








生物技术通报 ›› 2025, Vol. 41 ›› Issue (1): 25-38.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0522
邹涛圳1( ), 李鹏飞1(
), 李鹏飞1( ), 李新冬1, 万欢2, 张燚3
), 李新冬1, 万欢2, 张燚3
收稿日期:2024-05-31
出版日期:2025-01-26
发布日期:2025-01-22
通讯作者:
李鹏飞,男,博士,讲师,研究方向:微藻生物质能源及藻类资源化;E-mail: lpffighter@163.com作者简介:邹涛圳,男,硕士研究生,研究方向:微藻诱变产油;E-mail: gdztz8823@163.com
基金资助:
ZOU Tao-zhen1( ), LI Peng-fei1(
), LI Peng-fei1( ), LI Xin-dong1, WAN Huan2, ZHANG Yi3
), LI Xin-dong1, WAN Huan2, ZHANG Yi3
Received:2024-05-31
Published:2025-01-26
Online:2025-01-22
摘要:
微藻油脂作为一种潜在的可再生能源和生物燃料资源,在解决能源危机及促进绿色发展方面具有重要意义。藻种特性影响微藻培育、油脂提取和转化等多个环节,选择适宜的原始藻株进行定向育种,有望突破生产过程中总体油脂产率偏低的瓶颈。胁迫培养通过改变微藻的外部生长条件来引起其内部生理和代谢变化,从而促进油脂的积累,本质上是利用微藻自身的应激性反应,需要在油脂积累和生长平衡之间找到最佳点。诱变技术通过物理或化学手段引起微藻细胞损伤,本质上是一种外应力作用下的随机突变,需要从中筛选出具有优良性状的突变株。基因工程育种通过分子生物学手段,定向改造微藻的基因组,具备高精度、高成本和高复杂性。探索高脂藻株培养与资源化理念的结合,可以实现更经济环保的生物质能原料微藻生产模式,推动生物质能产业发展。论文概述了微藻油脂合成的机理及其调控策略,总结了促微藻产油的培育方法,包括胁迫、诱变、基因工程及高脂藻株与资源化生产的联动,强调培育高脂藻株对于实现可持续生物燃料生产的重要作用。通过列举各培育手段在当前微藻油脂高产研究的技术重点和作用机理,说明了当前微藻产油的研究方向和瓶颈,未来的研究可能致力于产油微藻油脂代谢调控网络的发掘完善、高通量育种方法的创新及资源化培养体系的优化。
邹涛圳, 李鹏飞, 李新冬, 万欢, 张燚. 微藻油脂合成及高脂藻株培育研究进展[J]. 生物技术通报, 2025, 41(1): 25-38.
ZOU Tao-zhen, LI Peng-fei, LI Xin-dong, WAN Huan, ZHANG Yi. Research Progress in Microalgal Lipid Synthesis and Cultivation of High-lipid Strain[J]. Biotechnology Bulletin, 2025, 41(1): 25-38.
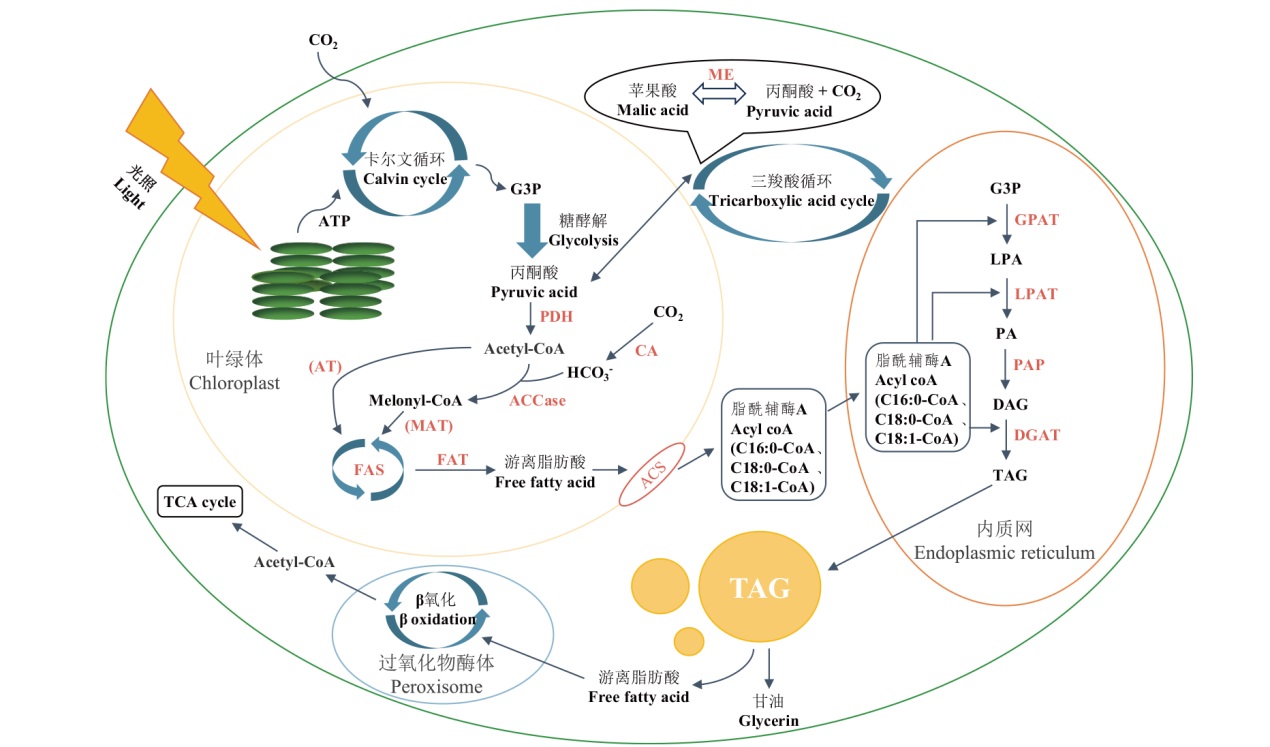
图2 微藻胞内TAG合成与分解主要路径 ATP:三磷酸腺苷;G3P:三磷酸甘油醛;PDH:丙酮酸脱氢酶系;Acetyl-CoA:乙酰辅酶A;Melonyl-CoA:丙二酸单酰辅酶A;ACCase:乙酰辅酶A羧化酶;MAT:丙二酸单酰辅酶A转酰酶;AT:乙酰辅酶A转酰酶;FAS:脂肪酸合酶复合体;FAT:酰基ACP硫酯酶;ACS:脂酰辅酶A合成酶;CA:碳酸酐酶;ME:苹果酸酶;GPAT:三磷酸甘油醛转酰酶;LPA:溶血磷脂酸;LPAT:溶血磷脂酸转酰酶;PA:磷脂酸;PAP:磷脂酸磷酸酶;DAG:二酰甘油;DGAT:二酰甘油转酰酶;TAG:三酰甘油
Fig. 2 Major pathways of intracellular TAG synthesis and catabolism in microalgae ATP: Adenosine triphosphate; G3P: glyceraldehyde 3-phosphate; PDH: pyruvate dehydrogenase complex; ACCase: acetyl-CoA carboxylase; MAT: malonyl-CoA transacetylase; AT: acetyl-CoA transacetylase; FAS: fatty acid synthase complex; FAT: fatty acyl-ACP thioesterase; ACS: acyl-CoA synthetase; CA: carbonic anhydrase; ME: malic enzyme; GPAT: glycerol-3-phosphate acyltransferases; LPA: lysophosphatidic acid; LPAT: lysophosphatidic acid transacetylase; PA: phosphatidic acid; PAP: phosphatidic acid phosphatase; DAG: diacyl glycerol; DGAT: diacyl glycerol transacetylase; TAG: triacylglycerol
| 藻种 Algae species | 光照条件 Light conditions | 培养基 Culture medium | 生物量增长率 Biomass growth rate/(mg·L-1·d-1) | 油脂含量 Oil content/% | 参考文献 Reference |
|---|---|---|---|---|---|
| Phaeodactylum tricornutum | 光暗交替 | 优化的f/2 | 10.49 | 25.00 | [ |
| Graesiella emersonii | 光暗交替 | BG-11 | 44.06 | 29.50 | [ |
| Nannochloropsis sp. | 光暗交替 | f/2 | 88.00 | 37.90 | [ |
| Dunaliella tertiolecta | 光暗交替 | f/2 | — | 30.90 | [ |
| Isochrysis galbana | 光暗交替 | f/2 | — | 35.60 | [ |
| Chlorella vulgari | 连续光照 | BG-11 | 153 | 27.20 | [ |
| Scenedesmus obliquus | 连续光照 | BG-11 | 120 | 22.20 | [ |
表1 实验室规模下常见产油藻种的生理特性
Table 1 Physiological characterization of common oil-producing algal species at laboratory scale
| 藻种 Algae species | 光照条件 Light conditions | 培养基 Culture medium | 生物量增长率 Biomass growth rate/(mg·L-1·d-1) | 油脂含量 Oil content/% | 参考文献 Reference |
|---|---|---|---|---|---|
| Phaeodactylum tricornutum | 光暗交替 | 优化的f/2 | 10.49 | 25.00 | [ |
| Graesiella emersonii | 光暗交替 | BG-11 | 44.06 | 29.50 | [ |
| Nannochloropsis sp. | 光暗交替 | f/2 | 88.00 | 37.90 | [ |
| Dunaliella tertiolecta | 光暗交替 | f/2 | — | 30.90 | [ |
| Isochrysis galbana | 光暗交替 | f/2 | — | 35.60 | [ |
| Chlorella vulgari | 连续光照 | BG-11 | 153 | 27.20 | [ |
| Scenedesmus obliquus | 连续光照 | BG-11 | 120 | 22.20 | [ |
| [1] | Shokravi H, Shokravi Z, Heidarrezaei M, et al. Fourth generation biofuel from genetically modified algal biomass: challenges and future directions[J]. Chemosphere, 2021, 285: 131535. |
| [2] | Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review[J]. Renew Sustain Energy Rev, 2010, 14(1): 217-232. |
| [3] | Raheem A, Prinsen P, Vuppaladadiyam AK, et al. A review on sustainable microalgae based biofuel and bioenergy production: recent developments[J]. J Clean Prod, 2018, 181: 42-59. |
| [4] | Hoang AT, Sirohi R, Pandey A, et al. Biofuel production from microalgae: challenges and chances[J]. Phytochem Rev, 2023, 22(4): 1089-1126. |
| [5] | Yu WL, Ansari W, Schoepp NG, et al. Modifications of the metabolic pathways of lipid and triacylglycerol production in microalgae[J]. Microb Cell Fact, 2011, 10: 91. |
| [6] | Rodionova MV, Poudyal RS, Tiwari I, et al. Biofuel production: challenges and opportunities[J]. Int J Hydrog Energy, 2017, 42(12): 8450-8461. |
| [7] | Mariadhas A, Sathish Kumar B, Kabilan K, et al. Technical insights of microalgae derived bio-diesel on its performance and emission characteristics, techno-economics and practicability huddles[J]. Fuel, 2023, 349: 128744. |
| [8] | Rawat J, Gupta PK, Pandit S, et al. Latest expansions in lipid enhancement of microalgae for biodiesel production: an update[J]. Energies, 2022, 15(4): 1550. |
| [9] | 李俊磊, 张红兵. 基因工程方法增加微藻脂质积累研究进展[J]. 应用化工, 2020, 49(9): 2385-2387, 2392. |
| Li JL, Zhang HB. Advances in genetic engineering methods to increase lipid accumulation in microalgae[J]. Appl Chem Ind, 2020, 49(9): 2385-2387, 2392. | |
| [10] | 郭宝文, 李煦, 宗保宁, 等. 微藻固碳实现CO2减排与生物质增值[J]. 石油学报: 石油加工, 2023, 39(3): 668-678. |
| Guo BW, Li X, Zong BN, et al. Carbon fixation by microalgae to achieve CO2 emission reduction and biomass valorization[J]. Acta Petrolei Sin Petrol Process Sect, 2023, 39(3): 668-678. | |
| [11] | Yang YN, Ge SH, Pan YT, et al. Screening of microalgae species and evaluation of algal-lipid stimulation strategies for biodiesel production[J]. Sci Total Environ, 2023, 857(Pt 1): 159281. |
| [12] | Mandal MK, Chanu NK, Chaurasia N. Exogenous addition of indole acetic acid and kinetin under nitrogen-limited medium enhances lipid yield and expression of glycerol-3-phosphate acyltransferase & diacylglycerol acyltransferase genes in indigenous microalgae: a potential approach for biodiesel production[J]. Bioresour Technol, 2020, 297: 122439. |
| [13] | Behl K, SeshaCharan P, Joshi M, et al. Multifaceted applications of isolated microalgae Chlamydomonas sp. TRC-1 in wastewater remediation, lipid production and bioelectricity generation[J]. Bioresour Technol, 2020, 304: 122993. |
| [14] | Goswami RK, Agrawal K, Verma P. Microalgae Dunaliella as biofuel feedstock and β-carotene production: an influential step towards environmental sustainability[J]. Energy Convers Manag X, 2022, 13: 100154. |
| [15] | Ding Y, Wen XB, Peng XN, et al. Surfactants as fungal parasite control agents in oleaginous microalga, Graesiella sp. WBG-1, mass culture[J]. Algal Res, 2019, 41: 101539. |
| [16] | Hawrot-Paw M, Ratomski P, Koniuszy A, et al. Fatty acid profile of microalgal oils as a criterion for selection of the best feedstock for biodiesel production[J]. Energies, 2021, 14(21): 7334. |
| [17] | Liu JY, Song YM, Qiu W. Oleaginous microalgae Nannochloropsis as a new model for biofuel production: review & analysis[J]. Renew Sustain Energy Rev, 2017, 72: 154-162. |
| [18] | Morais KCC, Conceição D, Vargas JVC, et al. Enhanced microalgae biomass and lipid output for increased biodiesel productivity[J]. Renew Energy, 2021, 163: 138-145. |
| [19] | Li DW, Balamurugan S, Yang YF, et al. Transcriptional regulation of microalgae for concurrent lipid overproduction and secretion[J]. Sci Adv, 2019, 5(1): eaau3795. |
| [20] | Jung JH, Sirisuk P, Ra CH, et al. Effects of green LED light and three stresses on biomass and lipid accumulation with two-phase culture of microalgae[J]. Process Biochem, 2019, 77: 93-99. |
| [21] | Kabir F, Gulfraz M, Raja GK, et al. Screening of native hyper-lipid producing microalgae strains for biomass and lipid production[J]. Renew Energy, 2020, 160: 1295-1307. |
| [22] | Song XT, Liu BF, Kong FY, et al. Overview on stress-induced strategies for enhanced microalgae lipid production: application, mechanisms and challenges[J]. Resour Conserv Recycl, 2022, 183: 106355. |
| [23] |
Moghimifam R, Niknam V, Ebrahimzadeh H, et al. The influence of different CO2 concentrations on the biochemical and molecular response of two isolates of Dunaliella sp.(ABRIINW-CH2 and ABRIINW-SH33)[J]. J Appl Phycol, 2020, 32(1): 175-187.
doi: 10.1007/s10811-019-01914-6 |
| [24] | 卢鸿翔. 核诱变及碳胁迫促进微藻光合作用及生长固碳的机理研究[D]. 杭州: 浙江大学, 2018. |
| Lu HX. Mechanisms on improving photosynthetic characterization and carbon fixation rate of microalgae by nuclear radiation and CO2 stress[D]. Hangzhou: Zhejiang University, 2018. | |
| [25] | Levitan O, Dinamarca J, Zelzion E, et al. Remodeling of intermediate metabolism in the diatom Phaeodactylum tricornutum under nitrogen stress[J]. Proc Natl Acad Sci U S A, 2015, 112(2): 412-417. |
| [26] | Wei Q, Yao JJ, Chen RG, et al. Low-frequency ultrasound and nitrogen limitation induced enhancement in biomass production and lipid accumulation of Tetradesmus obliquus FACHB-12[J]. Bioresour Technol, 2022, 358: 127387. |
| [27] | Nagappan S, Devendran S, Tsai PC, et al. Metabolomics integrated with transcriptomics and proteomics: evaluation of systems reaction to nitrogen deficiency stress in microalgae[J]. Process Biochem, 2020, 91: 1-14. |
| [28] | Kamalanathan M, Pierangelini M, Shearman LA, et al. Impacts of nitrogen and phosphorus starvation on the physiology of Chla-mydomonas reinhardtii[J]. J Appl Phycol, 2016, 28(3): 1509-1520. |
| [29] | Yu SJ, Hu H, Zheng H, et al. Effect of different phosphorus concentrations on biodiesel production from Isochrysis zhangjiangensis under nitrogen sufficiency or deprivation condition[J]. Appl Microbiol Biotechnol, 2019, 103(12): 5051-5059. |
| [30] | Yang FF, Xiang WZ, Li T, et al. Transcriptome analysis for phosphorus starvation-induced lipid accumulation in Scenedesmus sp[J]. Sci Rep, 2018, 8(1): 16420. |
| [31] | He QN, Yang HJ, Wu L, et al. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae[J]. Bioresour Technol, 2015, 191: 219-228. |
| [32] | Huang Y, Li PR, Huang Y, et al. A synchronous photoautotrophic-heterotrophic biofilm cultivation mode for Chlorella vulgaris biomass and lipid simultaneous accumulation[J]. J Clean Prod, 2022, 336: 130453. |
| [33] | 孙建瑞, 赵君峰, 符丹丹, 等. 不同光质对衣藻(Chla-mydomonas sp. 212)生长及油脂积累的影响[J]. 应用与环境生物学报, 2020, 26(4): 1016-1022. |
| Sun JR, Zhao JF, Fu DD, et al. Effects of different lights on the growth and lipid accumulation of Chlamydomonas sp. 212[J]. Chin J Appl Environ Biol, 2020, 26(4): 1016-1022. | |
| [34] | Jiang HM, Gao KS. Effects of lowering temperature during culture on the production of polyunsaturated fatty acids in the marine diatom Phaeodactylum tricornutum(Bacillariophyceae)[J]. J Phycol, 2004, 40(4): 651-654. |
| [35] | 韩飞. 高温胁迫与超声刺激促进微藻油脂积累的过程及机理[D]. 济南: 山东大学, 2016. |
| Han F. The mechanism of microalgae lipid accumulation induced by high-temperature stress and ultrasonic stimulation[D]. Jinan: Shandong University, 2016. | |
| [36] | Cao JY, Kong ZY, Ye MW, et al. Comprehensive comparable study of metabolomic and transcriptomic profiling of Isochrysis galbana exposed to high temperature, an important diet microalgal species[J]. Aquaculture, 2020, 521: 735034. |
| [37] | 戴文娜, 童旭, 张琴, 等. 一株荒漠产油微藻的筛选及其生长和产油的pH响应[J]. 中国油脂, 2020, 45(5): 82-87. |
| Dai WN, Tong X, Zhang Q, et al. Screening of a desert oil-producing microalgae and its growth and oil production response toward pH[J]. China Oils Fats, 2020, 45(5): 82-87. | |
| [38] | 王垿, 孙昕, 李鹏飞, 等. 双对栅藻FACHB-78甘油三酯积累的盐胁迫条件优化[J]. 中国环境科学, 2019, 39(12): 5248-5253. |
| Wang X, Sun X, Li PF, et al. Optimization of salt stress condition for accumulation of triglycerides in Scenedesmus bijuga FACHB-78[J]. China Environ Sci, 2019, 39(12): 5248-5253. | |
| [39] | Jiang LQ, Zhang LJ, Nie CL, et al. Lipid productivity in limnetic Chlorella is doubled by seawater added with anaerobically digested effluent from kitchen waste[J]. Biotechnol Biofuels, 2018, 11: 68. |
| [40] | Zalogin TR, Pick U. Azide improves triglyceride yield in microalgae[J]. Algal Res, 2014, 3: 8-16. |
| [41] | Rachutin Zalogin T, Pick U. Inhibition of nitrate reductase by azide in microalgae results in triglycerides accumulation[J]. Algal Res, 2014, 3: 17-23. |
| [42] |
王依霖, 莫创荣, 许雪棠, 等. 2, 4-二氯苯氧乙酸与盐胁迫联合提高小球藻的产脂量[J]. 食品与发酵工业, 2022, 48(24): 105-110.
doi: 10.13995/j.cnki.11-1802/ts.031174 |
| Wang YL, Mo CR, Xu XT, et al. 2, 4-dichlorophenoxyacetic acid combined with salt stress increased lipid production of Chlorella vulgaris[J]. Food Ferment Ind, 2022, 48(24): 105-110. | |
| [43] | 李文娜, 邢向英, 董庆霖, 等. 分散性多糖分散绿藻Chroococ-cidiorella tianjinensis促进其生长和脂类合成的研究[J]. 可再生能源, 2023, 41(9): 1152-1158. |
| Li WN, Xing XY, Dong QL, et al. Static cultivation of the green alga Chroococcidiorella tianjinensis with the dispersing polysaccharide[J]. Renew Energy Resour, 2023, 41(9): 1152-1158. | |
| [44] |
李喜明, 赵永腾, 余旭亚. 褪黑素调控缺氮胁迫下单针藻中油脂积累的影响[J]. 食品与发酵工业, 2019, 45(2): 39-44.
doi: 10.13995/j.cnki.11-1802/ts.017735 |
| Li XM, Zhao YT, Yu XY. Effects of melatonin on regulating lipid accumulation in Monoraphidium sp. QLY-1 under nitrogen deficiency stress[J]. Food Ferment Ind, 2019, 45(2): 39-44. | |
| [45] |
付峰, 隋正红, 孙利芹, 等. 藻类诱变育种技术研究进展[J]. 生物技术通报, 2018, 34(10): 58-63.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0094 |
|
Fu F, Sui ZH, Sun LQ, et al. Research advance on the algal mutation breeding technologies[J]. Biotechnol Bull, 2018, 34(10): 58-63.
doi: 10.13560/j.cnki.biotech.bull.1985.2018-0094 |
|
| [46] | Sung YJ, Patel AK, Yu BS, et al. Sedimentation rate-based screening of oleaginous microalgae for utilization as a direct combustion fuel[J]. Bioresour Technol, 2019, 293: 122045. |
| [47] | Lo E, Arora N, Philippidis GP. Physiological insights into enhanced lipid accumulation and temperature tolerance by Tetraselmis sueci-ca ultraviolet mutants[J]. Sci Total Environ, 2022, 839: 156361. |
| [48] | 梁英, 闫译允, 赖秋璇, 等. 微藻诱变育种研究进展[J]. 中国海洋大学学报: 自然科学版, 2020, 50(6): 19-32. |
| Liang Y, Yan YY, Lai QX, et al. Researching advances in microalgal mutation breeding[J]. Period Ocean Univ China, 2020, 50(6): 19-32. | |
| [49] | Choi JI, Yoon M, Joe M, et al. Development of microalga Scened-esmus dimorphus mutant with higher lipid content by radiation breeding[J]. Bioprocess Biosyst Eng, 2014, 37(12): 2437-2444. |
| [50] | Ma YB, Wang ZY, Zhu M, et al. Increased lipid productivity and TAG content in Nannochloropsis by heavy-ion irradiation mutagenesis[J]. Bioresour Technol, 2013, 136: 360-367. |
| [51] | Zheng XC, Niu HL, Yu JJ, et al. Responses of Alpha-linolenic acid strain(C-12)from Chlorella sp. L166 to low temperature plasma treatment[J]. Bioresour Technol, 2021, 336: 125291. |
| [52] | Li PF, Sun X, Sun Z, et al. Biochemical and genetic changes revealing the enhanced lipid accumulation in Desmodesmus sp. mutated by atmospheric and room temperature plasma[J]. Renew Energy, 2021, 172: 368-381. |
| [53] | Almarashi JQM, El-Zohary SE, Ellabban MA, et al. Enhancement of lipid production and energy recovery from the green microalga Chlorella vulgaris by inoculum pretreatment with low-dose cold atmospheric pressure plasma(CAPP)[J]. Energy Convers Manag, 2020, 204: 112314. |
| [54] | Hoekman SK, Broch A, Robbins C, et al. Review of biodiesel composition, properties, and specifications[J]. Renew Sustain Energy Rev, 2012, 16(1): 143-169. |
| [55] | Benavente-Valdés JR, Aguilar C, Contreras-Esquivel JC, et al. Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species[J]. Biotechnol Rep, 2016, 10: 117-125. |
| [56] | 刘林聪. 微拟球藻(Nannochloropsis gaditana CCMP527)高产油脂藻株的诱变筛选研究[D]. 重庆: 西南大学, 2017. |
| Liu LC. Study on mutation screening of Nannochloropsis gaditana CCMP527 with high oil-producing algae[D]. Chongqing: Southwest University, 2017. | |
| [57] | Sun X, Li PF, Liu XS, et al. Strategies for enhanced lipid production of Desmodesmus sp. mutated by atmospheric and room temperature plasma with a new efficient screening method[J]. J Clean Prod, 2020, 250: 119509. |
| [58] | Sun X, Meng LS, Li PF, et al. Increasing lipid production of Des-modesmus sp. through atmospheric and room temperature plasma orientated with malonic acid: performance and biochemical mechanism[J]. J Clean Prod, 2022, 342: 130911. |
| [59] | Zheng GX, Gu FR, Cui YT, et al. A microfluidic droplet array demonstrating high-throughput screening in individual lipid-producing microalgae[J]. Anal Chim Acta, 2022, 1227: 340322. |
| [60] | Cabanelas ITD, van der Zwart M, Kleinegris DMM, et al. Sorting cells of the microalga Chlorococcum littorale with increased triacylglycerol productivity[J]. Biotechnol Biofuels, 2016, 9(1): 183. |
| [61] |
Cernac A, Benning C. WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis[J]. Plant J, 2004, 40(4): 575-585.
doi: 10.1111/j.1365-313X.2004.02235.x pmid: 15500472 |
| [62] |
孙翰, 刘进. 真核微藻脂质代谢工程的研究进展和展望[J]. 合成生物学, 2023, 4(6): 1140-1160.
doi: 10.12211/2096-8280.2023-044 |
| Sun H, Liu J. Research progress and prospects in lipid metabolic engineering of eukaryotic microalgae[J]. Synthetic Biology J, 2023, 4(6): 1140-1160. | |
| [63] | Sun H, Ren YY, Mao XM, et al. Harnessing C/N balance of Chro-mochloris zofingiensis to overcome the potential conflict in microalgal production[J]. Commun Biol, 2020, 3(1): 186. |
| [64] | Sproles AE, Fields FJ, Smalley TN, et al. Recent advancements in the genetic engineering of microalgae[J]. Algal Res, 2021, 53: 102158. |
| [65] | Crozet P, Navarro FJ, Willmund F, et al. Birth of a photosynthetic chassis: a MoClo toolkit enabling synthetic biology in the microalga Chlamydomonas reinhardtii[J]. ACS Synth Biol, 2018, 7(9): 2074-2086. |
| [66] | Huang KX, Vadiveloo A, Zhou JL, et al. Integrated culture and harvest systems for improved microalgal biomass production and wastewater treatment[J]. Bioresour Technol, 2023, 376: 128941. |
| [67] | Alzgool HA, Alfraihat AS, Alzghool H. Reinforced-concrete bond with brine and olive oil mill wastewater[J]. Civ Eng J, 2022, 8(2): 319-333. |
| [68] | Gao F, Yang HL, Li C, et al. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp[J]. Bioresour Technol, 2019, 282: 118-124. |
| [69] | Li PF, Zou TZ, Sun X, et al. Bioremediation of domestic wastewaters integrated with enhanced biodiesel production using Desmodesmus sp. mutated by atmospheric and room temperature plasma[J]. J Environ Chem Eng, 2023, 11(5): 110957. |
| [70] | Li J, Tang XX, Pan KH, et al. Application study for the high CO2 tolerant Chlorella strain by flue gas culture: evaluation of growth performance and adaptive mechanisms[J]. Chem Eng J, 2024, 479: 147700. |
| [71] | Song CF, Qiu YT, Xie ML, et al. Novel regeneration and utilization concept using rich chemical absorption solvent as a carbon source for microalgae biomass production[J]. Ind Eng Chem Res, 2019, 58(27): 11720-11727. |
| [72] | Pavlik D, Zhong YK, Daiek C, et al. Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system[J]. Algal Res, 2017, 25: 413-420. |
| [73] | Song CF, Han XX, Yin QR, et al. Performance intensification of CO2 absorption and microalgae conversion(CAMC)hybrid system via low temperature plasma(LTP)treatment[J]. Sci Total Environ, 2021, 801: 149791. |
| [74] | Suparmaniam U, Lam MK, Uemura Y, et al. Insights into the microalgae cultivation technology and harvesting process for biofuel production: a review[J]. Renew Sustain Energy Rev, 2019, 115: 109361. |
| [75] | Harun R, Singh M, Forde GM, et al. Bioprocess engineering of microalgae to produce a variety of consumer products[J]. Renew Sustain Energy Rev, 2010, 14(3): 1037-1047. |
| [76] | Yin ZH, Zhu LD, Li SX, et al. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: environmental pollution control and future directions[J]. Bioresour Technol, 2020, 301: 122804. |
| [77] | Nguyen TDP, Le TVA, Show PL, et al. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent[J]. Bioresour Technol, 2019, 272: 34-39. |
| [78] | Vandamme D, Foubert I, Fraeye I, et al. Flocculation of Chlorella vulgaris induced by high pH: role of magnesium and calcium and practical implications[J]. Bioresour Technol, 2012, 105: 114-119. |
| [79] | Ma GX, Meng QY, Mu RM, et al. Obtaining mutant Chlorella vul-garis strains with excellent self-flocculation properties and high wastewater treatment efficiency via atmospheric and room temperature plasma technique[J]. J Water Process Eng, 2023, 53: 103644. |
| [1] | 白龙,李春美,吕途,杜颖,杨玥,田沈. 生物转化能源草制取纤维素乙醇的研究进展[J]. 生物技术通报, 2017, 33(5): 50-56. |
| [2] | 廖春丽;万亚涛;李亚平;姬晓娜;单林娜;. 溶藻菌株NP23的紫外诱变选育[J]. , 2012, 0(06): 174-178. |
| [3] | 石彩蕊;王义强;陈介南;张伟涛;. 产β-葡萄糖苷酶微生物育种研究进展[J]. , 2011, 0(03): 59-65. |
| [4] | 赵川;罗建军;陈少华;胡美英;. 微生物菌种改良筛选新技术研究进展[J]. , 2009, 0(S1): 118-121. |
| [5] | 孙国凤;. 生物柴油的国际发展现状和前景[J]. , 2007, 0(03): 169-170. |
| [6] | 胡明;李晓宇;马平;朱宝成. 抗真菌蛋白研究进展[J]. , 2004, 0(03): 13-17. |
| [7] | . 国内信息[J]. , 2002, 0(02): 15-55. |
| [8] | 李碧荣;王连琴. 我国转基因动物研究概况[J]. , 1994, 0(03): 8-10. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||