








生物技术通报 ›› 2023, Vol. 39 ›› Issue (9): 300-310.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0169
丁丽1( ), 都婷婷1, 唐琼英1, 高权新1, 易少奎1(
), 都婷婷1, 唐琼英1, 高权新1, 易少奎1( ), 杨国梁1,2(
), 杨国梁1,2( )
)
收稿日期:2023-02-28
出版日期:2023-09-26
发布日期:2023-10-24
通讯作者:
易少奎,男,博士,副教授,研究方向:水产动物遗传与育种;E-mail: 02844@zjhu.edu.cn;作者简介:丁丽,女,硕士研究生,研究方向:水产遗传与育种;E-mail: dingli104619430849@163.com
基金资助:
DING Li1( ), DU Ting-ting1, TANG Qiong-ying1, GAO Quan-xin1, YI Shao-kui1(
), DU Ting-ting1, TANG Qiong-ying1, GAO Quan-xin1, YI Shao-kui1( ), YANG Guo-liang1,2(
), YANG Guo-liang1,2( )
)
Received:2023-02-28
Published:2023-09-26
Online:2023-10-24
摘要:
蜕皮是罗氏沼虾重要的生理过程,为了探究罗氏沼虾蜕皮周期中内分泌调控与蜕皮通路中相关基因在蜕皮周期中的表达模式,阐明罗氏沼虾蜕皮的分子调节通路。本研究测定了罗氏沼虾肝胰腺和血淋巴组织中蜕皮周期内蜕皮相关酶活性(谷氨酰胺合成酶,β-N乙酰氨基葡萄糖苷酶和几丁质酶)与蜕皮激素含量,并通过RT-qPCR分析了蜕皮信号通路中Mr-ETHR、Mr-FTZ-F1以及RXR、ECR和MIH基因在罗氏沼虾不同蜕皮周期内的表达模式。酶活测定结果表明,谷氨酰胺合成酶在血淋巴组织中活力高于肝胰腺组织(P<0.05);β-N乙酰氨基葡萄糖苷酶在肝胰腺和血淋巴组织中蜕皮前期的活力远高于蜕皮后期(P<0.05)。在肝胰腺中几丁质酶在蜕皮后期活力显著高于其他时期(P<0.05)。肝胰腺和血淋巴组织中蜕皮激素含量在蜕皮间期最低,蜕皮后期达到最高,呈上升趋势。通过PCR扩增与测序验证获得了Mr-ETHR、Mr-FTZ-F1基因ORF全长序列,Mr-ETHR基因ORF全长1 173 bp,编码390个氨基酸;Mr-FTZ-F1基因ORF全长为1 206 bp,编码401个氨基酸。荧光定量结果表明Mr-ETHR、Mr-RXR和Mr-ECR在蜕皮后期表达量达到最高,而Mr-FTZ-F1在蜕皮前期表达量达到最高。Mr-MIH在蜕皮间期表达量达到最高,蜕皮后期表达量最低,呈下降趋势。聚类分析及相关性分析结果表明Mr-ETHR与Mr-RXR和Mr-ECR表达模式相近且呈极显著正相关(r=0.7030,P<0.01;r=0.8680,P<0.01),Mr-FTZ-F1和Mr-MIH表达模式相近且呈极显著正相关(r=0.6665,P<0.01),而Mr-FTZ-F1与Mr-ECR和Mr-ETHR呈极显著负相关(r=-0.8339,P<0.01;r=-0.6275,P<0.01)。研究表明罗氏沼虾的蜕皮过程受谷氨酰胺合成酶、β-N乙酰氨基葡萄糖苷酶和几丁质酶的调节,Mr-ETHR、Mr-RXR、Mr-ECR、Mr-FTZ-F1和Mr-MIH参与调控罗氏沼虾蜕皮过程,Mr-ETHR、Mr-RXR和Mr-ECR在蜕皮信号通路中起正向调节作用,而Mr-FTZ-F1和Mr-MIH起负调节作用。本研究结果为甲壳动物蜕皮信号通路下游基因的蜕皮调控机制研究提供了基础资料。
丁丽, 都婷婷, 唐琼英, 高权新, 易少奎, 杨国梁. 罗氏沼虾蜕皮周期中内分泌调控和蜕皮信号通路相关基因的表达分析[J]. 生物技术通报, 2023, 39(9): 300-310.
DING Li, DU Ting-ting, TANG Qiong-ying, GAO Quan-xin, YI Shao-kui, YANG Guo-liang. Analyses of Endocrine Regulation and Expression of Genes Related to the Molting Signaling Pathway in the Molting Cycle of Macrobrachium rosenbergii[J]. Biotechnology Bulletin, 2023, 39(9): 300-310.

图1 节肢动物蜕皮调控过程示意图 MIH受体是G蛋白偶联受体,当MIH与受体的结合时,细胞内cAMP增加,激活了细胞膜上的Ca2+通道,Ca2+进入细胞内并激活了钙调蛋白(CaM),CaM去磷酸化一氧化氮合酶,激活NOS,以精氨酸为底物形成NO,而后激活鸟苷酸环化酶I型(GC-I),GC-I使GTP转化为cGTP,激活细胞内的cGTP依赖的蛋白激酶,活性的PKA进入细胞核,调节蜕皮激素20E的合成,而20E入核后,与异源二聚体(ECR/USP/RXR)结合后,启动早期应答基因,如E75、E74和Br-C等,随后调控早晚期基因,如HR3、HR4和HR38等,最后这些早晚期应答基因调控晚期应答基因,如FTZ-F1,最终完成蜕皮。内分泌Inka细胞分泌蜕皮启动激素(ETH)作用蜕皮启动激素受体(ETHR),启动蜕皮
Fig. 1 Schematic diagram of the regulation process of molting in arthropods MIH receptor is G protein-coupled receptor. When MIH binds to the receptor, intracellular cAMP increases, activating Ca2+ channels on the cell membrane, and Ca2+ enters the cell and activates calmodulin(CaM). CaM dephosphorylates nitric oxide synthase activates NOS, then NO is formed with arginine as a substrate, and then this activates guanylate cyclase type I(GC-I), GC-I converts GTP to cGTP, and activates cGTP-dependent protein kinases in cells. The active PKA enters the nucleus to regulate the synthesis of ecdyhormone, and after 20E enters the nucleus, it binds to heterodimer(ECR/USP/RXR)to initiate early response genes, such as E75, E74 and Br-C, and then regulates early-late genes, such as HR3, HR4 and HR38, and finally these early and late response genes regulate late response genes, such as FTZ-F1, and finally complete molting. Endocrine Inka cells secrete ecdy-initiating hormone(ETH)acting on the ecdy-initiating hormone receptor(ETHR), initiating molt
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp | 退火温度Annealing temperature/℃ |
|---|---|---|---|
| FTZ-F1-F-1 | CCTATTATGCTGCGGGAGT | 649 | 55 |
| FTZ-F1-R-1 | CACCGTTGATGTGCTTGTA | ||
| FTZ-F1-F-2 | TGTCCGTACTGTCGCTTTC | 627 | 52 |
| FTZ-F1-R-2 | ACCAACTCCCGCAGCATA | ||
| FTZ-F1-F-3 | CCTATTATGCTGCGGGAGT | 595 | 55 |
| FTZ-F1-R-3 | GAAGCTCTGGCAGTAAATCC | ||
| ETHR-F-1 | GTCCCGTTGCTGGTTCTT | 535 | 55 |
| ETHR-R-1 | ATGACGCTGACCCTGTTG | ||
| ETHR-F-2 | CGATGACGCTGACCCTGT | 537 | 55 |
| ETHR-R-2 | AAGCCAAGGCGATAACAG | ||
| ETHR-F-3 | ATAGTGCAGCCGCAGATT | 635 | 53.5 |
| ETHR-R-3 | GGATCACCTGCACCAACGTA | ||
| RTFTZ-F1-F | GGATCACCTGCACCAACGTA | 120 | 57 |
| RTFTZ-F1-R | GGAAACGATCTGCGAACTGC | ||
| RTETHR-F | GTCCCGTTGCTGGTTCTT | 165 | 55 |
| RTETHR-R | GTCTCGCTCGCATTTGTG | ||
| RTMIH-F | AGCCCTGAGTGTCTGTCC | 123 | 57 |
| RTMIH-R | CCTTGCGTTGTCTGGTT | ||
| RTRXR-F | GATCGGCAGTCCCCTTTGAA | 109 | 57 |
| RTRXR-R | TTGGACACACTGGGAGAAGC | ||
| RTECR-F | AGAGCCGCATAAAGTGGAGA | 134 | 57 |
| RTECR-R | CTCAGGTCGGTCAGGATGTT | ||
| 18S-F | TATACGCTAGTGGAGCTGGAA | 313 | 60 |
| 18S-R | GGGGAGGTAGTGACGAAAAAT |
表1 实验所用引物序列
Table 1 Primer sequences used in the experiment
| 引物名称Primer name | 引物序列Primer sequence(5'-3') | 产物长度Product length/bp | 退火温度Annealing temperature/℃ |
|---|---|---|---|
| FTZ-F1-F-1 | CCTATTATGCTGCGGGAGT | 649 | 55 |
| FTZ-F1-R-1 | CACCGTTGATGTGCTTGTA | ||
| FTZ-F1-F-2 | TGTCCGTACTGTCGCTTTC | 627 | 52 |
| FTZ-F1-R-2 | ACCAACTCCCGCAGCATA | ||
| FTZ-F1-F-3 | CCTATTATGCTGCGGGAGT | 595 | 55 |
| FTZ-F1-R-3 | GAAGCTCTGGCAGTAAATCC | ||
| ETHR-F-1 | GTCCCGTTGCTGGTTCTT | 535 | 55 |
| ETHR-R-1 | ATGACGCTGACCCTGTTG | ||
| ETHR-F-2 | CGATGACGCTGACCCTGT | 537 | 55 |
| ETHR-R-2 | AAGCCAAGGCGATAACAG | ||
| ETHR-F-3 | ATAGTGCAGCCGCAGATT | 635 | 53.5 |
| ETHR-R-3 | GGATCACCTGCACCAACGTA | ||
| RTFTZ-F1-F | GGATCACCTGCACCAACGTA | 120 | 57 |
| RTFTZ-F1-R | GGAAACGATCTGCGAACTGC | ||
| RTETHR-F | GTCCCGTTGCTGGTTCTT | 165 | 55 |
| RTETHR-R | GTCTCGCTCGCATTTGTG | ||
| RTMIH-F | AGCCCTGAGTGTCTGTCC | 123 | 57 |
| RTMIH-R | CCTTGCGTTGTCTGGTT | ||
| RTRXR-F | GATCGGCAGTCCCCTTTGAA | 109 | 57 |
| RTRXR-R | TTGGACACACTGGGAGAAGC | ||
| RTECR-F | AGAGCCGCATAAAGTGGAGA | 134 | 57 |
| RTECR-R | CTCAGGTCGGTCAGGATGTT | ||
| 18S-F | TATACGCTAGTGGAGCTGGAA | 313 | 60 |
| 18S-R | GGGGAGGTAGTGACGAAAAAT |
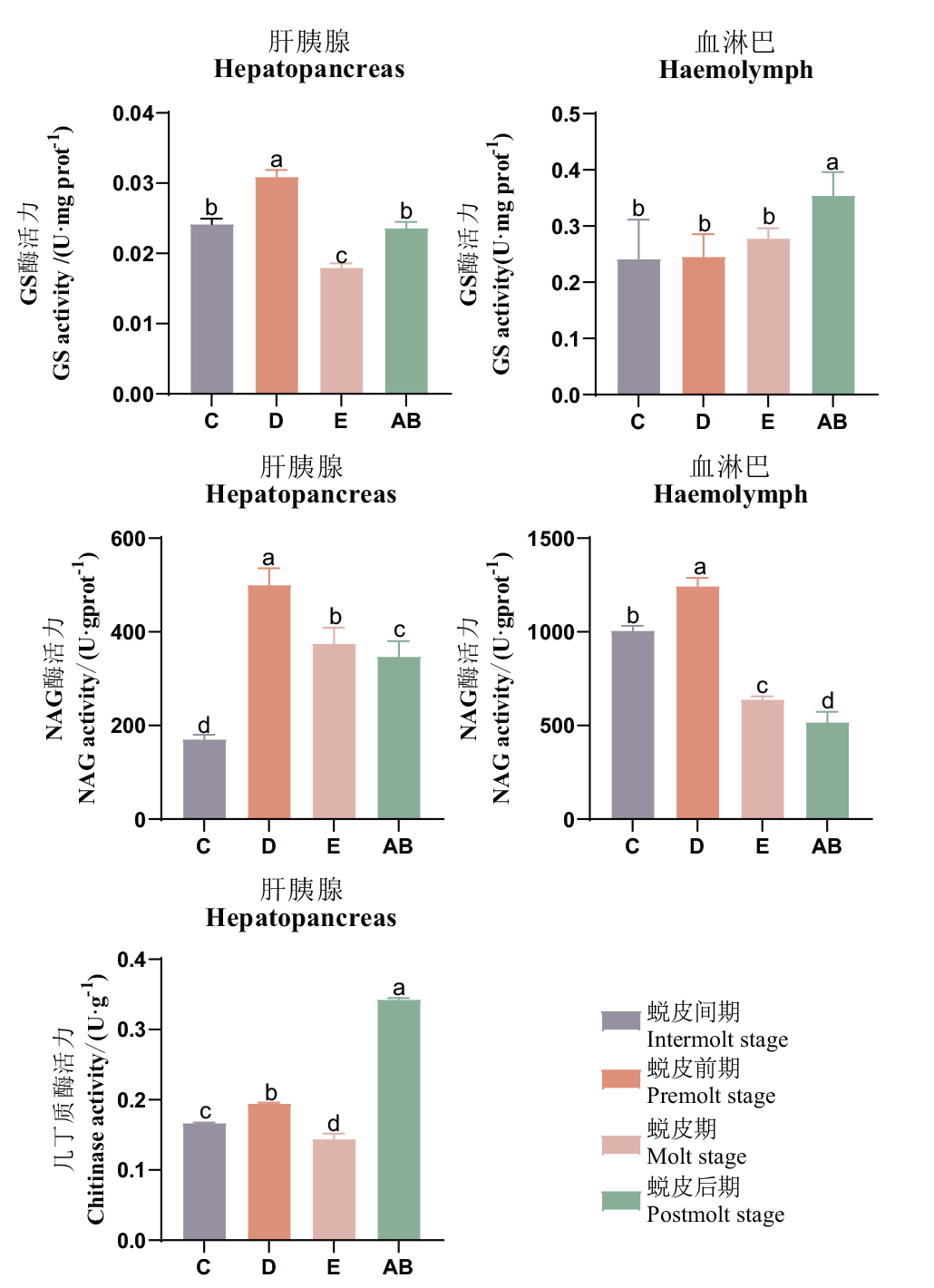
图2 罗氏沼虾蜕皮周期中蜕皮相关酶活性变化 C:蜕皮间期;D:蜕皮前期;E:蜕皮期;AB:蜕皮后期;不同小写字母代表显著差异(P<0.05),下同
Fig. 2 Dynamics in molting-related enzyme activity in M. rosenbergiiat at different molting cycles C: Intermolt stage. D: Premolt stage. E: Molt stage. AB: Postmolt stage. Different letters indicated significant differences(P<0.05), The same below
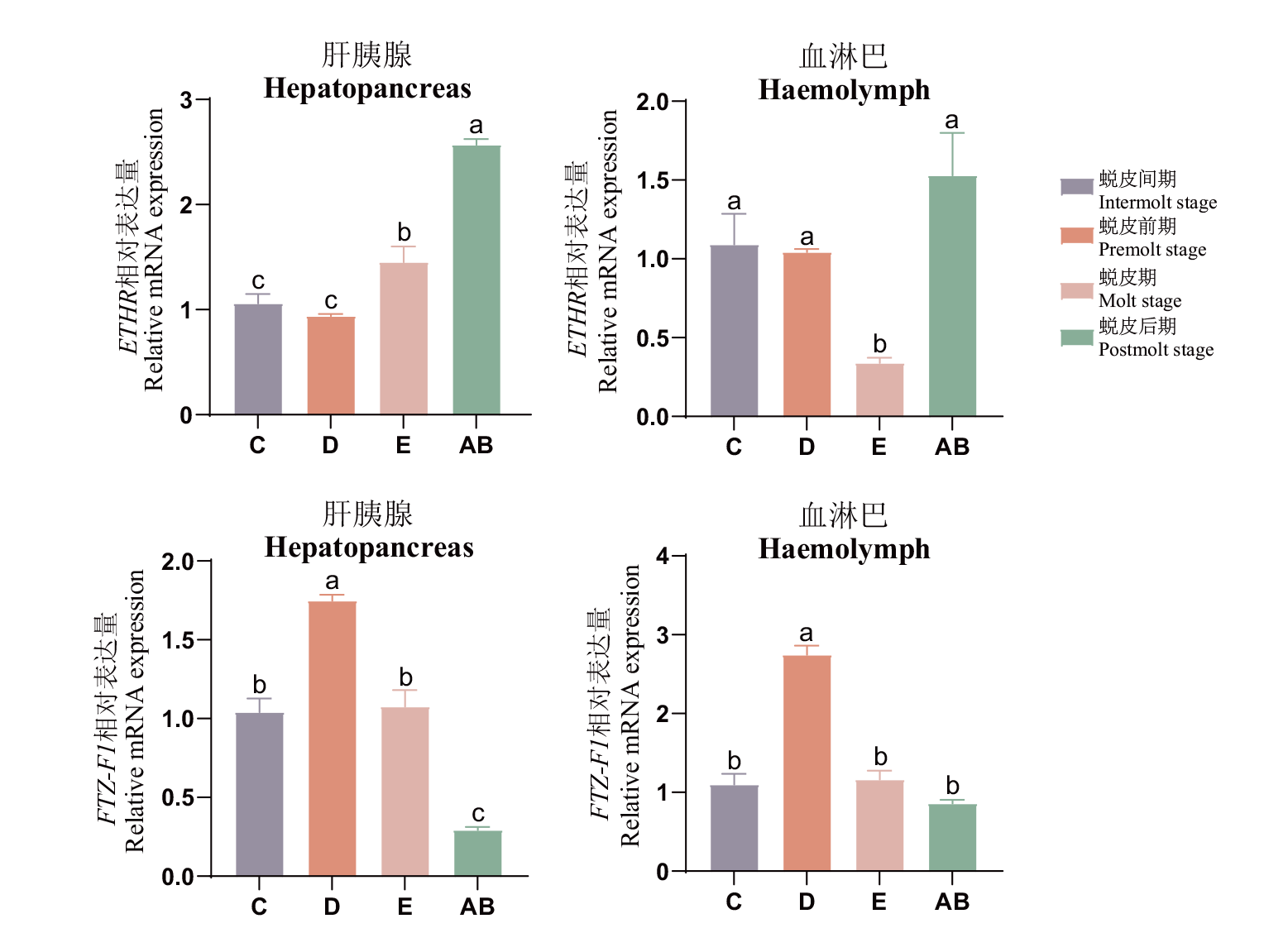
图5 在不同蜕皮时期内Mr-ETHR、Mr-FTZ-F1基因在肝胰腺和血淋巴组织中的表达情况
Fig. 5 Expressions of Mr-ETHR and Mr-FTZ-F1 in hepatopancreatic and hemolymphatic tissues at different molting stages

图6 在不同蜕皮时期内罗氏沼虾ETHR、FTZ-F1、RXR、ECR和MIH基因的表达情况
Fig. 6 Expressions of Mr-ETHR, Mr-FTZ-F1, Mr-RXR, Mr-ECR and Mr-MIH genes in M. rosenbergii at different molting cycles
| [1] |
Liu SB, Li XY, Wang XD, et al. Molting, tissue calcium-phosphorus deposition and immunity of juvenile Chinese mitten crab(Eriocheir sinensis)fed different levels of calcium and vitamin D3[J]. Aquaculture, 2022, 554: 738124.
doi: 10.1016/j.aquaculture.2022.738124 URL |
| [2] | 叶成凯, 卢志杰, Sarath Babu V, 等. 罗氏沼虾几丁质酶3B基因的克隆及其在蜕皮周期中的表达[J]. 水产学报, 2019, 43(4): 751-762. |
| Ye CK, Lu ZJ, Sarath B, et al. Cloning and expression analysis of chitinase-3B from giant freshwater prawn(Macrobrachium rosenbergii)during molting cycle[J]. J Fish China, 2019, 43(4): 751-762. | |
| [3] |
Raghavan SDA, Ayanath A. Effect of ecdysteroids on oogenesis in the freshwater crab Travancoriana schirnerae Bott, 1969(Crustacea: Gecarcinucidae)[J]. Braz J Biol Sci, 2019, 6(12): 87-101.
doi: 10.21472/bjbs.061208 URL |
| [4] | Head TB, Mykles DL, Tomanek L. Proteomic analysis of the crustacean molting gland(Y-organ)over the course of the molt cycle[J]. Comp Biochem Physiol D Genom Proteom, 2019, 29: 193-210. |
| [5] |
Kim HW, Lee SG, Mykles DL. Ecdysteroid-responsive genes, RXR and E75, in the tropical land crab, Gecarcinus lateralis: differential tissue expression of multiple RXR isoforms generated at three alternative splicing sites in the hinge and ligand-binding domains[J]. Mol Cell Endocrinol, 2005, 242(1-2): 80-95.
doi: 10.1016/j.mce.2005.08.001 URL |
| [6] |
Chang ES, Mykles DL. Regulation of crustacean molting: a review and our perspectives[J]. Gen Comp Endocrinol, 2011, 172(3): 323-330.
doi: 10.1016/j.ygcen.2011.04.003 URL |
| [7] |
Mykles DL. Ecdysteroid metabolism in crustaceans[J]. J Steroid Biochem Mol Biol, 2011, 127(3-5): 196-203.
doi: 10.1016/j.jsbmb.2010.09.001 URL |
| [8] |
Van Lommel J, Lenaerts C, Delgouffe C, et al. Knockdown of ecdysone receptor in male desert locusts affects relative weight of accessory glands and mating behavior[J]. J Insect Physiol, 2022, 138: 104368.
doi: 10.1016/j.jinsphys.2022.104368 URL |
| [9] | 柳鹏飞, 王伟伟, 凌晓霏, 等. 保幼激素和蜕皮激素调控昆虫变态发育机制的进展[J]. 基因组学与应用生物学, 2021, 40(Z1): 2054-2062. |
| Liu PF, Wang WW, Lin XF, et al. Advances in molecular mechanism of juvenile hormone and molting hormone-mediated metamorphosis[J]. Genomics and Applied Biology, 2021, 40(Z1): 2054-2062. | |
| [10] |
Gu SH, Chen CH, Lin PL. Changes in expressions of ecdysteroidogenic enzyme and ecdysteroid signaling genes in relation to Bombyx embryonic development[J]. J Exp Zool A Ecol Integr Physiol, 2021, 335(5): 477-488.
doi: 10.1002/jez.v335.5 URL |
| [11] |
Street SM, Eytcheson SA, LeBlanc GA. The role of nuclear receptor E75 in regulating the molt cycle of Daphnia magna and consequences of its disruption[J]. PLoS One, 2019, 14(8): e0221642.
doi: 10.1371/journal.pone.0221642 URL |
| [12] |
Zhu L, Zhang W, Li G, et al. Molecular characterization of ecdysis triggering hormone and its receptor in citrus red mite(Panonychus citri)[J]. Comp Biochem Physiol A Mol Integr Physiol, 2019, 230: 100-105.
doi: 10.1016/j.cbpa.2019.01.003 URL |
| [13] |
Shahin R, Fujimoto S, Kawasaki H. Cuticular protein genes showing peaks at different stages are probably regulated by different ecdysone responsive transcription factors during larval-pupal transformation[J]. Gene, 2022, 809: 146002.
doi: 10.1016/j.gene.2021.146002 URL |
| [14] |
Cho KH, Daubnerová I, Park Y, et al. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila[J]. Dev Biol, 2014, 385(2): 253-262.
doi: 10.1016/j.ydbio.2013.11.003 URL |
| [15] |
Zhang WN, Ma L, Liu XY, et al. Dissecting the roles of FTZ-F1 in larval molting and pupation, and the sublethal effects of methoxyfenozide on Helicoverpa armigera[J]. Pest Manag Sci, 2021, 77(3): 1328-1338.
doi: 10.1002/ps.v77.3 URL |
| [16] |
Liu ZQ, Nanda S, Yang CX, et al. RNAi suppression of the nuclear receptor FTZ-F1 impaired ecdysis, pupation, and reproduction in the 28-spotted potato ladybeetle, Henosepilachna vigintioctopunctata[J]. Pestic Biochem Physiol, 2022, 182: 105029.
doi: 10.1016/j.pestbp.2021.105029 URL |
| [17] |
Yuan HW, Zhang WY, Fu Y, et al. MnFtz-f1 is required for molting and ovulation of the oriental river prawn Macrobrachium nipponense[J]. Front Endocrinol, 2021, 12: 798577.
doi: 10.3389/fendo.2021.798577 URL |
| [18] |
Shen CH, Xu QY, Fu KY, et al. Two splice isoforms of Leptinotarsa ecdysis triggering hormone receptor have distinct roles in larva-Pupa transition[J]. Front Physiol, 2020, 11: 593962.
doi: 10.3389/fphys.2020.593962 URL |
| [19] |
Shi Y, Liu TY, Jiang HB, et al. The ecdysis triggering hormone system, via ETH/ETHR-B, is essential for successful reproduction of a major pest insect, Bactrocera dorsalis(Hendel)[J]. Front Physiol, 2019, 10: 151.
doi: 10.3389/fphys.2019.00151 URL |
| [20] |
Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors: the transmembrane segments and second extracellular loop[J]. Annu Rev Pharmacol Toxicol, 2002, 42: 437-467.
pmid: 11807179 |
| [21] | 丁兰, 徐胜南. 罗氏沼虾养殖技术探索与示范[J]. 水产养殖, 2022, 43(4): 64-66. |
| Ding L, Xu SN. Exploration and demonstration of Macrobrachium rosenbergii culture technology[J]. J Aquac, 2022, 43(4): 64-66. | |
| [22] |
Schumann I, Kenny N, Hui J, et al. Halloween genes in panarthropods and the evolution of the early moulting pathway in Ecdysozoa[J]. R Soc Open Sci, 2018, 5(9): 180888.
doi: 10.1098/rsos.180888 URL |
| [23] |
Benhalima K, Moriyasu M, Hébert M. A technique for identifying the early-premolt stage in the male snow crab Chionoecetes opilio(Brachyura: Majidae)in Baie des Chaleurs, southern Gulf of St. Lawrence[J]. Can J Zool, 1998, 76(4): 609-617.
doi: 10.1139/z97-239 URL |
| [24] |
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method[J]. Methods, 2001, 25(4): 402-408.
doi: 10.1006/meth.2001.1262 pmid: 11846609 |
| [25] |
Chen CJ, Chen H, Zhang Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data[J]. Mol Plant, 2020, 13(8): 1194-1202.
doi: S1674-2052(20)30187-8 pmid: 32585190 |
| [26] |
Kim C, Kültz D. An osmolality/salinity-responsive enhancer 1(OSRE1)in intron 1 promotes salinity induction of tilapia glutamine synthetase[J]. Sci Rep, 2020, 10: 12103.
doi: 10.1038/s41598-020-69090-z |
| [27] |
Qiu LG, Shi X, Yu SM, et al. Changes of ammonia-metabolizing enzyme activity and gene expression of two strains in shrimp litopenaeus vannamei under ammonia stress[J]. Front Physiol, 2018, 9: 211.
doi: 10.3389/fphys.2018.00211 URL |
| [28] | 孙武卫. 低盐胁迫下凡纳滨对虾消减cDNA文库构建及谷氨酰胺合成酶cDNA克隆和表达[D]. 湛江: 广东海洋大学. |
| Sun WW. Construction of subtractive cDNA library of litopenaeus vannamei and cloning and expression of glutamine synthase cDNA under low salt stress[D]. Zhanjiang: Guangdong Ocean University. | |
| [29] |
McKinnon E, Hargittai PT, Grossfeld RM, et al. Glutamine cycle enzymes in the crayfish giant nerve fiber: implications for axon-to-glia signaling[J]. Glia, 1995, 14(3): 198-208.
pmid: 7591031 |
| [30] | 贾玉萍. 中国明对虾SWD抗菌肽、谷氨酰胺合成酶和Ras基因的表达与功能研究[D]. 济南: 山东大学, 2008. |
| Jia YP. Study on the expression and function of SWD antimicrobial peptide, glutamine synthase and ras gene in fenneropenaeus China[D]. Jinan: Shandong University, 2008. | |
| [31] | 陈劲松, 周发林, 江世贵, 等. 斑节对虾谷氨酰胺合成酶基因的克隆及氨氮胁迫对其时空表达的影响[J]. 上海海洋大学学报, 2016, 25(4): 497-507. |
| Chen JS, Zhou FL, Jiang SG, et al. Molecular cloning and the expression analysis of glutamine synthetase(GS)in Penaeus monodon under the condition of ammonia nitrogen stress[J]. J Shanghai Ocean Univ, 2016, 25(4): 497-507. | |
| [32] | 李少飞. 中国对虾氨氮代谢酶基因的cDNA克隆及其在氨氮解毒代谢过程中的作用[D]. 大连: 大连海洋大学, 2014. |
| Li SF. CDNA cloning of ammonia-nitrogen metabolizing enzyme gene of Penaeus China and its role in detoxification and metabolism of ammonia-nitrogen[D]. Dalian: Dalian Ocean University, 2014. | |
| [33] |
Dukariya G, Kumar A. Distribution and biotechnological applications of chitinase: a review[J]. Ijbb, 2020, 8(2): 17-29.
doi: 10.13189/ijbb.2020.080201 URL |
| [34] | Soedirga LC, Hardoko H, Widianto NV. Production of N-acetylglucosamine from semi purified chitinase of Mucor circinelloides that immobilized by using agar[J]. J Perikanan Univ Gadjah Mada, 2019, 21(2): 99. |
| [35] |
Beygmoradi A, Homaei A, Hemmati R, et al. Identification of a novel tailor-made chitinase from white shrimp Fenneropenaeus merguiensis[J]. Colloids Surf B Biointerfaces, 2021, 203: 111747.
doi: 10.1016/j.colsurfb.2021.111747 URL |
| [36] | 吕艳杰, 关建义, 杜娟, 等. 日本沼虾N-乙酰-β-D-氨基葡萄糖苷酶基因克隆及KK-42对其表达的影响[J]. 水产学报, 2018, 42(5): 646-652. |
| Lü YJ, Guan JY, Du J, et al. Molecular cloning of N-acetyl-β-D-glucosaminidase(NAGase)gene and the effect of KK-42 on NAGase gene in Macrobrachium nipponense[J]. J Fish China, 2018, 42(5): 646-652. | |
| [37] |
Li XG, Xu ZQ, Zhou G, et al. Molecular characterization and expression analysis of five chitinases associated with molting in the Chinese mitten crab, Eriocheir sinensis[J]. Comp Biochem Physiol B Biochem Mol Biol, 2015, 187: 110-120.
doi: 10.1016/j.cbpb.2015.05.007 URL |
| [38] | 黄姝, 陈娇, 陈晓雯, 等. 中华绒螯蟹蜕壳周期内蜕皮激素和蜕壳相关基因的表达动态分析[J]. 农业生物技术学报, 2018, 26(1): 150-158. |
| Huang S, Chen J, Chen XW, et al. Dynamic analysis of ecdysteroid hormone content and molting related genes expression in the molting cycle of Chinese mitten crab(Eriocheir sinensis)[J]. J Agric Biotechnol, 2018, 26(1): 150-158. | |
| [39] | 张龙涛, 吕建建, 高保全, 等. 三疣梭子蟹 ftz-f 基因的克隆及相关核受体基因在蜕皮中的功能分析[J]. 海洋与湖沼, 2015, 46(6): 1390-1397. |
| Zhang LT, Lv JJ, Gao BQ, et al. Cloning of Portunus trituberculatus ftz-f1 cdna and expression analysis of related nuclear receptors during molting cycle[J]. Oceanol Limnol Sin, 2015, 46(6): 1390-1397. | |
| [40] |
Techa S, Chung JS. Ecdysteroids regulate the levels of molt-inhibiting hormone(MIH)expression in the blue crab, Callinectes sapidus[J]. PLoS One, 2015, 10(4): e0117278.
doi: 10.1371/journal.pone.0117278 URL |
| [41] | 王瑶, 杨志刚, 沈城, 等. 中华绒螯蟹RXR基因全长cDNA克隆及表达分析[J]. 水产学报, 2013, 37(12): 1761-1769. |
|
Wang Y, Yang ZG, Shen C, et al. Full-length cDNA cloning and expression analysis of RXR gene in Eriocheir sinensis[J]. Journal of Fisheries of China, 2013, 37(12): 1761-1769.
doi: 10.3724/SP.J.1231.2013.38754 URL |
|
| [42] |
Jose Priya TA, Li FH, Zhang JQ, et al. Molecular characterization and effect of RNA interference of retinoid X receptor(RXR)on E75 and chitinase gene expression in Chinese shrimp Fenneropenaeus chinensis[J]. Comp Biochem Physiol B Biochem Mol Biol, 2009, 153(1): 121-129.
doi: 10.1016/j.cbpb.2009.02.009 pmid: 19250973 |
| [1] | 李玉锋,戴习林,袁新程,周迅,胡彦杰,丁福江. 四个罗氏沼虾抗病选择系抗病力比较分析[J]. 生物技术通报, 2017, 33(7): 203-209. |
| [2] | 戴习林, 高翔, 王海洋, 明磊, 江宗冰, 丁福江. 幼体发育速度不同的罗氏沼虾遗传结构分析[J]. 生物技术通报, 2016, 32(2): 192-197. |
| [3] | 戴习林;邓平平;施永海;何安元;蒋飞;丁福江;. 罗氏沼虾SSR标记再开发及其影响因素初探[J]. , 2012, 0(10): 142-149. |
| [4] | 杨哲;高路;陈慧卿;陈克平;. 一种新的家蚕信息素相关蛋白基因BmP218的生物信息学分析及功能预测[J]. , 2009, 0(S1): 315-319. |
| [5] | 杨晓菁;王玉凤;. 一种简便的适合抱卵孵化甲壳动物的转基因方法[J]. , 2008, 0(S1): 287-290. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||