








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (6): 68-80.doi: 10.13560/j.cnki.biotech.bull.1985.2023-1196
Previous Articles Next Articles
CAI Nan1( ), FANG Jing-ping1, CHEN Bi-lian1,2, HE Yong-jin1,2(
), FANG Jing-ping1, CHEN Bi-lian1,2, HE Yong-jin1,2( )
)
Received:2023-12-19
Online:2024-06-26
Published:2024-05-14
Contact:
HE Yong-jin
E-mail:cainan327@163.com;yongjinhe@fjnu.edu.cn
CAI Nan, FANG Jing-ping, CHEN Bi-lian, HE Yong-jin. Research Progress in Carbon Sequestration by High-valued Isochrysis Strain[J]. Biotechnology Bulletin, 2024, 40(6): 68-80.
| 藻株 Microalgae strain | 生物量产量 Biomass yield/(g·L-1·d-1) | CO2来源 CO2 source | CO2固定效率 Fixed efficiency/(gCO2·L-1·d-1) | 参考文献 Reference |
|---|---|---|---|---|
| 四爿藻 Tetraselmis sp. | 0.42 | 燃煤电厂;CO2(10%-15%, V/V) | 0.19 | [ |
| 微拟球藻 Nannochloropsis sp. | 0.27 | 燃煤电厂:CO2(13%, V/V) | 0.51 | [ |
| 小球藻Chlorella vulgaris | 0.28 | 钢铁厂;CO2(10%-15%, V/V) | 0.45 | [ |
| 螺旋藻Spirulina | 0.22 | 煤化工烟气:CO2(10%, V/V) | 0.32 | [ |
| 杜氏盐藻 NIES-2257 Dunaliella salina NIES-2257 | 0.23 | 5% CO2 | 0.11 | [ |
| 等鞭金藻 Isochrysis sp. | 0.32 | 燃煤电厂;CO2(10%-15%, V/V) | 0.61 | [ |
Table 1 Microalgae carbon-fixing efficiency
| 藻株 Microalgae strain | 生物量产量 Biomass yield/(g·L-1·d-1) | CO2来源 CO2 source | CO2固定效率 Fixed efficiency/(gCO2·L-1·d-1) | 参考文献 Reference |
|---|---|---|---|---|
| 四爿藻 Tetraselmis sp. | 0.42 | 燃煤电厂;CO2(10%-15%, V/V) | 0.19 | [ |
| 微拟球藻 Nannochloropsis sp. | 0.27 | 燃煤电厂:CO2(13%, V/V) | 0.51 | [ |
| 小球藻Chlorella vulgaris | 0.28 | 钢铁厂;CO2(10%-15%, V/V) | 0.45 | [ |
| 螺旋藻Spirulina | 0.22 | 煤化工烟气:CO2(10%, V/V) | 0.32 | [ |
| 杜氏盐藻 NIES-2257 Dunaliella salina NIES-2257 | 0.23 | 5% CO2 | 0.11 | [ |
| 等鞭金藻 Isochrysis sp. | 0.32 | 燃煤电厂;CO2(10%-15%, V/V) | 0.61 | [ |
| CO2捕获技术 CO2 capture technology | 优点 Advantage | 缺点 Disadvantage | 参考文献 Reference |
|---|---|---|---|
| 地质封存 Geological sequestration | 1. CO2性质稳定,封存长; 2. CO2注入油田或气田可提高采收率 | 1. 地质运动会导致CO2泄露,形成毁灭性的窒息区域,引发土壤、大气环境变化; 2. 地质封层需要改造蒸汽储存、运输、在线地震勘探设备,机械造价和运行成本高; 3. 目前我国CO2地质封存技术仍处于示范阶段,亟待深入研究 | [ |
| 物理化学吸附 Physicochemical adsorption | 1. CO2封存安全、稳定; 2. CO2封存的同时还可将浓缩的CO2用于生产水泥、生物塑料等产品,经济效益高; 3. 固碳工艺相对成熟 | 1. 吸附剂材料消耗大,成本高昂; 2. 反应器运行成本高; 3. 许多化学试剂有毒且挥发性强,对操作要求高且易造成环境问题 | [ |
| 植物固碳 Carbon sequestration in plants | 1. 固碳效率高; 2. 无需使用化学品,固碳过程安全、无毒; 3. 固碳成本低 | 1. 耗时长、占用土地面积大; 2. 固碳效果受外部条件影响大 | [ |
| 微藻生物固碳 Microalgae biocarbon sequestration | 1. 固碳效率高,是陆生植物的10-50倍; 2. 微藻生长速度快,易于培养; 3. 微藻对极端环境耐受性高,适用于多种环境下培养; 4. 微藻能在固碳同时合成生物质,并应用于能源、食品和饲料等行业 | 1. 微藻生物固碳起步较晚,某些工艺/技术仍处于起步阶段; 2. 微藻培养需安装光生物反应器,耗费高; 3. 微藻固碳目前市场规模小,还有广大待挖掘区域 | [ |
Table 2 CO2 capture technology and its advantages and disadvantages
| CO2捕获技术 CO2 capture technology | 优点 Advantage | 缺点 Disadvantage | 参考文献 Reference |
|---|---|---|---|
| 地质封存 Geological sequestration | 1. CO2性质稳定,封存长; 2. CO2注入油田或气田可提高采收率 | 1. 地质运动会导致CO2泄露,形成毁灭性的窒息区域,引发土壤、大气环境变化; 2. 地质封层需要改造蒸汽储存、运输、在线地震勘探设备,机械造价和运行成本高; 3. 目前我国CO2地质封存技术仍处于示范阶段,亟待深入研究 | [ |
| 物理化学吸附 Physicochemical adsorption | 1. CO2封存安全、稳定; 2. CO2封存的同时还可将浓缩的CO2用于生产水泥、生物塑料等产品,经济效益高; 3. 固碳工艺相对成熟 | 1. 吸附剂材料消耗大,成本高昂; 2. 反应器运行成本高; 3. 许多化学试剂有毒且挥发性强,对操作要求高且易造成环境问题 | [ |
| 植物固碳 Carbon sequestration in plants | 1. 固碳效率高; 2. 无需使用化学品,固碳过程安全、无毒; 3. 固碳成本低 | 1. 耗时长、占用土地面积大; 2. 固碳效果受外部条件影响大 | [ |
| 微藻生物固碳 Microalgae biocarbon sequestration | 1. 固碳效率高,是陆生植物的10-50倍; 2. 微藻生长速度快,易于培养; 3. 微藻对极端环境耐受性高,适用于多种环境下培养; 4. 微藻能在固碳同时合成生物质,并应用于能源、食品和饲料等行业 | 1. 微藻生物固碳起步较晚,某些工艺/技术仍处于起步阶段; 2. 微藻培养需安装光生物反应器,耗费高; 3. 微藻固碳目前市场规模小,还有广大待挖掘区域 | [ |
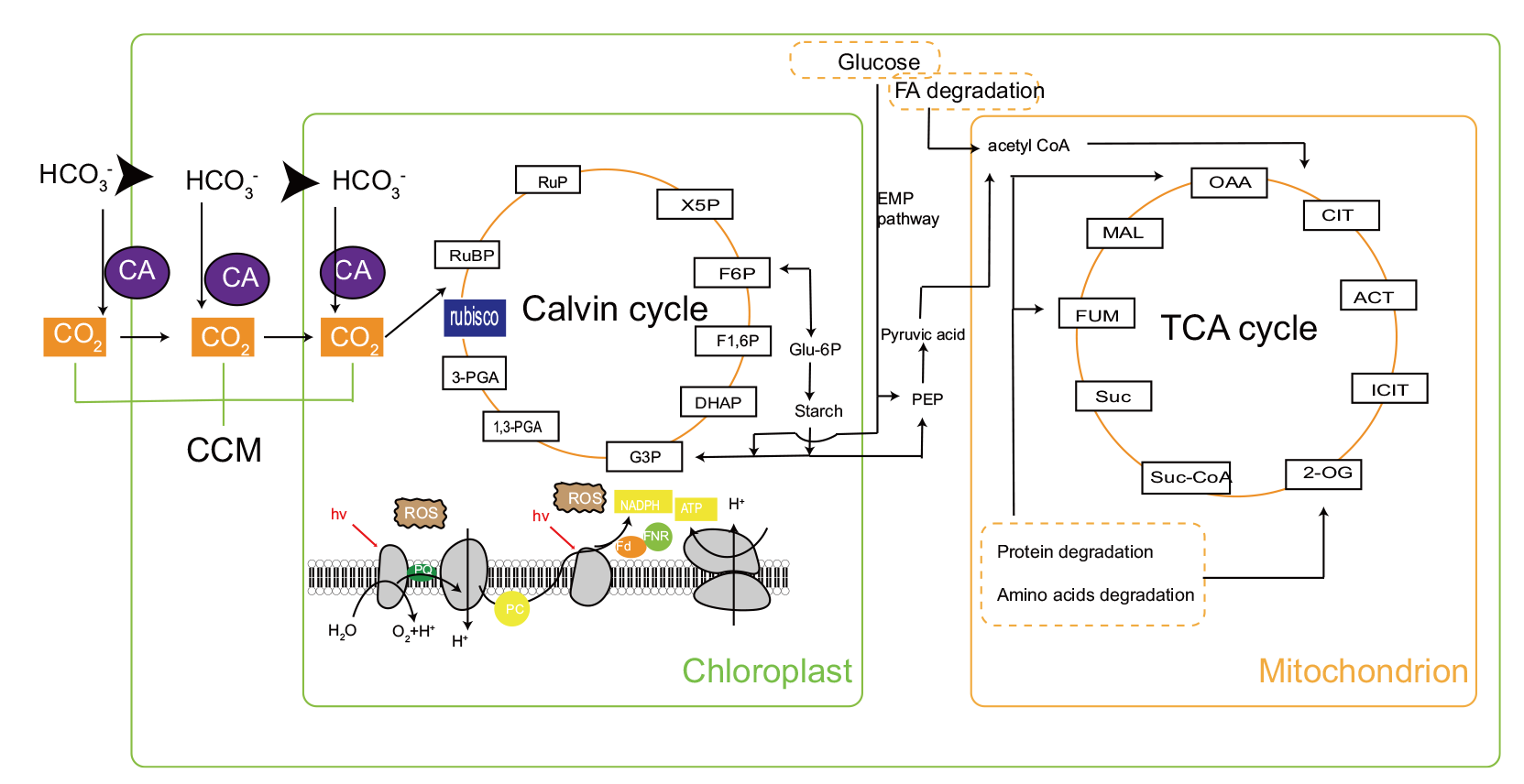
Fig. 2 Carbon flux metabolic pathway in Isochrysis CA: Carbonic anhydrase. CCM: CO2-concentrating mechanism. RuBP: Ribulose bisphosphate. Rubisco: Ribulose bisphosphate carboxylase. 3-PGA: 3-phosphoglycerate. 1,3-PGA: 1,3-disphosphoglycerate. G3P: Glyceraldehyde 3-phosphate. DHAP: Dihydroxyacetone phosphate. F-1,6P: Fructose-1,6bisphoshate. F-6P: Fructose6-phosphate. X5P: Xylulose 5-phosphate. RuP: Ribulose 5-phosphate. Glu-6P: Glucose-6-phosphate. ROS: Reactive oxygen species. NADPH: Nicotinamide adenine dinucleotide phosphate. FNR: Ferredoxin-NADP+-oxidoreductase. PQ: Plastoquinone. PC: Plastocyanin. ATP: Adenosine triphosphate. Fd: Ferredoxin. OAA: Oxaloacetic acid. MAL: Malic acid. FUM: Fumaric acid. Suc: Succinic acid. Suc-CoA: Succinyl-CoA-synthetase. 2-OG: α-ketoglutaric acid. ICIT: Isocitricacid. ACT: Cis-aconitic acid. CIT: Citric acid. FA: Fatty acid. Acetyl-CoA: Acetyl-coenzyme A
| 藻株 Microalgae strain | n-3 多不饱和脂肪酸 n-3 polyunsaturated acids/(mg·g-1 oil) | |||||
|---|---|---|---|---|---|---|
| ALA(C18:3n-3) | SDA(C18:4n-3) | EPA(C20:5n-3) | DPA(C22:5n-3) | DHA(C22:6n-3) | ||
| 等鞭金藻Isochrysis T-Iso | 29.00 ± 4.00 | 43.00 ± 10.00 | 2.80 ± 0.70 | - | 46.00 ± 14.00 | |
| 微拟球藻Nannochloropsis oculata | 0.30 ± 0.03 | 0.30 ± 0.10 | 175.00 ± 12.00 | - | - | |
| 巴夫藻Pavlova Lutheri | 10.00 ± 0.30 | 17.00 ± 0.50 | 92.00 ± 2.00 | - | 40.90 ± 0.90 | |
| 硅藻Thalassiosira Preudonana | 1.90 ± 0.10 | 20.40 ± 0.80 | 81.00 ± 2.00 | 1.82 ± 0.01 | 20.90 ± 0.80 | |
| 鱼油Fish oil | 7.70 ± 0.20 | 29.00 ± 1.00 | 184.00 ± 5.00 | 16.80 ± 0.30 | 105.20 ± 0.70 | |
Table 3 n-3 polyunsaturated fatty acid content in several microalgae and fish oil
| 藻株 Microalgae strain | n-3 多不饱和脂肪酸 n-3 polyunsaturated acids/(mg·g-1 oil) | |||||
|---|---|---|---|---|---|---|
| ALA(C18:3n-3) | SDA(C18:4n-3) | EPA(C20:5n-3) | DPA(C22:5n-3) | DHA(C22:6n-3) | ||
| 等鞭金藻Isochrysis T-Iso | 29.00 ± 4.00 | 43.00 ± 10.00 | 2.80 ± 0.70 | - | 46.00 ± 14.00 | |
| 微拟球藻Nannochloropsis oculata | 0.30 ± 0.03 | 0.30 ± 0.10 | 175.00 ± 12.00 | - | - | |
| 巴夫藻Pavlova Lutheri | 10.00 ± 0.30 | 17.00 ± 0.50 | 92.00 ± 2.00 | - | 40.90 ± 0.90 | |
| 硅藻Thalassiosira Preudonana | 1.90 ± 0.10 | 20.40 ± 0.80 | 81.00 ± 2.00 | 1.82 ± 0.01 | 20.90 ± 0.80 | |
| 鱼油Fish oil | 7.70 ± 0.20 | 29.00 ± 1.00 | 184.00 ± 5.00 | 16.80 ± 0.30 | 105.20 ± 0.70 | |
| [1] | 潘家华, 陈梦玫, 刘保留. “双碳” 战略助推中国落实联合国可持续发展目标的作用机理[J]. 阅江学刊, 2024, 16(1): 60-70, 172-173. |
| Pan JH, Chen MM, Liu BL. The mechanisms of facilitating China's implementation of the united nations sustainable development goals through the “dual carbon” strategy[J]. Yuejiang Acad J, 2024, 16(1): 60-70, 172-173. | |
| [2] | Tripathi S, Choudhary S, Meena A, et al. Carbon capture, storage, and usage with microalgae: a review[J]. Environ Chem Lett, 2023, 21(4): 2085-2128. |
| [3] | 康佳宁, 张云龙, 彭凇, 等. 实现碳中和目标的CCUS产业发展展望[J]. 北京理工大学学报:社会科学版, 2024, 26(2):68-75. |
| Kang JN, Zhang YL, Peng S, et al. Prospect of CCUS industry development to achieve carbon neutrality[J]. J Beijing Inst Technol Soc Sci Ed, 2024, 26(2):68-75. | |
| [4] | Li P, Pan SY, Pei SL, et al. Challenges and perspectives on carbon fixation and utilization technologies: an overview[J]. Aerosol Air Qual Res, 2016, 16(6): 1327-1344. |
| [5] | 莫壮洪, 朱俊英, 荣峻峰, 等. 微藻生物固碳技术在碳中和中的应用及潜在价值[J]. 石油炼制与化工, 2024, 55(1): 98-111, 4. |
| Mo ZH, Zhu JY, Rong JF, et al. Application and potential value of microalgae bio-carbon fixation technology in carbon neutrality[J]. Petrol Process Petrochem, 2024, 55(1): 98-111, 4. | |
| [6] |
崔金玉, 张爱娣, 栾国栋, 等. 微藻光驱固碳合成技术的发展现状与未来展望[J]. 合成生物学, 2022, 3(5): 884-900.
doi: 10.12211/2096-8280.2022-005 |
| Cui JY, Zhang AD, Luan GD, et al. Engineering microalgae for photosynthetic biosynthesis: progress and prospect[J]. Synth Biol J, 2022, 3(5): 884-900. | |
| [7] | Xu XZ, Gu XG, Wang ZY, et al. Progress, challenges and solutions of research on photosynthetic carbon sequestration efficiency of microalgae[J]. Renew Sustain Energy Rev, 2019, 110: 65-82. |
| [8] |
Matos J, Cardoso C, Gomes A, et al. Bioprospection of Isochrysis galbana and its potential as a nutraceutical[J]. Food Funct, 2019, 10(11): 7333-7342.
doi: 10.1039/c9fo01364d pmid: 31646314 |
| [9] | Yahya L, Harun R, Abdullah LC. Screening of native microalgae species for carbon fixation at the vicinity of Malaysian coal-fired power plant[J]. Sci Rep, 2020, 10(1): 22355. |
| [10] | Zheng MM, Ji XW, He YJ, et al. Simultaneous fixation of carbon dioxide and purification of undiluted swine slurry by culturing Chlorella vulgaris MBFJNU-1[J]. Algal Res, 2020, 47: 101866. |
| [11] | Fan JH, Xu H, Luo YC, et al. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella[J]. Appl Microbiol Biotechnol, 2015, 99(5): 2451-2462. |
| [12] | Sydney EB, Sturm W, de Carvalho JC, et al. Potential carbon dioxide fixation by industrially important microalgae[J]. Bioresour Technol, 2010, 101(15): 5892-5896. |
| [13] | de Morais MG, Costa JAV. Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor[J]. J Biotechnol, 2007, 129(3): 439-445. |
| [14] | Kishi M, Toda T. Carbon fixation properties of three alkalihalophilic microalgal strains under high alkalinity[J]. J Appl Phycol, 2018, 30(1): 401-410. |
| [15] |
Kumar A, Ergas S, Yuan X, et al. Enhanced CO2 fixation and biofuel production via microalgae: recent developments and future directions[J]. Trends Biotechnol, 2010, 28(7): 371-380.
doi: 10.1016/j.tibtech.2010.04.004 pmid: 20541270 |
| [16] | Nguyen LN, Vu MT, Vu HP, et al. Microalgae-based carbon capture and utilization: a critical review on current system developments and biomass utilization[J]. Crit Rev Environ Sci Technol, 2023, 53(2): 216-238. |
| [17] | Zhao BT, Su YX, Zhang YX, et al. Carbon dioxide fixation and biomass production from combustion flue gas using energy microalgae[J]. Energy, 2015, 89: 347-357. |
| [18] | Realmonte G, Drouet L, Gambhir A, et al. An inter-model assessment of the role of direct air capture in deep mitigation pathways[J]. Nat Commun, 2019, 10(1): 3277. |
| [19] | Wang XX, Song CS. Carbon capture from flue gas and the atmosphere: a perspective[J]. Front Energy Res, 2020, 8: 560849. |
| [20] | Ali M, Jha NK, Pal N, et al. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook[J]. Earth Sci Rev, 2022, 225: 103895. |
| [21] | Bui M, Adjiman CS, Bardow A, et al. Carbon capture and storage(CCS): the way forward[J]. Energy Environ Sci, 2018, 11(5): 1062-1176. |
| [22] | Varshney P, Mikulic P, Vonshak A, et al. Extremophilic micro-algae and their potential contribution in biotechnology[J]. Bioresour Technol, 2015, 184: 363-372. |
| [23] |
Spalding MH. Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters[J]. J Exp Bot, 2008, 59(7): 1463-1473.
pmid: 17597098 |
| [24] | Sayre R. Microalgae: the potential for carbon capture[J]. BioScience, 2010, 60(9): 722-727. |
| [25] | Li SN, Li X, Ho SH. How to enhance carbon capture by evolution of microalgal photosynthesis?[J]. Sep Purif Technol, 2022, 291: 120951. |
| [26] | 程亚田, 汤皓, 孙丽丽, 等. 植物源二萜类化合物微生物合成研究进展[J]. 生物工程学报, 2023, 39(6): 2265-2283. |
| Cheng YT, Tang H, Sun LL, et al. Advances on the microbial synthesis of plant-derived diterpenoids[J]. Chin J Biotechnol, 2023, 39(6): 2265-2283. | |
| [27] | Li YL, Sun H, Wang YN, et al. Integrated metabolic tools reveal carbon alternative in Isochrysis zhangjiangensis for fucoxanthin improvement[J]. Bioresour Technol, 2022, 347: 126401. |
| [28] | Kapoore RV, Padmaperuma G, Maneein S, et al. Co-culturing microbial consortia: approaches for applications in biomanufacturing and bioprocessing[J]. Crit Rev Biotechnol, 2022, 42(1): 46-72. |
| [29] | Kao CY, Chen TY, Chang YB, et al. Utilization of carbon dioxide in industrial flue gases for the cultivation of microalga Chlorella sp[J]. Bioresour Technol, 2014, 166: 485-493. |
| [30] | Zhou YC, He YJ, Guo X, et al. Pilot-scale remediation of rare earth elements ammonium wastewater by Chlamydomonas sp. YC in summer under outdoor conditions[J]. Bioresour Technol, 2023, 372: 128674. |
| [31] | Zheng MM, Dai JX, Ji XW, et al. An integrated semi-continuous culture to treat original swine wastewater and fix carbon dioxide by an indigenous Chlorella vulgaris MBFJNU-1 in an outdoor photobioreactor[J]. Bioresour Technol, 2021, 340: 125703. |
| [32] | Xia YJ, Sekine M, Hirahara M, et al. Effects of concentration and frequency of CO2 supply on productivity of marine microalga Isochrysis galbana[J]. Algal Res, 2023, 70: 102985. |
| [33] | Liu JY, Song YM, Qiu W. Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis[J]. Renew Sustain Energy Rev, 2017, 72: 154-162. |
| [34] | Baer S, Heining M, Schwerna P, et al. Optimization of spectral light quality for growth and product formation in different microalgae using a continuous photobioreactor[J]. Algal Res, 2016, 14: 109-115. |
| [35] | Ramanna L, Rawat I, Bux F. Light enhancement strategies improve microalgal biomass productivity[J]. Renew Sustain Energy Rev, 2017, 80: 765-773. |
| [36] | Michael C, Del Ninno M, Gross M, et al. Use of wavelength-selective optical light filters for enhanced microalgal growth in different algal cultivation systems[J]. Bioresour Technol, 2015, 179: 473-482. |
| [37] | Seo YH, Cho C, Lee JY, et al. Enhancement of growth and lipid production from microalgae using fluorescent paint under the solar radiation[J]. Bioresour Technol, 2014, 173: 193-197. |
| [38] |
Zehentbauer FM, Moretto C, Stephen R, et al. Fluorescence spectroscopy of Rhodamine 6G: concentration and solvent effects[J]. Spectrochim Acta A Mol Biomol Spectrosc, 2014, 121: 147-151.
doi: 10.1016/j.saa.2013.10.062 pmid: 24239710 |
| [39] | 方静平, 陈钦常, 黄鹭强. 岩藻黄素生物合成途径及其对光照响应研究进展[J]. 福建师范大学学报: 自然科学版, 2021, 37(5): 96-108. |
| Fang JP, Chen QC, Huang LQ. A review of biosynthesis pathway of fucoxanthin and fucoxanthin production in response to light[J]. J Fujian Norm Univ Nat Sci Ed, 2021, 37(5): 96-108. | |
| [40] | Wang SK, Stiles AR, Guo C, et al. Microalgae cultivation in photobioreactors: an overview of light characteristics[J]. Eng Life Sci, 2014, 14(6): 550-559. |
| [41] | Xu PL, Li J, Qian J, et al. Recent advances in CO2 fixation by microalgae and its potential contribution to carbon neutrality[J]. Chemosphere, 2023, 319: 137987. |
| [42] | Guardini Z, Dall’Osto L, Barera S, et al. High carotenoid mutants of Chlorella vulgaris show enhanced biomass yield under high irradiance[J]. Plants, 2021, 10(5): 911. |
| [43] | Sirohi R, Kumar Pandey A, Ranganathan P, et al. Design and applications of photobioreactors- a review[J]. Bioresour Technol, 2022, 349: 126858. |
| [44] |
Pulz O. Photobioreactors: production systems for phototrophic microorganisms[J]. Appl Microbiol Biotechnol, 2001, 57(3): 287-293.
doi: 10.1007/s002530100702 pmid: 11759675 |
| [45] | Thomas DM, Mechery J, Paulose SV. Carbon dioxide capture strategies from flue gas using microalgae: a review[J]. Environ Sci Pollut Res Int, 2016, 23(17): 16926-16940. |
| [46] | Wang LL, Zhao RQ, Wang Q, et al. Novel bioreactor with inclined baffles in cost-efficiently increasing algal biomass and carbon fixation[J]. Energy, 2022, 247: 123453. |
| [47] | Ye Q, Cheng J, Guo WB, et al. Serial lantern-shaped draft tube enhanced flashing light effect for improving CO2 fixation with microalgae in a gas-lift circumflux column photobioreactor[J]. Bioresour Technol, 2018, 255: 156-162. |
| [48] | Ali Kubar A, Cheng J, Kumar S, et al. Developing a Zigzag-baffled column photobioreactor to increase mass-transfer, CO2 fixation and biomass yield during A. platensis cultivation[J]. J CO2 Util, 2022, 63: 102126. |
| [49] |
Genkov T, Meyer M, Griffiths H, et al. Functional hybrid rubisco enzymes with plant small subunits and algal large subunits: engineered rbcS cDNA for expression in chlamydomonas[J]. J Biol Chem, 2010, 285(26): 19833-19841.
doi: 10.1074/jbc.M110.124230 pmid: 20424165 |
| [50] | Mueller-Cajar O, Stotz M, Wendler P, et al. Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase[J]. Nature, 2011, 479(7372): 194-199. |
| [51] | Wei L, Wang QT, Xin Y, et al. Enhancing photosynthetic biomass productivity of industrial oleaginous microalgae by overexpression of RuBisCO activase[J]. Algal Res, 2017, 27: 366-375. |
| [52] | Xie D, Ji XW, Zhou YC, et al. Chlorella vulgaris cultivation in pilot-scale to treat real swine wastewater and mitigate carbon dioxide for sustainable biodiesel production by direct enzymatic transesterification[J]. Bioresour Technol, 2022, 349: 126886. |
| [53] |
Nomura T, Kikuchi M, Kubodera A, et al. Proton-donative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl(DPPH)[J]. Biochem Mol Biol Int, 1997, 42(2): 361-370.
pmid: 9238535 |
| [54] | Sabia A, Clavero E, Pancaldi S, et al. Effect of different CO2 concentrations on biomass, pigment content, and lipid production of the marine diatom Thalassiosira pseudonana[J]. Appl Microbiol Biotechnol, 2018, 102(4): 1945-1954. |
| [55] | Li JW, Zhao XQ, Chang JS, et al. A two-stage culture strategy for Scenedesmus sp. FSP3 for CO2 fixation and the simultaneous production of lutein under light and salt stress[J]. Molecules, 2022, 27(21): 7497. |
| [56] | Lin JY, Effendi SSW, Ng IS. Enhanced carbon capture and utilization(CCU)using heterologous carbonic anhydrase in Chlamydomonas reinhardtii for lutein and lipid production[J]. Bioresour Technol, 2022, 351: 127009. |
| [57] | Katiyar R, Arora A. Health promoting functional lipids from microalgae pool: a review[J]. Algal Res, 2020, 46: 101800. |
| [58] | Bonfanti C, Cardoso C, Afonso C, et al. Potential of microalga Isochrysis galbana: bioactivity and bioaccessibility[J]. Algal Res, 2018, 29: 242-248. |
| [59] | Balakrishnan J, Dhavamani S, Sadasivam SG, et al. Omega-3-rich Isochrysis sp. biomass enhances brain docosahexaenoic acid levels and improves serum lipid profile and antioxidant status in Wistar rats[J]. J Sci Food Agric, 2019, 99(13): 6066-6075. |
| [60] |
Ryckebosch E, Bruneel C, Termote-Verhalle R, et al. Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil[J]. Food Chem, 2014, 160: 393-400.
doi: 10.1016/j.foodchem.2014.03.087 pmid: 24799253 |
| [61] | He YJ, Wu T, Sun H, et al. Comparison of fatty acid composition and positional distribution of microalgae triacylglycerols for human milk fat substitutes[J]. Algal Res, 2019, 37: 40-50. |
| [62] | He YJ, Lin G, Rao XZ, et al. Microalga Isochrysis galbana in feed for Trachinotus ovatus: effect on growth performance and fatty acid composition of fish fillet and liver[J]. Aquac Int, 2018, 26(5): 1261-1280. |
| [63] | 农业农村部. 中华人民共和国农业农村部公告第692号[J]. 广东饲料, 2023, 32(7): 5-6. |
| Ministry of Agriculture and Rural Affairs. Announcement No.692 of the ministry of agriculture and rural affairs of the People's republic of China[J]. Guangdong Feed, 2023, 32(7): 5-6. | |
| [64] | 晁红娟, 雷占兰, 刘爱琴, 等. Omega-3多不饱和脂肪酸性质、功能及主要应用[J]. 中国食品添加剂, 2019, 30(10): 122-130. |
| Chao HJ, Lei ZL, Liu AQ, et al. Properties, functions and main applications of Omega-3 polyunsaturated fatty acids[J]. China Food Addit, 2019, 30(10): 122-130. | |
| [65] | Huang AY, Wu SC, Gu WH, et al. Provision of carbon skeleton for lipid synthesis from the breakdown of intracellular protein and soluble sugar in Phaeodactylum tricornutum under high CO2[J]. BMC Biotechnol, 2019, 19(1): 53. |
| [66] | Yun HS, Ji MK, Park YT, et al. Microalga, Acutodesmus obliquus KGE 30 as a potential candidate for CO2 mitigation and biodiesel production[J]. Environ Sci Pollut Res Int, 2016, 23(17): 17831-17839. |
| [67] | Kumar K, Banerjee D, Das D. Carbon dioxide sequestration from industrial flue gas by Chlorella sorokiniana[J]. Bioresour Technol, 2014, 152: 225-233. |
| [68] | Gao FZ, Sá M, Cabanelas ITD, et al. Improved fucoxanthin and docosahexaenoic acid productivities of a sorted self-settling Tisochrysis lutea phenotype at pilot scale[J]. Bioresour Technol, 2021, 325: 124725. |
| [69] | Li DG, Chen N, Zhou ZH, et al. Isochrysis sp. cultivation in pilot-scale to concurrently produce sustainable triacylglycerols for human milk fat substitutes and fucoxanthin[J]. Algal Res, 2023, 69: 102937. |
| [70] | Zhang PY, Sun Q, Dong Y, et al. Effects of different bicarbonate on spirulina in CO2 absorption and microalgae conversion hybrid system[J]. Front Bioeng Biotechnol, 2023, 10: 1119111. |
| [71] | Liu Y, Wei D, Chen WN. Oleaginous microalga Coccomyxa subellipsoidea as a highly effective cell factory for CO2 fixation and high-protein biomass production by optimal supply of inorganic carbon and nitrogen[J]. Front Bioeng Biotechnol, 2022, 10: 921024. |
| [72] | 赵震宇, 姚舜, 杨朔鹏, 等. “双碳” 目标下:中国CCUS发展现状、存在问题及建议[J]. 环境科学, 2023, 44(2): 1128-1138. |
| Zhao ZY, Yao S, Yang SP, et al. Under goals of carbon peaking and carbon neutrality: status, problems, and suggestions of CCUS in China[J]. Environ Sci, 2023, 44(2): 1128-1138. |
| [1] | WANG Lu, LIU Meng-yu, ZHANG Fu-yuan, JI Shou-kun, WANG Yun, ZHANG Ying-jie, DUAN Chun-hui, LIU Yue-qin, YAN Hui. Isolation and Identification of Rumen Skatole-degrading Bacteria and Analysis on Their Degradation Characteristics [J]. Biotechnology Bulletin, 2024, 40(3): 305-311. |
| [2] | CHEN Zhong-yuan, WANG Yu-hong, DAI Wei-jun, ZHANG Yan-min, YE Qian, LIU Xu-ping, TAN Wen-Song, ZHAO Liang. Mechanism Investigation of Ferric Ammonium Citrate on Transfection for Suspended HEK293 Cells [J]. Biotechnology Bulletin, 2023, 39(9): 311-318. |
| [3] | DING Kai-xin, WANG Li-chun, TIAN Guo-kui, WANG Hai-yan, LI Feng-yun, PAN Yang, PANG Ze, SHAN Ying. Research Progress in Uniconazole Alleviating Plant Drought Damage [J]. Biotechnology Bulletin, 2023, 39(6): 1-11. |
| [4] | ZHANG Long-xi, LYU Lin, ZHANG Huan-huan, ZHOU Jin-cheng, CHE Wu-nan, DONG Hui. Research Progress in the Application of RNAi Technology in Parasitoid Wasps [J]. Biotechnology Bulletin, 2023, 39(12): 99-108. |
| [5] | LI Xin-yue, ZHOU Ming-hai, FAN Ya-chao, LIAO Sha, ZHANG Feng-li, LIU Chen-guang, SUN Yue, ZHANG Lin, ZHAO Xin-qing. Research Progress in the Improvement of Microbial Strain Tolerance and Efficiency of Biological Manufacturing Based on Transporter Engineering [J]. Biotechnology Bulletin, 2023, 39(11): 123-136. |
| [6] | SHAN Qi, JIA Hui-shu, YAO Wen-bo, LIU Wei-can, LI Hai-yan. Research Progress in Biological Functions of miR396-GRF Module in Plants and Its Potential Application Values [J]. Biotechnology Bulletin, 2022, 38(10): 34-44. |
| [7] | LIU Hai-guang, LUO Zhen, DONG He-zhong. Research Progress on the Regulation of NO3- Uptake and Transport in Plant [J]. Biotechnology Bulletin, 2021, 37(6): 192-201. |
| [8] | HAO Jun-yao, MA Fu-qiang, YANG Guang-yu. Functional Analysis of Key Residues in the Active Center of Creatinase from Alcaligenes sp. KS-85 [J]. Biotechnology Bulletin, 2021, 37(3): 75-83. |
| [9] | LU Yu-fang, SHI Wei-ming. Rhizospheric Chemical Signals and Soil Nutrient Transformation [J]. Biotechnology Bulletin, 2020, 36(9): 14-24. |
| [10] | WANG Xiao-fang, HOU Yu-gang, YANG Ke-ming, WANG Jia-ning, WEI Zhong, XU Yang-chun, SHEN Qi-rong. Isolation of Specific Phage of Ralstonia solanacearum and Its Effects on Control of Soil-borne Bacterial Wilt Disease [J]. Biotechnology Bulletin, 2020, 36(9): 194-201. |
| [11] | XU Mei-hui, ZHANG Yao-jie, TANG Ke-xuan, MIAO Zhi-qi. Optimization of GoldenBraid Digestion-ligation Reaction System [J]. Biotechnology Bulletin, 2020, 36(9): 266-274. |
| [12] | HAN Mei, YUAN Chao, GUO Ting-ting, WU Yi, YUE Yao-jing, YANG Bo-hui. Research Progress on Handmade Cloning in Mammalian [J]. Biotechnology Bulletin, 2020, 36(3): 54-61. |
| [13] | WU Jia-jin, ZHU Sen-lin, ZHOU Mi, SUN Hui-zeng. Research Progress and Trends on Rumen Microbiota in Dairy Cows [J]. Biotechnology Bulletin, 2020, 36(2): 27-38. |
| [14] | QIU Jin, HUANG Huo-qing, YAO Bin, LUO Hui-ying. Improvement of Catalytic Activity of Amylase from Bacillus amyloliquefaciens and Its High Expression in Bacillus subtilis [J]. Biotechnology Bulletin, 2019, 35(9): 134-143. |
| [15] | DENG Xiao-fen, YANG Xiao-jia, YI Tian-hong, FENG Ying, KE Xiao, LAI Wei-li. Optimization of Electrotransfection Conditions of Genes for Fusion Protein and Antibody to CHO-S Cells [J]. Biotechnology Bulletin, 2019, 35(4): 223-228. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||