








生物技术通报 ›› 2024, Vol. 40 ›› Issue (5): 300-309.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1006
收稿日期:2023-10-30
出版日期:2024-05-26
发布日期:2024-04-25
通讯作者:
黄俊琼,女,教授,研究方向:医学免疫;E-mail: junqiongh@aliyun.com作者简介:谢爽,男,硕士,研究方向:医学免疫;E-mail: 1172143123@qq.com
基金资助:
XIE Shuang1( ), CHEN Yao2, HUANG Jun-qiong1(
), CHEN Yao2, HUANG Jun-qiong1( )
)
Received:2023-10-30
Published:2024-05-26
Online:2024-04-25
摘要:
【目的】评估预先存在的H5Nx交叉反应性免疫记忆,为人类H5禽流感病毒的防治和广谱疫苗的研发提供理论数据。【方法】利用生物信息学方法筛选人源H5Nx禽流感病毒与季节性流感病毒的共同保守的交叉反应性CD4+ T细胞表位,进一步分析保守表位嵌套CD8+ T细胞表位和B细胞表位情况以及在中国人群中的覆盖率。【结果】92.3%(513/555)的H5Nx CD4+ T细胞表位在季节性甲型流感病毒中具有交叉反应性,其中172个表位嵌套CD8+ T细胞表位和5个表位嵌套B细胞表位。这些表位与相应HLA-DRB1等位基因在全世界人群中的覆盖率为88.65%。【结论】由于反复接触季节性甲型流感病毒,人群中存在一定水平的的H5Nx交叉反应性免疫记忆,能够为H5Nx感染提供部分保护。
谢爽, 陈瑶, 黄俊琼. H5Nx禽流感病毒交叉反应性CD4+ T细胞表位预测[J]. 生物技术通报, 2024, 40(5): 300-309.
XIE Shuang, CHEN Yao, HUANG Jun-qiong. Prediction of H5Nx Avian Influenza Virus Cross-reactivity CD4+ T-cell Epitopes[J]. Biotechnology Bulletin, 2024, 40(5): 300-309.
| 蛋白片段Protein segment | 蛋白序列数 Protein sequence number | |||||||
|---|---|---|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | H5N1 | H5N6 | H5N8 | Total | ||
| HA | 1994 | 43360 | 62270 | 517 | 37 | 1 | 108179 | |
| NA | 2803 | 39867 | 56908 | 477 | 37 | 1 | 100093 | |
| M1 | 1645 | 28497 | 44559 | 394 | 37 | 1 | 75133 | |
| M2 | 1645 | 28496 | 44569 | 404 | 37 | 1 | 75152 | |
| NS1 | 872 | 22279 | 33622 | 391 | 37 | 1 | 57202 | |
| NEP | 872 | 22279 | 33624 | 389 | 37 | 1 | 57202 | |
| NP | 881 | 22047 | 35759 | 384 | 37 | 1 | 59109 | |
| PA | 877 | 22522 | 35229 | 386 | 37 | 1 | 59052 | |
| PB1 | 871 | 20601 | 34524 | 371 | 37 | 1 | 56405 | |
| PB2 | 900 | 21837 | 34855 | 371 | 37 | 1 | 58001 | |
表1 sH1N1、pH1N1、H3N2、H5N1、H5N6和H5N8病毒蛋白质序列分析数据集
Table 1 Protein sequence analysis datasets of sH1N1, pH-1N1, H3N2, H5N1, H5N6 and H5N8 virus subtypes
| 蛋白片段Protein segment | 蛋白序列数 Protein sequence number | |||||||
|---|---|---|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | H5N1 | H5N6 | H5N8 | Total | ||
| HA | 1994 | 43360 | 62270 | 517 | 37 | 1 | 108179 | |
| NA | 2803 | 39867 | 56908 | 477 | 37 | 1 | 100093 | |
| M1 | 1645 | 28497 | 44559 | 394 | 37 | 1 | 75133 | |
| M2 | 1645 | 28496 | 44569 | 404 | 37 | 1 | 75152 | |
| NS1 | 872 | 22279 | 33622 | 391 | 37 | 1 | 57202 | |
| NEP | 872 | 22279 | 33624 | 389 | 37 | 1 | 57202 | |
| NP | 881 | 22047 | 35759 | 384 | 37 | 1 | 59109 | |
| PA | 877 | 22522 | 35229 | 386 | 37 | 1 | 59052 | |
| PB1 | 871 | 20601 | 34524 | 371 | 37 | 1 | 56405 | |
| PB2 | 900 | 21837 | 34855 | 371 | 37 | 1 | 58001 | |
| 蛋白片段 Protein segment | 氨基酸序列同源性 Amino acid sequence identity/% | |||||
|---|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | H5N1 | H5N6 | ||
| HA | 50.5-56.2 | 47.1-55.4 | 32.8-55.6 | 88.4-94.2 | 92.5-99.3 | |
| NA | 16.7-39.4 | 32.6-39.7 | 19.1-37.0 | 38.2-43.1 | 18.7-22.6 | |
| M1 | 92.2-96.8 | 91.3-95.5 | 90.0-96.8 | 92.5-97.1 | 93.4-100.0 | |
| M2 | 73.7-91.4 | 80.7-91.4 | 72.4-93.6 | 79.1-92.2 | 80.7-100.0 | |
| NS1 | 76.2-86.2 | 72.3-80.7 | 72.6-86.7 | 80.6-89.8 | 58.5-93.2 | |
| NEP | 85.8-93.2 | 79.9-89.6 | 82.9-91.4 | 82.5-92.3 | 76.9-96.6 | |
| NP | 90.5-96.3 | 90.3-94.5 | 88.5-95.7 | 96.4-98.4 | 93.4-97.6 | |
| PA | 91.6-96.7 | 93.5-97.0 | 92.2-97.0 | 94.5-98.3 | 95.1-98.0 | |
| PB1 | 93.9-97.3 | 93.9-96.4 | 93.7-98.0 | 96.0-98.3 | 96.0-98.5 | |
| PB2 | 93.5-96.1 | 92.9-96.9 | 87.7-97.2 | 95.0-97.9 | 95.7-98.4 | |
表2 A/Astrakhan/3212/2020(H5N8)与sH1N1、pH1N1和H3N2病毒氨基酸序列同源性
Table 2 Amino acid sequence identity of A/Astrakhan/3212/2020(H5N8)with sH1N1, pH1N1 and H3N2 virus subtypes
| 蛋白片段 Protein segment | 氨基酸序列同源性 Amino acid sequence identity/% | |||||
|---|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | H5N1 | H5N6 | ||
| HA | 50.5-56.2 | 47.1-55.4 | 32.8-55.6 | 88.4-94.2 | 92.5-99.3 | |
| NA | 16.7-39.4 | 32.6-39.7 | 19.1-37.0 | 38.2-43.1 | 18.7-22.6 | |
| M1 | 92.2-96.8 | 91.3-95.5 | 90.0-96.8 | 92.5-97.1 | 93.4-100.0 | |
| M2 | 73.7-91.4 | 80.7-91.4 | 72.4-93.6 | 79.1-92.2 | 80.7-100.0 | |
| NS1 | 76.2-86.2 | 72.3-80.7 | 72.6-86.7 | 80.6-89.8 | 58.5-93.2 | |
| NEP | 85.8-93.2 | 79.9-89.6 | 82.9-91.4 | 82.5-92.3 | 76.9-96.6 | |
| NP | 90.5-96.3 | 90.3-94.5 | 88.5-95.7 | 96.4-98.4 | 93.4-97.6 | |
| PA | 91.6-96.7 | 93.5-97.0 | 92.2-97.0 | 94.5-98.3 | 95.1-98.0 | |
| PB1 | 93.9-97.3 | 93.9-96.4 | 93.7-98.0 | 96.0-98.3 | 96.0-98.5 | |
| PB2 | 93.5-96.1 | 92.9-96.9 | 87.7-97.2 | 95.0-97.9 | 95.7-98.4 | |
| 蛋白片段 Protein segment | (A)预测的CD4+ T细胞表位 (A)Predicted CD4+ T-cell epitopes | (B)保守表位(%=B/A*100) (B)Conserved predicted epitopes(% = B/A*100) | (C)交叉反应性表位(%=C/B*100) (C)Cross-reactivity epitopes(%= C/B*100) | 已验证的表位(来源:IEDB,免疫表位数据库) Experimentally defined epitopes/% (Source: IEDB, immune epitope database) | ||
|---|---|---|---|---|---|---|
| (D)CD4+ T细胞表位(%=D/C*100) (D)CD4+ T-cell epitopes(%=D/C*100) | (E)CD8+ T细胞表位(%=E/D*100) (E)CD8+ T-cell epitopes(%=E/D*100) | (F)B细胞表位(%=F/E*100) (F)B-cell epitopes(%=F/E*100) | ||||
| HA | 102 | 24 | 7 | 7 | 7 | 2 |
| NA | 56 | 0 | 0 | 0 | 0 | 0 |
| M1 | 77 | 47 | 47 | 40 | 29 | 3 |
| M2 | 8 | 0 | 0 | 0 | 0 | 0 |
| NS1 | 46 | 4 | 4 | 4 | 0 | 0 |
| NEP | 29 | 8 | 6 | 4 | 4 | 0 |
| NP | 118 | 98 | 75 | 57 | 49 | 0 |
| PA | 122 | 104 | 104 | 32 | 21 | 0 |
| PB1 | 158 | 138 | 138 | 63 | 42 | 0 |
| PB2 | 179 | 132 | 132 | 27 | 20 | 0 |
| Total | 895 | 555(62.0) | 513(92.4) | 234(45.6) | 172(73.5) | 5(2.9) |
表3 A/Astrakhan/3212/2020(H5N8)的CD4+ T细胞预测表位
Table 3 Predicted CD4+ T-cell epitopes of A/Astrakhan/3212/2020(H5N8)
| 蛋白片段 Protein segment | (A)预测的CD4+ T细胞表位 (A)Predicted CD4+ T-cell epitopes | (B)保守表位(%=B/A*100) (B)Conserved predicted epitopes(% = B/A*100) | (C)交叉反应性表位(%=C/B*100) (C)Cross-reactivity epitopes(%= C/B*100) | 已验证的表位(来源:IEDB,免疫表位数据库) Experimentally defined epitopes/% (Source: IEDB, immune epitope database) | ||
|---|---|---|---|---|---|---|
| (D)CD4+ T细胞表位(%=D/C*100) (D)CD4+ T-cell epitopes(%=D/C*100) | (E)CD8+ T细胞表位(%=E/D*100) (E)CD8+ T-cell epitopes(%=E/D*100) | (F)B细胞表位(%=F/E*100) (F)B-cell epitopes(%=F/E*100) | ||||
| HA | 102 | 24 | 7 | 7 | 7 | 2 |
| NA | 56 | 0 | 0 | 0 | 0 | 0 |
| M1 | 77 | 47 | 47 | 40 | 29 | 3 |
| M2 | 8 | 0 | 0 | 0 | 0 | 0 |
| NS1 | 46 | 4 | 4 | 4 | 0 | 0 |
| NEP | 29 | 8 | 6 | 4 | 4 | 0 |
| NP | 118 | 98 | 75 | 57 | 49 | 0 |
| PA | 122 | 104 | 104 | 32 | 21 | 0 |
| PB1 | 158 | 138 | 138 | 63 | 42 | 0 |
| PB2 | 179 | 132 | 132 | 27 | 20 | 0 |
| Total | 895 | 555(62.0) | 513(92.4) | 234(45.6) | 172(73.5) | 5(2.9) |
| 蛋白片段 Protein segment | H5Nx预测表位 H5Nx predicted epitopes | 与H5Nx的交叉表位 Cross-epitopes with H5Nx | |||
|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | |||
| HA head | 8 | 0 | 0 | 0 | |
| HA stem | 16 | 7 | 4 | 0 | |
| NA | 0 | 0 | 0 | 0 | |
| M1 | 47 | 41 | 45 | 40 | |
| M2 | 0 | 0 | 0 | 0 | |
| NS1 | 4 | 4 | 4 | 4 | |
| NEP | 8 | 6 | 6 | 6 | |
| NP | 98 | 61 | 66 | 52 | |
| PA | 104 | 95 | 96 | 93 | |
| PB1 | 138 | 129 | 122 | 138 | |
| PB2 | 132 | 120 | 128 | 121 | |
| Total | 555 | 463 | 471 | 454 | |
表4 H5Nx的CD4+ T细胞表位与季节性甲型流感病毒的交叉反应性
Table 4 Cross-reactivity between CD4+ T-cell epitopes of H5Nx and seasonal IAV
| 蛋白片段 Protein segment | H5Nx预测表位 H5Nx predicted epitopes | 与H5Nx的交叉表位 Cross-epitopes with H5Nx | |||
|---|---|---|---|---|---|
| sH1N1 | pH1N1 | H3N2 | |||
| HA head | 8 | 0 | 0 | 0 | |
| HA stem | 16 | 7 | 4 | 0 | |
| NA | 0 | 0 | 0 | 0 | |
| M1 | 47 | 41 | 45 | 40 | |
| M2 | 0 | 0 | 0 | 0 | |
| NS1 | 4 | 4 | 4 | 4 | |
| NEP | 8 | 6 | 6 | 6 | |
| NP | 98 | 61 | 66 | 52 | |
| PA | 104 | 95 | 96 | 93 | |
| PB1 | 138 | 129 | 122 | 138 | |
| PB2 | 132 | 120 | 128 | 121 | |
| Total | 555 | 463 | 471 | 454 | |
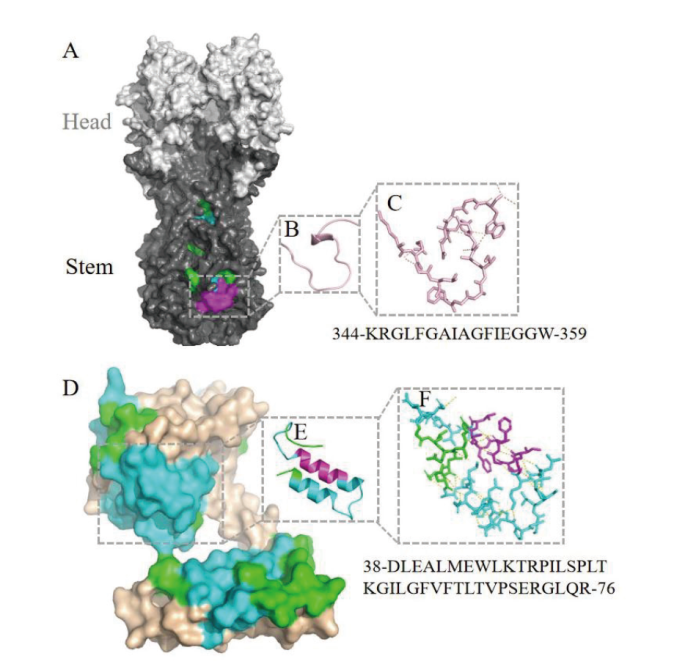
图2 A/Astrakhan/3212/2020(H5N8)HA-A和M1-D嵌套表位的3D模拟 A: HA头部和茎部分别用浅灰色和深灰色标记;绿色标记CD4+ T细胞表位,浅蓝色标记嵌套CD8+ T细胞表位,紫色标记嵌套表位B 细胞表位; D: M蛋白其余氨基酸用棕色标记;B、C和 E、F: 分别是与CD8+ T细胞和 B 细胞表位嵌套的 CD4+ T 细胞表位的卡通和棒表示,虚线代表氢键
Fig. 2 Locating nested epitopes in 3D model of A/Astrakhan/3212/2020(H5N8)HA-A and M1-D A marks the HA head and stem domains in light gray and dark gray respectively; green marks CD4+ T-cell epitopes, light blue marks nested CD8+ T-cell epitopes, and purple marks nested B-cell epitopes; D marks other amino acid residues of M1 in brown; B, C and E, F are respectively cartoon and sticks representation of CD4+ T-cell epitopes nested with both CD8+ T-cell and B-cell epitopes. The dashed lines represent hydrogen bonds

图4 IAV交叉反应性CD4+ T细胞表位的人口覆盖率 A:所有种族群体;B:H5N8 中的8种蛋白质。已确定的交叉反应性 CD4+ T 细胞表位提供了广泛的人群覆盖范围。根据每个 HLA II 类限制 DRB1 等位基因的结合数据,计算理论群体覆盖率。显示了可能的表位-HLA 等位基因组合的数量作为每个种族群体的分数(%)和每个蛋白质(%)的函数
Fig. 4 Population coverage of identified cross-reactivity CD4+ T-cell epitopes A: All ethnicities groups. B: Eight proteins in H5N8. The identified cross-reactivity CD4+ T-cell epitopes provide broad population coverage. Based on the binding data for each HLA class II-restricted DRB1 alleles, theoretical population coverage was calculated. The number of possible epitope-HLA allele combinations as a function of the fraction of each ethnic population(%)and each protein(%)is shown
| [1] | Authority EFS, Adlhoch C, Fusaro A, et al. Avian influenza overview December 2022-March 2023[J]. EFSA J, 2023, 21(3): e07917. |
| [2] | Bruno A, Alfaro-Núñez A, de Mora D, et al. First case of human infection with highly pathogenic H5 avian Influenza A virus in South America: a new zoonotic pandemic threat for 2023?[J]. J Travel Med, 2023, 30(5): taad032. |
| [3] | Shi JZ, Zeng XY, Cui PF, et al. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies[J]. Emerg Microbes Infect, 2023, 12(1): 2155072. |
| [4] | World Health Organization. Influenza(avian and other zoonotic)[EB/OL].[2023-08-21]. https://www.who.int/teams/global-influenza-programme/avian-influenza. |
| [5] |
Zhao KK, Gu M, Zhong L, et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China[J]. Vet Microbiol, 2013, 163(3-4): 351-357.
doi: 10.1016/j.vetmic.2012.12.025 pmid: 23375651 |
| [6] | Pyankova OG, Susloparov IM, Moiseeva AA, et al. Isolation of clade 2.3.4.4b a(H5N8), a highly pathogenic avian influenza virus, from a worker during an outbreak on a poultry farm, Russia, December 2020[J]. Eurosurveillance, 2021, 26(24): 2100439. |
| [7] | Boyden AW, Legge KL, Waldschmidt TJ. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses[J]. PLoS One, 2012, 7(7): e40733. |
| [8] | Moise L, Meyers LM, Jang H, et al. Novel H7N9 influenza immunogen design enhances mobilization of seasonal influenza T cell memory in H3N2 pre-immune mice[J]. Hum Vaccin Immunother, 2022, 18(4): 2082191. |
| [9] |
Strutt TM, McKinstry KK, Marshall NB, et al. Multipronged CD4+ T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus[J]. Immunol Rev, 2013, 255(1): 149-164.
doi: 10.1111/imr.12088 pmid: 23947353 |
| [10] |
Wilkinson TM, Li CKF, Chui CSC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans[J]. Nat Med, 2012, 18(2): 274-280.
doi: 10.1038/nm.2612 pmid: 22286307 |
| [11] | Garcia K, Teyton L, Wilson I. Structural basis of T cell recognition[J]. Annual review of immunology, 1999,17:369-397. |
| [12] |
Quiñones-Parra S, Grant E, Loh L, et al. Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities[J]. Proc Natl Acad Sci USA, 2014, 111(3): 1049-1054.
doi: 10.1073/pnas.1322229111 pmid: 24395804 |
| [13] |
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets[J]. Mol Biol Evol, 2016, 33(7): 1870-1874.
doi: 10.1093/molbev/msw054 pmid: 27004904 |
| [14] | Lin HH, Zhang GL, Tongchusak S, et al. Evaluation of MHC-II peptide binding prediction servers: applications for vaccine research[J]. BMC Bioinformatics, 2008, 9(Suppl 12): S22. |
| [15] | Bui HH, Sidney J, Li W, et al. Development of an epitope conservancy analysis tool to facilitate the design of epitope-based diagnostics and vaccines[J]. BMC Bioinformatics, 2007, 8: 361. |
| [16] |
Duvvuri VR, Duvvuri B, Jamnik V, et al. T cell memory to evolutionarily conserved and shared hemagglutinin epitopes of H1N1 viruses: a pilot scale study[J]. BMC Infect Dis, 2013, 13: 204.
doi: 10.1186/1471-2334-13-204 pmid: 23641949 |
| [17] | Duvvuri VR, Marchand-Austin A, Eshaghi A, et al. Potential T cell epitopes within swine-origin triple reassortant influenza A(H3N2)variant virus which emerged in 2011: an immunoinformatics study[J]. Vaccine, 2012, 30(42): 6054-6063. |
| [18] | Gaevert JA, Luque Duque D, Lythe G, et al. Quantifying T cell cross-reactivity: influenza and coronaviruses[J]. Viruses, 2021, 13(9): 1786. |
| [19] | Fatoba AJ, Maharaj L, Adeleke VT, et al. Immunoinformatics prediction of overlapping CD8+ T-cell, IFN-γ and IL-4 inducer CD4+ T-cell and linear B-cell epitopes based vaccines against COVID-19(SARS-CoV-2)[J]. Vaccine, 2021, 39(7): 1111-1121. |
| [20] | Kalodimou G, Veit S, Jany S, et al. A soluble version of Nipah virus glycoprotein G delivered by vaccinia virus MVA activates specific CD8 and CD4 T cells in mice[J]. Viruses, 2019, 12(1): 26. |
| [21] |
Doytchinova IA, Flower DR. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines[J]. BMC Bioinformatics, 2007, 8: 4.
pmid: 17207271 |
| [22] | Kowal K, Tkaczyk-Wlizło A, Jusiak M, et al. Canis MitoSNP database: a functional tool useful for comparative analyses of human and canine mitochondrial genomes[J]. J Appl Genet, 2023, 64(3): 515-520. |
| [23] |
Oyarzun P, Kobe B. Computer-aided design of T-cell epitope-based vaccines: addressing population coverage[J]. Int J Immunogenet, 2015, 42(5): 313-321.
doi: 10.1111/iji.12214 pmid: 26211755 |
| [24] |
Hertz T, Oshansky CM, Roddam PL, et al. HLA targeting efficiency correlates with human T-cell response magnitude and with mortality from influenza A infection[J]. Proc Natl Acad Sci USA, 2013, 110(33): 13492-13497.
doi: 10.1073/pnas.1221555110 pmid: 23878211 |
| [25] | Cui YF, Li YL, Li MH, et al. Evolution and extensive reassortment of H5 influenza viruses isolated from wild birds in China over the past decade[J]. Emerg Microbes Infect, 2020, 9(1): 1793-1803. |
| [26] |
Peng XM, Liu FM, Wu HB, et al. Amino acid substitutions HA A150V, PA A343T, and PB2 E627K increase the virulence of H5N6 influenza virus in mice[J]. Front Microbiol, 2018, 9: 453.
doi: 10.3389/fmicb.2018.00453 pmid: 29593694 |
| [27] | Sun YP, Hu Z, Zhang XX, et al. An R195K mutation in the PA-X protein increases the virulence and transmission of influenza A virus in mammalian hosts[J]. J Virol, 2020, 94(11): e01817-e01819. |
| [28] | Zhao DM, Liang LB, Wang S, et al. Glycosylation of the hemagglutinin protein of H5N1 influenza virus increases its virulence in mice by exacerbating the host immune response[J]. J Virol, 2017, 91(7): e02215-e02216. |
| [29] | El-Shesheny R, Moatasim Y, Mahmoud SH, et al. Highly pathogenic avian influenza a(H5N1)virus clade 2.3.4.4b in wild birds and live bird markets, Egypt[J]. Pathogens, 2022, 12(1): 36. |
| [30] |
Marchenko V, Goncharova N, Susloparov I, et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia[J]. Virology, 2018, 525: 216-223.
doi: S0042-6822(18)30303-9 pmid: 30296682 |
| [31] |
Auladell M, Jia XX, Hensen LC, et al. Recalling the future: immunological memory toward unpredictable influenza viruses[J]. Front Immunol, 2019, 10: 1400.
doi: 10.3389/fimmu.2019.01400 pmid: 31312199 |
| [32] |
Quiñones-Parra SM, Clemens EB, Wang ZF, et al. A role of influenza virus exposure history in determining pandemic susceptibility and CD8+ T cell responses[J]. J Virol, 2016, 90(15): 6936-6947.
doi: 10.1128/JVI.00349-16 pmid: 27226365 |
| [33] | Zhong WM, Liu F, Dong LB, et al. Significant impact of sequence variations in the nucleoprotein on CD8 T cell-mediated cross-protection against influenza A virus infections[J]. PLoS One, 2010, 5(5): e10583. |
| [34] | Andrews SF, Joyce MG, Chambers MJ, et al. Preferential induction of cross-group influenza A hemagglutinin stem-specific memory B cells after H7N9 immunization in humans[J]. Sci Immunol, 2017, 2(13): eaan2676. |
| [35] | DiPiazza A, Richards K, Poulton N, et al. Avian and human seasonal influenza hemagglutinin proteins elicit CD4 T cell responses that are comparable in epitope abundance and diversity[J]. Clin Vaccine Immunol, 2017, 24(3): e00548-16. |
| [36] | Rattan A, Richards KA, Knowlden ZAG, et al. Protein vaccination directs the CD4+ T cell response toward shared protective epitopes that can be recalled after influenza virus infection[J]. J Virol, 2019, 93(20): e00947-19. |
| [37] | Liu J, Wu B, Zhang SH, et al. Conserved epitopes dominate cross-CD8+ T-cell responses against influenza A H1N1 virus among Asian populations[J]. Eur J Immunol, 2013, 43(8): 2055-2069. |
| [38] | Tan ACL, Deliyannis G, Bharadwaj M, et al. The design and proof of concept for a CD8+ T cell-based vaccine inducing cross-subtype protection against influenza A virus[J]. Immunol Cell Biol, 2013, 91(1): 96-104. |
| [39] |
van de Sandt CE, Kreijtz JHCM, de Mutsert G, et al. Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus[J]. J Virol, 2014, 88(3): 1684-1693.
doi: 10.1128/JVI.02843-13 pmid: 24257602 |
| [40] | van de Sandt CE, Kreijtz JHCM, Geelhoed-Mieras MM, et al. Differential recognition of influenza A viruses by M158-66 epitope-specific CD8+ T cells is determined by extraepitopic amino acid residues[J]. J Virol, 2015, 90(2): 1009-1022. |
| [41] | van de Sandt CE, Pronk MR, van Baalen CA, et al. Variation at extra-epitopic amino acid residues influences suppression of influenza virus replication by M158-66 epitope-specific CD8+ T lymphocytes[J]. J Virol, 2018, 92(11): e00232-18. |
| [42] | Dhakal S, Loube J, Misplon JA, et al. Effect of an adenovirus-vectored universal influenza virus vaccine on pulmonary pathophysiology in a mouse model[J]. J Virol, 2021, 95(9): e02359-20. |
| [43] | Terajima M, Cruz J, Leporati AM, et al. Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients[J]. J Virol, 2008, 82(18): 9283-9287. |
| [44] | van de Sandt CE, Sagong KA, Pronk MR, et al. H1N1pdm09 influenza virus and its descendants lack extra-epitopic amino acid residues associated with reduced recognition by M158-66-specific CD8+ T cells[J]. J Infect Dis, 2018, 218(4): 581-585. |
| [45] |
Lohia N, Baranwal M. Conserved peptides containing overlapping CD4+ and CD8+ T-cell epitopes in the H1N1 influenza virus: an immunoinformatics approach[J]. Viral Immunol, 2014, 27(5): 225-234.
doi: 10.1089/vim.2013.0135 pmid: 24821387 |
| [46] |
Naylor PH, Egan JE, Berinstein NL. Peptide based vaccine approaches for cancer-a novel approach using a WT-1 synthetic long peptide and the IRX-2 immunomodulatory regimen[J]. Cancers, 2011, 3(4): 3991-4009.
doi: 10.3390/cancers3043991 pmid: 24213121 |
| [47] |
Gong X, Yin H, Shi YH, et al. Conserved stem fragment from H3 influenza hemagglutinin elicits cross-clade neutralizing antibodies through stalk-targeted blocking of conformational change during membrane fusion[J]. Immunol Lett, 2016, 172: 11-20.
doi: 10.1016/j.imlet.2016.02.006 pmid: 26875772 |
| [48] |
Claes F, Morzaria SP, Donis RO. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses-how is the asian hpai H5 lineage maintained[J]. Current opinion in virology, 2016, 16, 158-163.
doi: S1879-6257(16)30009-8 pmid: 26991931 |
| [49] | Lee DH, Bertran K, Kwon JH, et al. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4[J]. Journal of veterinary science, 2017, 18, 269-280. |
| [50] | Chen P, Xie JF, Lin Q et al. A study of the relationship between human infection with avian influenza a(H5N6)and environmental avian influenza viruses in Fujian, China[J]. BMC infectious diseases, 2019, 19, 762. |
| [51] | Li YT, Su YCF, Smith GJD. H5Nx viruses emerged during the suppression of H5N1 virus populations in poultry[J]. Microbiol Spectr, 2021, 9(2): e0130921. |
| [1] | 王颖. 发现胰岛素与GAD之间的分子联系[J]. , 1996, 0(02): 19-20. |
| [2] | . 淋巴因子和干扰素[J]. , 1989, 0(12): 102-103. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||