity[J]. J Bacteriol, 1998, 180(24): 6793.
doi: 10.1128/JB.180.24.6793-6793.1998 URL |
| [65] |
Bollmann A, Lewis K, Epstein SS. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates[J]. Appl Environ Microbiol, 2007, 73(20): 6386-6390.
doi: 10.1128/AEM.01309-07 URL |
| [66] |
Whitesides GM. The origins and the future of microfluidics[J]. Nature, 2006, 442(7101): 368-373.
doi: 10.1038/nature05058 |
| [67] |
Du GS, Fang Q, den Toonder JMJ. Microfluidics for cell-based high throughput screening platforms—a review[J]. Anal Chimica Acta, 2016, 903: 36-50.
doi: 10.1016/j.aca.2015.11.023 URL |
| [68] |
Autebert J, Coudert B, Bidard FC, et al. Microfluidic: an innovative tool for efficient cell sorting[J]. Methods, 2012, 57(3): 297-307.
doi: 10.1016/j.ymeth.2012.07.002 pmid: 22796377 |
| [69] |
Li MQ, Xu J, Romero-Gonzalez M, et al. Single cell Raman spectroscopy for cell sorting and imaging[J]. Curr Opin Biotechnol, 2012, 23(1): 56-63.
doi: 10.1016/j.copbio.2011.11.019 URL |
| [70] | 黄彩虹, 易定容, 金福江, 叶一青. 单细胞分离方法及仪器研究进展[J]. 仪器仪表学报, 2020, 41(5): 140-153. |
| Huang CH, Yi DR, Jin FJ, Ye YQ. Progress on single cell isolation methods and instruments[J]. Chinese Journal of Scientific Instrument, 2020, 41(5): 140-153. | |
| [71] |
Rubin BE, Diamond S, Cress BF, et al. Species- and site-specific genome editing in complex bacterial communities[J]. Nat Microbiol, 2021, 7(1): 34-47.
doi: 10.1038/s41564-021-01014-7 pmid: 34873292 |
| [1] | 叶娜, 张晓兰, 包鹏甲, 王兴东, 阎萍, 潘和平. 单细胞测序技术及其在毛囊发育中的应用[J]. 生物技术通报, 2021, 37(10): 245-256. |
| [2] | 朱允华;李俭;方俊;田云;卢向阳;. 宏基因组技术在开发极端环境未培养微生物中的应用[J]. , 2011, 0(09): 52-58. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||