








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 322-331.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0751
史京辉( ), 陈文慧, 陆坤, 郑婷婷, 任志远, 鲍国庆, 王敏, 骆健美(
), 陈文慧, 陆坤, 郑婷婷, 任志远, 鲍国庆, 王敏, 骆健美( )
)
收稿日期:2023-08-07
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
骆健美,女,博士,教授,研究方向:工业微生物改造;E-mail: luojianmei@tust.edu.cn作者简介:史京辉,男,研究方向:甾体羟化酶的改造;E-mail: sjh18636348386@163.com
基金资助:
SHI Jing-hui( ), CHEN Wen-hui, LU Kun, ZHENG Ting-ting, REN Zhi-yuan, BAO Guo-qing, WANG Min, LUO Jian-mei(
), CHEN Wen-hui, LU Kun, ZHENG Ting-ting, REN Zhi-yuan, BAO Guo-qing, WANG Min, LUO Jian-mei( )
)
Received:2023-08-07
Published:2024-01-26
Online:2024-02-06
摘要:
【目的】11α,17α-双羟基黄体酮是重要的甾体激素类药物中间体,应用价值大。赭曲霉(Aspergillus ochraceus)CICC 41473的11α羟化酶CYP68J5是转化17α-羟基黄体酮生成11α,17α-双羟基黄体酮的关键酶,但其催化性能有待于提升。【方法】在课题组前期确定的关键氨基酸位点D118、F216和M488基础上,通过定点饱和突变和底物转化实验进行优良突变体的筛选;使用AutoDock进行酶与底物的分子对接,并通过Discovery Studio和Gromacs分别研究分子间相互作用力和分子动力学模拟。【结果】突变体D118V、F216W、M488L和M488W具有催化性能,其中,优良突变体D118V的活性最高,其在底物浓度0.5 g/L时,生产强度为431.66 mg/(L·d),较野生型提高了2.12倍,这可能是因为当118位的天冬氨酸突变为缬氨酸后,该位点与底物之间产生了新的疏水相互作用,增强了酶的底物结合口袋的疏水性。分子动力学模拟结果表明酶的整体构象更稳定,酶与底物的结合更紧密。【结论】通过定点饱和突变获得了催化性能显著提升的优良突变体D118V,研究结果为甾体11α羟化酶的改造提供了应用案例和理论指导。
史京辉, 陈文慧, 陆坤, 郑婷婷, 任志远, 鲍国庆, 王敏, 骆健美. 定点饱和突变提高赭曲霉11α羟化酶的催化性能[J]. 生物技术通报, 2024, 40(1): 322-331.
SHI Jing-hui, CHEN Wen-hui, LU Kun, ZHENG Ting-ting, REN Zhi-yuan, BAO Guo-qing, WANG Min, LUO Jian-mei. Site-directed Saturation Mutagenesis to Improve the Catalytic Performance of 11α-hydroxylase from Aspergillus ochraceus[J]. Biotechnology Bulletin, 2024, 40(1): 322-331.
| 引物名称Primer name | 引物序列Primer sequence(5'-3') |
|---|---|
| D118-F | NNKTCTCATGGTTATATTCCTGGTTTTG |
| D118-R | ATCAGTAGTTGGAGTTTCAAAATCCATTC |
| F216-F | NNKGGTGTTGGTGATAAATTGAG |
| F216-R | AGCCAAAGCAGCATATTGAGAAGAAG |
| M488-F | NNKACTTATTTGGCTGATCCAAACAC |
| M488-R | ACCAATATTCAATGGTTGTGGTTTAAAAC |
表1 饱和突变的PCR引物
Table 1 PCR primers for the saturated mutation
| 引物名称Primer name | 引物序列Primer sequence(5'-3') |
|---|---|
| D118-F | NNKTCTCATGGTTATATTCCTGGTTTTG |
| D118-R | ATCAGTAGTTGGAGTTTCAAAATCCATTC |
| F216-F | NNKGGTGTTGGTGATAAATTGAG |
| F216-R | AGCCAAAGCAGCATATTGAGAAGAAG |
| M488-F | NNKACTTATTTGGCTGATCCAAACAC |
| M488-R | ACCAATATTCAATGGTTGTGGTTTAAAAC |
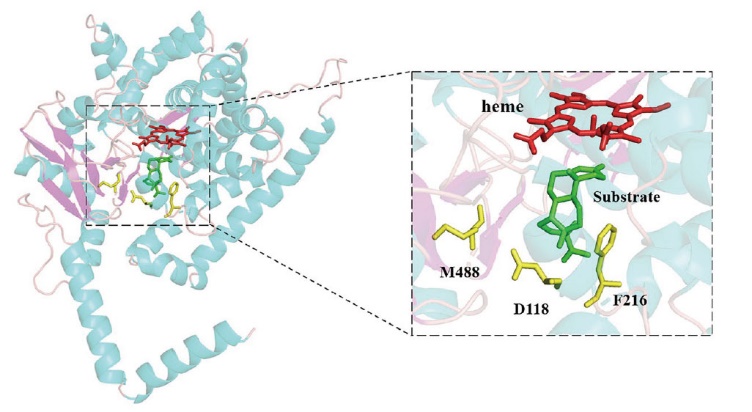
图1 CYP68J5与底物对接结构中D118V、F216和M488三个位点的分布情况 CYP68J5的α螺旋、β折叠和无规则卷曲分别用亮蓝色、亮紫色和粉红色表示,血红素(heme)、关键氨基酸位点和底物分别用深红色、黄色和绿色表示
Fig. 1 Distribution of the D118V, F216 and M488 in the docking structure of CYP68J5 with substrate The α-helix, β-folding and random curl of CYP68J5 are colored in bright blue, bright purple and pink, respectively, while the heme, key amino acid sites and substrates are colored in dark red, yellow and green, respectively
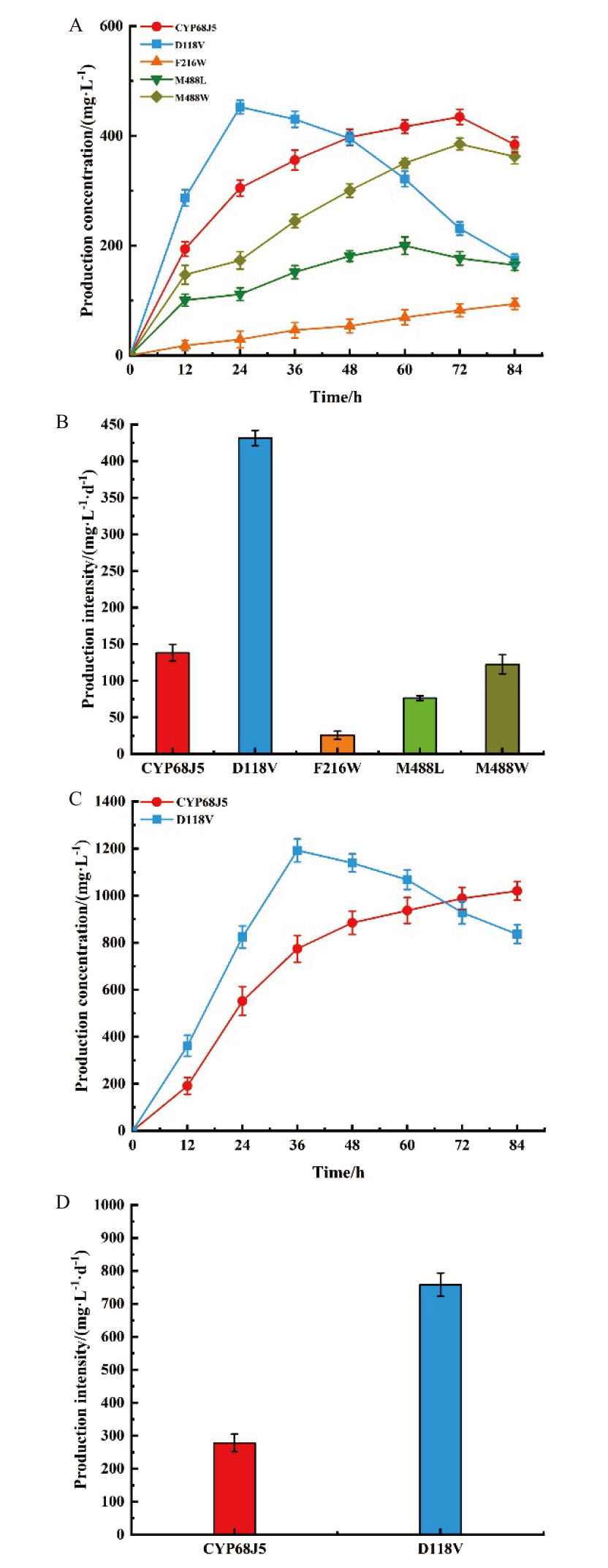
图3 表达CYP68J5及其突变体D118V、F216W、M488L和M488W的酿酒酵母在0.5 g/L底物浓度(A, B)和2.0 g/L底物浓度(C, D)的产物生成曲线和生产强度
Fig. 3 S. cerevisiae expressing CYP68J5 and its mutants D118V, F216W, M488L, and M488W at 0.5 g/L substrate concentration product formation curves and production intensities(A, B)and 2.0 g/L substrate concentration(C, D)
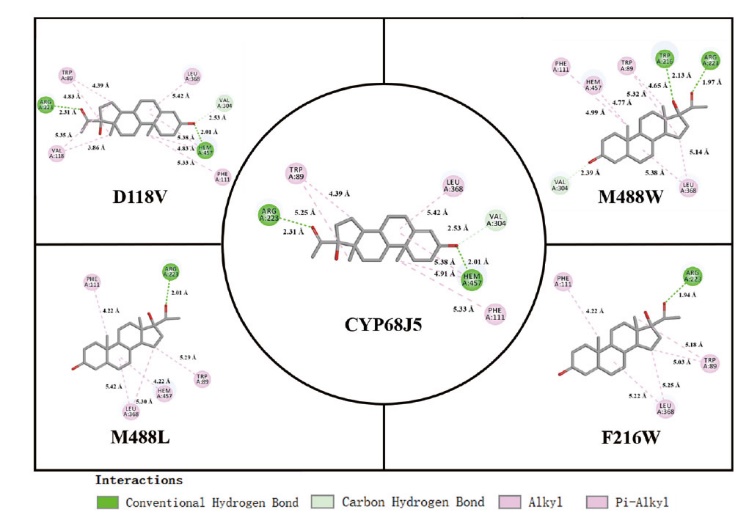
图4 使用Discovery Studio绘制的CYP68J5及其突变体D118V、F216W、M488L和M488W残基和血红素(heme)与底物的分子间相互作用2D图
Fig. 4 2D plot of the intermolecular interactions of CYP68J5 and its the mutant D118V, F216W, M488L, and M488W residues and heme with substrates using Discovery Studio

图5 CYP68J5和优良突变体D118V的分子动力学模拟 A:均方根偏差;B:均方根波动;C:回转半径;D:溶剂可及表面积;E:氢键个数;F:自由能
Fig. 5 Molecular dynamics simulations of CYP68J5 and its elite mutant D118V A: Root mean square deviation; B: root mean square fluctuation; C: radius of gyration; D: solvent accessible surface area; E: number of hydrogen bonds; F: free energy
| [1] |
Stouthart AJ, Lucassen EC, van Strien FJ, et al. Stress responsiveness of the pituitary-interrenal axis during early life stages of common carp(Cyprinus carpio)[J]. J Endocrinol, 1998, 157(1): 127-137.
pmid: 9614366 |
| [2] | 张峥斌. 一锅法连续发酵制备Δ1-11α,17α-二羟基黄体酮的方法: CN104711311A[P]. 2015-06-17. |
| Zhang ZB. Preparation of Δ1-11α,17α-dihydroxyprogesterone by continuous fermentation in a one-pot method: 10471131-1A[P]. 2015-06-17. | |
| [3] |
Fotsch C, Wang MH. Blockade of glucocorticoid excess at the tissue level: inhibitors of 11beta-hydroxysteroid dehydrogenase type 1 as a therapy for type 2 diabetes[J]. J Med Chem, 2008, 51(16): 4851-4857.
doi: 10.1021/jm800369f pmid: 18652443 |
| [4] |
Pedersen KB, Geng CD, Vedeckis WV. Three mechanisms are involved in glucocorticoid receptor autoregulation in a human T-lymphoblast cell line[J]. Biochemistry, 2004, 43(34): 10851-10858.
pmid: 15323545 |
| [5] |
Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs[J]. N Engl J Med, 2005, 353(16): 1711-1723.
doi: 10.1056/NEJMra050541 URL |
| [6] | 张喜春, 韩振海, XoджaйoBa ЛТ, 等. 植物体内甾醇的合成和生理作用[J]. 植物生理学通讯, 2001, 37(5): 452-457. |
| Zhang XC, Han ZH, XoджaйoBa ЛT, et al. Synthesis and physiological function of sterols in plants[J]. Plant Physiol Commun, 2001, 37(5): 452-457. | |
| [7] |
Canada KA, Iwashita S, Shim H, et al. Directed evolution of toluene ortho-monooxygenase for enhanced 1-naphthol synthesis and chlorinated ethene degradation[J]. J Bacteriol, 2002, 184(2): 344-349.
doi: 10.1128/JB.184.2.344-349.2002 pmid: 11751810 |
| [8] |
Bühler B, Schmid A. Process implementation aspects for biocatalytic hydrocarbon oxyfunctionalization[J]. J Biotechnol, 2004, 113(1-3): 183-210.
pmid: 15380656 |
| [9] |
Urlacher VB, Schmid RD. Recent advances in oxygenase-catalyzed biotransformations[J]. Curr Opin Chem Biol, 2006, 10(2): 156-161.
pmid: 16488653 |
| [10] |
van Beilen JB, Duetz WA, Schmid A, et al. Practical issues in the application of oxygenases[J]. Trends Biotechnol, 2003, 21(4): 170-177.
pmid: 12679065 |
| [11] |
艾露, 陈文慧, 史京辉, 等. 赭曲霉11α羟化酶的克隆表达及关键氨基酸位点分析[J]. 生物技术通报, 2023, 39(4): 114-123.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0572 |
| Ai L, Chen WH, Shi JH, et al. Cloning and expression of 11α hydroxylase from Aspergillus ochraceus and analysis of key amino acid sites[J]. Biotechnol Bull, 2023, 39(4): 114-123. | |
| [12] |
Koehn EM, Kohen A. Flavin-dependent thymidylate synthase: a novel pathway towards thymine[J]. Arch Biochem Biophys, 2010, 493(1): 96-102.
doi: 10.1016/j.abb.2009.07.016 pmid: 19643076 |
| [13] |
Hannemann F, Bichet A, Ewen KM, et al. Cytochrome P450 systems—biological variations of electron transport chains[J]. Biochim Biophys Acta, 2007, 1770(3): 330-344.
pmid: 16978787 |
| [14] |
Barnaba C, Ramamoorthy A. Picturing the membrane-assisted choreography of cytochrome P450 with lipid nanodiscs[J]. Chemphyschem, 2018, 19(20): 2603-2613.
doi: 10.1002/cphc.201800444 pmid: 29995333 |
| [15] | 候向江. 赭曲霉甾体11α-羟化酶基因异源表达研究[D]. 天津: 天津科技大学. |
| Hou XJ. Heterologous expression research of Aspergillus ochraceus steroid 11α-hydrolyase gene[D]. Tianjin: Tianjin University of Science & Technology. | |
| [16] |
Felpeto-Santero C, Galán B, García JL. Production of 11α-hydroxysteroids from sterols in a single fermentation step by Mycolicibacterium smegmatis[J]. Microb Biotechnol, 2021, 14(6): 2514-2524.
doi: 10.1111/1751-7915.13735 pmid: 33660943 |
| [17] | 林本凤, 职亚飞, 刘晓光, 等. 黑曲霉ATCC1015催化16α,17α-环氧黄体酮11α-羟基化及相关P450基因诱导表达[J]. 天津科技大学学报, 2017, 32(6): 8-14. |
| Lin BF, Zhi YF, Liu XG, et al. 11α-hydroxylation of 16α,17α-epoxy progesterone by Aspergillus niger ATCC1015 and induction expression of relevant cytochromes P450 genes[J]. J Tianjin Univ Sci Technol, 2017, 32(6): 8-14. | |
| [18] |
Wang RJ, Sui PC, Hou XJ, et al. Cloning and identification of a novel steroid 11α-hydroxylase gene from Absidia coerulea[J]. J Steroid Biochem Mol Biol, 2017, 171: 254-261.
doi: 10.1016/j.jsbmb.2017.04.006 URL |
| [19] |
Wang X, Yang XW, Jia X, et al. Determination of steroid hydroxylation specificity of an industrial strain Aspergillus ochraceus TCCC41060 by cytochrome P450 gene CYP68J5[J]. Ann Microbiol, 2020, 70(1): 45.
doi: 10.1186/s13213-020-01577-6 |
| [20] |
Qian M, Zeng YL, Mao SH, et al. Engineering of a fungal steroid 11α-hydroxylase and construction of recombinant yeast for improved production of 11α-hydroxyprogesterone[J]. J Biotechnol, 2022, 353: 1-8.
doi: 10.1016/j.jbiotec.2022.05.012 pmid: 35654275 |
| [21] |
Li SL, Chang YW, Liu YN, et al. A novel steroid hydroxylase from Nigrospora sphaerica with various hydroxylation capabilities to different steroid substrates[J]. J Steroid Biochem Mol Biol, 2023, 227: 106236.
doi: 10.1016/j.jsbmb.2022.106236 URL |
| [22] | 乔玉茜. 17α羟基黄体酮11α羟化菌株筛选及其转化工艺研究[D]. 天津: 天津科技大学, 2017. |
| Qiao YQ/X. Screeing and transformation conditions of 11α-hydroxylation of 17α-hydroxy progesterone[D]. Tianjin: Tianjin University of Science & Technology, 2017. | |
| [23] |
Genheden S, Ryde U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities[J]. Expert Opin Drug Discov, 2015, 10(5): 449-461.
doi: 10.1517/17460441.2015.1032936 URL |
| [24] | 曲戈, 袁波, 孙周通. 工业蛋白质理性设计与应用[J]. 生物工程学报, 2022, 38(11): 4068-4080. |
| Qu G, Yuan B, Sun ZT. Rational design and applications of industrial proteins[J]. Chin J Biotechnol, 2022, 38(11): 4068-4080. | |
| [25] |
Acevedo-Rocha CG, Gamble CG, Lonsdale R, et al. P450-catalyzed regio- and diastereoselective steroid hydroxylation: efficient directed evolution enabled by mutability landscaping[J]. ACS Catal, 2018, 8(4): 3395-3410.
doi: 10.1021/acscatal.8b00389 URL |
| [26] | Tao S, Gao Y, Li K, et al. Engineering substrate recognition sites of cytochrome P450 monooxygenase CYP116B3 from Rhodococcus ruber for enhanced regiospecific naphthalene hydroxylation[J]. Mol Catal, 2020, 493: 111089. |
| [27] |
Chen J, Fan FY, Qu G, et al. Identification of Absidia orchidis steroid 11β-hydroxylation system and its application in engineering Saccharomyces cerevisiae for one-step biotransformation to produce hydrocortisone[J]. Metab Eng, 2020, 57: 31-42.
doi: 10.1016/j.ymben.2019.10.006 URL |
| [28] |
Doğru EK, Güralp G, Uyar A, et al. Rational design of thermophilic CYP119 for progesterone hydroxylation by in silico mutagenesis and docking screening[J]. J Mol Graph Model, 2023, 118: 108323.
doi: 10.1016/j.jmgm.2022.108323 URL |
| [29] | Tong W, Yan QP, Tian SX, et al. Single-site modification of the P450-BM3 substrate-entrance facilitates the synthesis of optically pure pharmaceutically useful methyl trans-3-phenylglycidates[J]. Mol Catal, 2023, 547: 113354. |
| [30] |
Wang FH, Zhu ML, Song Z, et al. Reshaping the binding pocket of lysine hydroxylase for enhanced activity[J]. ACS Catal, 2020, 10(23): 13946-13956.
doi: 10.1021/acscatal.0c03841 URL |
| [1] | 王佳蕊, 孙培媛, 柯瑾, 冉彬, 李洪有. 苦荞糖基转移酶基因FtUGT143的克隆及表达分析[J]. 生物技术通报, 2023, 39(8): 204-212. |
| [2] | 艾露, 陈文慧, 史京辉, 任志远, 沈文琦, 杨嘉凝, 骆健美, 王敏. 赭曲霉11α羟化酶的克隆表达及关键氨基酸位点分析[J]. 生物技术通报, 2023, 39(4): 114-123. |
| [3] | 郝俊尧, 马富强, 杨广宇. 产碱杆菌Alcaligenes sp.KS-85来源肌酸酶活性中心的关键氨基酸功能研究[J]. 生物技术通报, 2021, 37(3): 75-83. |
| [4] | 孙熙麟, 蒋振彦, 刘志屹, 戴璐, 孙非, 黄伟. 氨基酸定点突变提高灵芝蛋白LZ-8热稳定性的研究[J]. 生物技术通报, 2020, 36(1): 23-28. |
| [5] | 乔晶, 崔晟榕, 石宏武, 罗祖良, 马小军. 罗汉果环阿屯醇合酶的同源建模、分子对接及催化环化的机理推测[J]. 生物技术通报, 2019, 35(2): 101-108. |
| [6] | 程杏安, 叶静敏, 蒋旭红, 刘展眉, 胡美英. 草地贪夜蛾组织蛋白酶B的基因克隆、序列分析、三维结构预测及其分子对接模拟[J]. 生物技术通报, 2018, 34(1): 183-194. |
| [7] | 吴丛梅, 李玲玲, 关晓侠, 刘新涛, 陈吉, 殷玉和. 肽类化合物对H37Ra抑制的优化筛选[J]. 生物技术通报, 2014, 0(8): 196-201. |
| [8] | 张国防, 李真真, 姚伟利, 王丽君, 朱显明. 基于流感病毒PAN蛋白的高通量药物筛选[J]. 生物技术通报, 2014, 0(2): 181-186. |
| [9] | 林春娇;杨立荣;徐刚;吴坚平;. R-2-卤代酸脱卤酶的同源模建及底物对映体选择性研究[J]. , 2011, 0(10): 191-198. |
| [10] | 胡雪松;李夏兰;. 阿魏酸酯酶基因克隆表达调控及其应用进展[J]. , 2009, 0(12): 11-16. |
| [11] | 朱威;张莲芬;雷楗勇;顾健德;金坚;. 羟喜树碱结合肽的研究[J]. , 2009, 0(01): 126-129. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||