








生物技术通报 ›› 2025, Vol. 41 ›› Issue (11): 35-46.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0656
收稿日期:2025-06-23
出版日期:2025-11-26
发布日期:2025-12-09
通讯作者:
纪晓俊,男,博士,教授,研究方向 :微生物代谢工程及合成生物技术;E-mail: xiaojunji@njtech.edu.cn作者简介:汪鑫,男,博士研究生,研究方向 :微生物代谢工程及合成生物技术;E-mail: xinwang@njtech.edu.cn
基金资助:
WANG Xin( ), SUN Tao, SUN Mei-li, WANG Kai-feng, JI Xiao-jun(
), SUN Tao, SUN Mei-li, WANG Kai-feng, JI Xiao-jun( )
)
Received:2025-06-23
Published:2025-11-26
Online:2025-12-09
摘要:
羟基酪醇(hydroxytyrosol)又称3,4-二羟基苯乙醇(3,4-dihydroxyphenylethanol),是一类具有代表性的天然酚类化合物,广泛存在于橄榄等地中海植物中,具有较高的生物活性。研究表明,羟基酪醇具有显著的抗氧化、抗菌、抗炎和抗衰老等多重生理功能,在食品保鲜与包装、天然调味剂、保健品以及功能性食品开发等领域展现出广阔的应用前景和商业价值。当前,羟基酪醇主要通过植物提取或化学合成方式获得,但植物提取方式受到资源限制且产量有限,化学合成则存在反应条件苛刻、产物纯化复杂以及环境污染严重等问题,难以满足日益增长的产业化需求。近年来,基于合成生物学策略构建工程微生物,实现羟基酪醇的绿色高效生物制造,成为该领域的研究热点之一。微生物合成不仅具有环境友好、可持续性强和成本较低等优势,还为高选择性和高纯度生产提供了可能。本文综述了羟基酪醇的生物合成途径及其合成生物学改造策略,重点介绍了利用各种微生物底盘细胞实现羟基酪醇从头生物合成的研究进展。最后,对未来进一步通过合成生物学手段优化羟基酪醇的生物合成途径进行了展望,以期为羟基酪醇的合成生物制造规模化奠定基础。
汪鑫, 孙涛, 孙美莉, 王凯峰, 纪晓俊. 生物合成功能性食品原料羟基酪醇的研究进展[J]. 生物技术通报, 2025, 41(11): 35-46.
WANG Xin, SUN Tao, SUN Mei-li, WANG Kai-feng, JI Xiao-jun. Advances in the Biosynthesis of Functional Food Ingredient Hydroxytyrosol[J]. Biotechnology Bulletin, 2025, 41(11): 35-46.
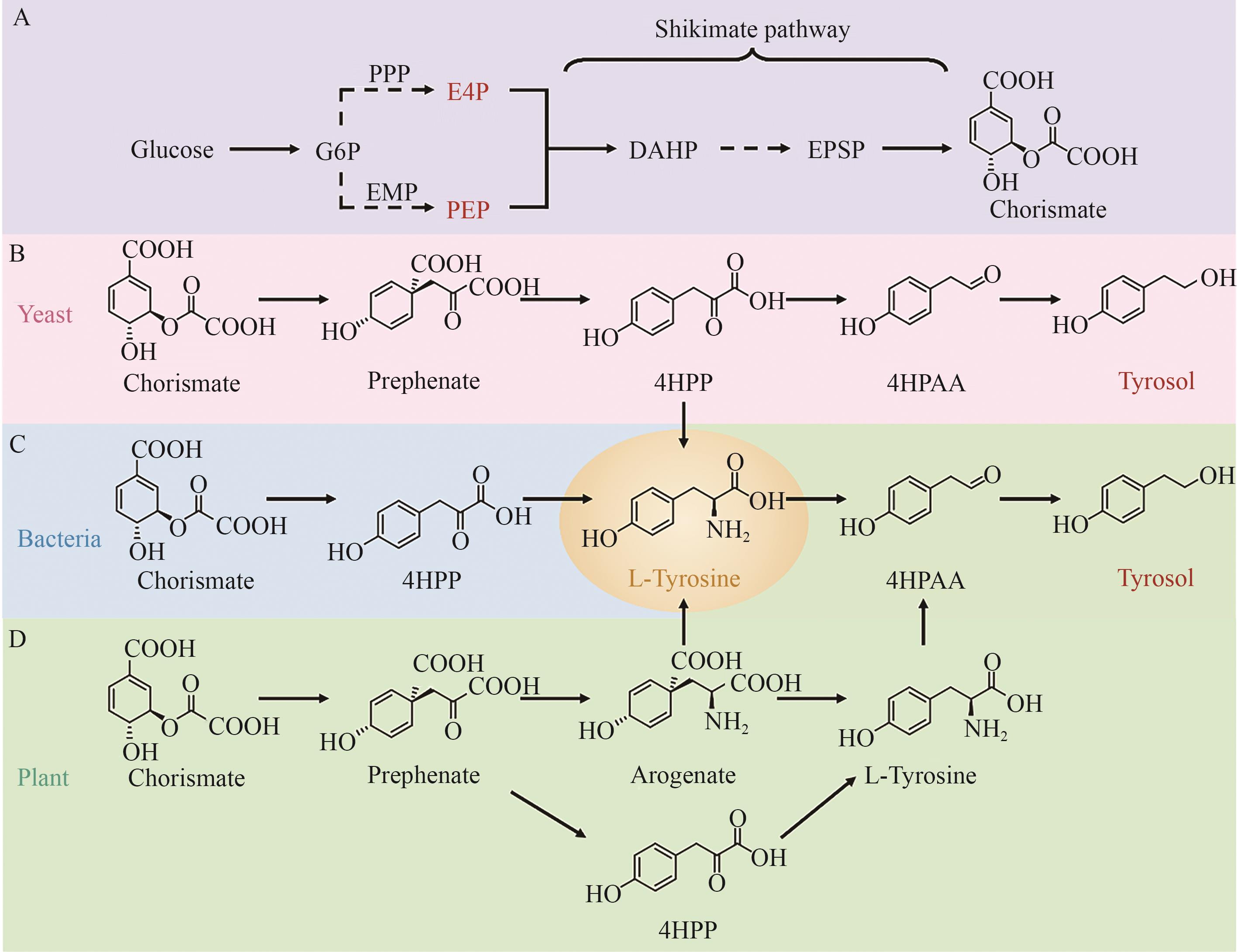
图2 酪醇的生物合成途径A:从葡萄糖到分支酸的生物合成途径;B:酵母中从分支酸到酪醇的合成途径;C:细菌中从分支酸到L-酪氨酸的合成途径;D:植物中从分支酸到酪醇的合成途径。Arogenate:阿罗酸;Chorismate:分支酸;DAHP:3-脱氧-D-阿拉伯庚酮糖-7-磷酸;EPSP:5-烯醇丙酮莽草酸-3-磷酸;E4P:赤藓糖-4-磷酸;Glucose:葡萄糖;G6P:葡萄糖-6-磷酸;Tyrosol:酪醇;L-Tyrosine:L-酪氨酸;PEP:磷酸烯醇式丙酮酸;Prephenate:预苯酸;4HPAA:4-羟基苯乙醛;4HPP:4-羟基苯丙酮酸
Fig. 2 Biosynthetic pathways of tyrosolA: Biosynthetic pathway from glucose to chorismite. B: Tyrosol biosynthesis from chorismate in yeast. C: L-tyrosine biosynthesis from chorismate in bacteria. D: Tyrosol biosynthesis from chorismate in plants. DAHP: 3-deoxy-D-arabinoheptulosonate-7-phosphate, EPSP: 5-enolpyruvylshikimate-3-phosphatesynthase, E4P: erythrose-4-phosphate, G6P: glucose-6-phosphate, PEP: phosphoenolpyruvate, 4HPAA: 2-(4-hydroxyphenyl) acetaldehyde, 4HPP: 4-hydroxyphenylpyruvic acid
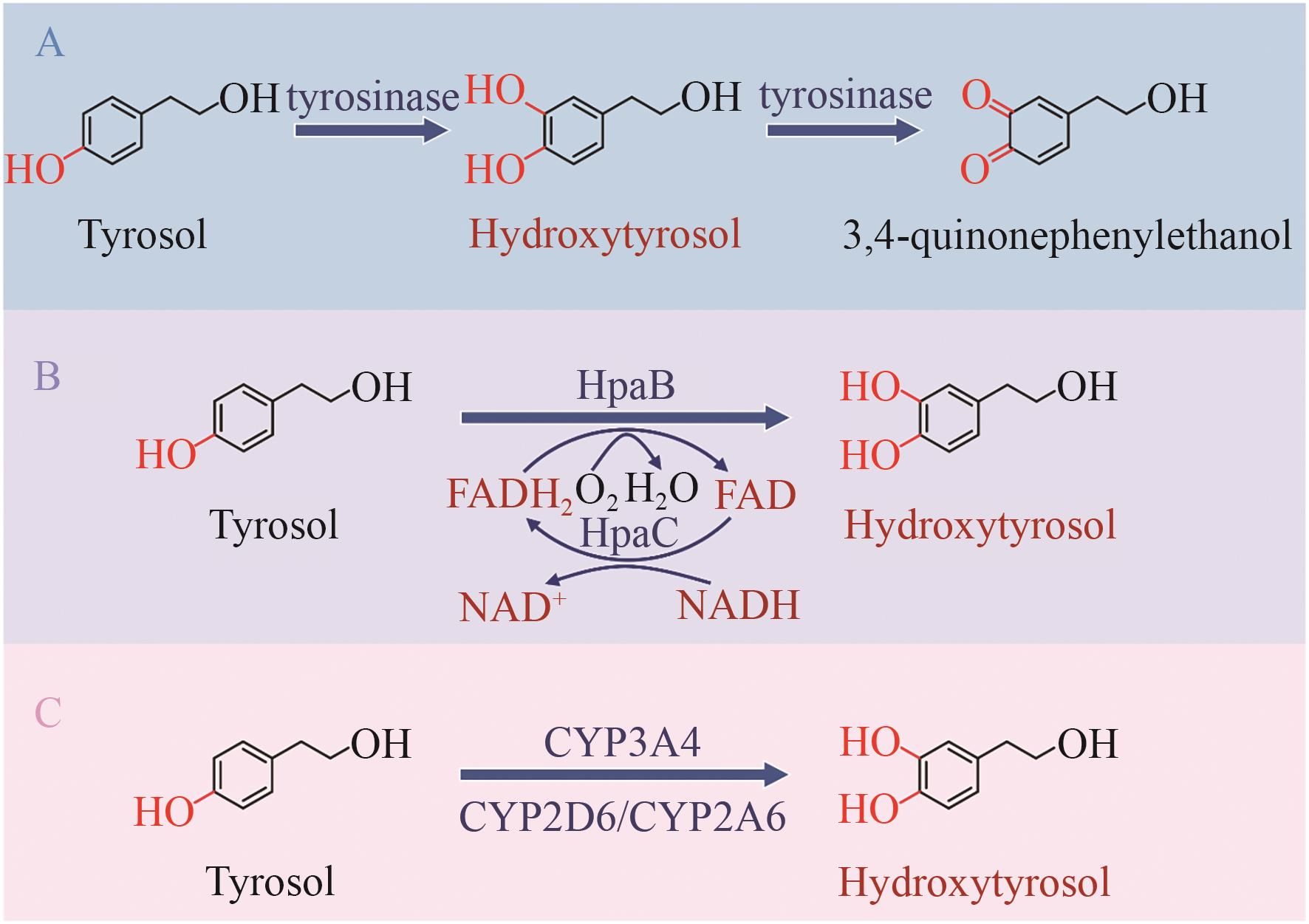
图3 酪醇到羟基酪醇的生物合成途径A:酪醇在酪氨酸酶的催化下生成羟基酪醇并进一步氧化为3,4-醌-苯乙醇;B:酪醇在双组分多酚氧化酶催化下生成羟基酪醇;C:酪醇在细胞色素P450酶的催化下生成羟基酪醇。CYP3A4/CYP2D6/CYP2A6:细胞色素P450酶;HpaBC:双组分核黄素依赖的单加氧酶;Hydroxytyrosol:羟基酪醇;tyrosinase;酪氨酸酶;Tyrosol:酪醇;3,4-quinonephenylethanol:3,4-醌-苯乙醇
Fig. 3 Biosynthetic pathway from tyrosol to hydroxytyrosolA: Tyrosol is catalyzed by tyrosinase to form hydroxytyrosol, which is further oxidized to 3,4-quinonephenylethanol. B: Tyrosol is converted to hydroxytyrosol via 4-hydroxyphenylacetate 3-hydroxylase. C: Tyrosol is converted to hydroxytyrosol via cytochrome P450 enzymes. CYP3A4/CYP2D6/CYP2A6: Cytochrome P450 enzymes; HpaBC: 4-hydroxyphenylacetate 3-hydroxylase
菌株 Strain | 底物 Substrate | 产量 Titer | 规模 Scale | 合成生物学策略 Metabolic engineering strategies | 参考文献Reference |
|---|---|---|---|---|---|
大肠杆菌 E. coli | 葡萄糖 | 0.08 mmol/L | 摇瓶 | 异源表达鼠来源的TH、人类来源的PCD和DHPR、猪来源的DDC、藤黄微球菌来源的TYO,敲除FEAB | [ |
| 葡萄糖 | 208.00 mg/L | 摇瓶 | 异源表达欧芹来源的AAS,过表达HPABC,敲除TYRR、PHEA和FEAB | [ | |
| 葡萄糖 | 268.30 mg/L | 摇瓶 | 异源表达罂粟来源的TDC和藤黄微球菌来源的TYO,过表达HPABC、AROGfbr 和TYRAfbr,敲除TYRR、 PHEA和FEAB | [ | |
| 甘油,葡萄糖 | (647±35)mg/L | 摇瓶 | 异源表达酿酒酵母来源的ARO10,过表达HPABC和ADH6,敲除FEAB | [ | |
| 葡萄糖 | 270.80 mg/L | 摇瓶 | 异源表达欧芹来源的AAS,青枯雷尔氏菌来源的TYR,过表达ALR-K、 AROA、 AROB、 AROC、 AROD、 AROE、 AROG、 AROL、 PPSA、 TKTA、 TYRA、 TYRB和YDIB | [ | |
| 葡萄糖 | 1.60 g/L | 摇瓶 | 异源表达酿酒酵母来源的ARO10D331C/V,过表达HPABC,敲除FEAB、PHEA、TYRB和TYRR | [ | |
| 葡萄糖 | 8.80 g/L | 3-L发酵罐 | 异源表达巴西固氮螺菌来源的IPDC,过表达AROGfbr 、 PPSA、 TKTA、 AROALC、AROEDB、 TYRAfbr、 YAHK、 HPABC,敲除FEAB、 PYKA、 PHEA、TYRB | [ | |
| 甘油 | 9.87 g/L | 5-L发酵罐 | 异源表达酿酒酵母来源的ARO10和ADH6和奇异变形杆菌来源的LAAD,过表达HPABC、 AROGfbr 、 TYRC,敲除PTSG、 CRR、 PHEA、 TYRR | [ | |
| 葡萄糖 | 4.97 g/L | 5-L发酵罐 | 异源表达解脂耶氏酵母来源的ARO10和PAR4,过表达AROGD146N、 TYRAM53I/A354R 、 HPABC、 GLF、 GLK,敲除PTSG、 PYKA、 MHPB、 PHEA和TYRB | [ | |
酿酒酵母 S. cerevisiae | 蔗糖,甘油 | 308.65 mg/L | 摇瓶 | 异源表达铜绿假单胞菌来源的HPAA/C、欧芹来源的AAS和短双歧杆菌来源的XFPK,过表达ARO4K229L 和ARO7G141S | [ |
| 葡萄糖 | 375.00 mg/L | 摇瓶 | 异源表达大肠杆菌来源的HPABC,过表达ARO4K229L | [ | |
| 葡萄糖 | 6.97 g/L | 5-L发酵罐 | 异源表达铜绿假单胞菌来源的HPAB、大肠杆菌来源的HPAC和TYRAM53I/A354V,过表达ARO3D154N、 ARO4K229L 、 ARO7G141S 、 ARO2、 TKL1、RKL1、 ARO10,敲除PDC1、 PHA2,下调TRP2 | [ | |
| 葡萄糖 | 639.84 mg/L | 摇瓶 | 异源表达大肠杆菌来源的TYRAM53I/A354V 、枯草芽胞杆菌来源的RIBBA、欧芹来源的AAS和短双歧杆菌来源的BbXFPKopt,过表达ARO4K229L 和ARO7G141S,敲除PDC1、 PHA2 | [ | |
酿酒酵母-大肠杆菌 S. cerevisiae- E. coli | 蔗糖 | 435.32 mg/L | 摇瓶 | 酿酒酵母:异源表达欧芹来源的AAS和短双歧杆菌来源的BbXFPKopt,过表达ARO4K229L 、 ARO7G141S;大肠杆菌:过表达HPABC | [ |
地衣芽胞杆菌 B. licheniformis | 葡萄糖 | 9.48 g/L | 5-L发酵罐 | 异源表达大肠杆菌来源的HPABC和乳酸杆菌来源的KIVD,过表达GLCU、 GLCK、 ZWF、 TKT、 AROGfbr 、 AROK、 TYRAfbr、 YUGJ,敲除PYK、 DHBC、 PHEA、 HISG、 DHAS和ADHA | [ |
表1 微生物从头合成羟基酪醇的研究进展
Table 1 Research progress on de novo synthesis of hydroxytyrosol by microorganisms
菌株 Strain | 底物 Substrate | 产量 Titer | 规模 Scale | 合成生物学策略 Metabolic engineering strategies | 参考文献Reference |
|---|---|---|---|---|---|
大肠杆菌 E. coli | 葡萄糖 | 0.08 mmol/L | 摇瓶 | 异源表达鼠来源的TH、人类来源的PCD和DHPR、猪来源的DDC、藤黄微球菌来源的TYO,敲除FEAB | [ |
| 葡萄糖 | 208.00 mg/L | 摇瓶 | 异源表达欧芹来源的AAS,过表达HPABC,敲除TYRR、PHEA和FEAB | [ | |
| 葡萄糖 | 268.30 mg/L | 摇瓶 | 异源表达罂粟来源的TDC和藤黄微球菌来源的TYO,过表达HPABC、AROGfbr 和TYRAfbr,敲除TYRR、 PHEA和FEAB | [ | |
| 甘油,葡萄糖 | (647±35)mg/L | 摇瓶 | 异源表达酿酒酵母来源的ARO10,过表达HPABC和ADH6,敲除FEAB | [ | |
| 葡萄糖 | 270.80 mg/L | 摇瓶 | 异源表达欧芹来源的AAS,青枯雷尔氏菌来源的TYR,过表达ALR-K、 AROA、 AROB、 AROC、 AROD、 AROE、 AROG、 AROL、 PPSA、 TKTA、 TYRA、 TYRB和YDIB | [ | |
| 葡萄糖 | 1.60 g/L | 摇瓶 | 异源表达酿酒酵母来源的ARO10D331C/V,过表达HPABC,敲除FEAB、PHEA、TYRB和TYRR | [ | |
| 葡萄糖 | 8.80 g/L | 3-L发酵罐 | 异源表达巴西固氮螺菌来源的IPDC,过表达AROGfbr 、 PPSA、 TKTA、 AROALC、AROEDB、 TYRAfbr、 YAHK、 HPABC,敲除FEAB、 PYKA、 PHEA、TYRB | [ | |
| 甘油 | 9.87 g/L | 5-L发酵罐 | 异源表达酿酒酵母来源的ARO10和ADH6和奇异变形杆菌来源的LAAD,过表达HPABC、 AROGfbr 、 TYRC,敲除PTSG、 CRR、 PHEA、 TYRR | [ | |
| 葡萄糖 | 4.97 g/L | 5-L发酵罐 | 异源表达解脂耶氏酵母来源的ARO10和PAR4,过表达AROGD146N、 TYRAM53I/A354R 、 HPABC、 GLF、 GLK,敲除PTSG、 PYKA、 MHPB、 PHEA和TYRB | [ | |
酿酒酵母 S. cerevisiae | 蔗糖,甘油 | 308.65 mg/L | 摇瓶 | 异源表达铜绿假单胞菌来源的HPAA/C、欧芹来源的AAS和短双歧杆菌来源的XFPK,过表达ARO4K229L 和ARO7G141S | [ |
| 葡萄糖 | 375.00 mg/L | 摇瓶 | 异源表达大肠杆菌来源的HPABC,过表达ARO4K229L | [ | |
| 葡萄糖 | 6.97 g/L | 5-L发酵罐 | 异源表达铜绿假单胞菌来源的HPAB、大肠杆菌来源的HPAC和TYRAM53I/A354V,过表达ARO3D154N、 ARO4K229L 、 ARO7G141S 、 ARO2、 TKL1、RKL1、 ARO10,敲除PDC1、 PHA2,下调TRP2 | [ | |
| 葡萄糖 | 639.84 mg/L | 摇瓶 | 异源表达大肠杆菌来源的TYRAM53I/A354V 、枯草芽胞杆菌来源的RIBBA、欧芹来源的AAS和短双歧杆菌来源的BbXFPKopt,过表达ARO4K229L 和ARO7G141S,敲除PDC1、 PHA2 | [ | |
酿酒酵母-大肠杆菌 S. cerevisiae- E. coli | 蔗糖 | 435.32 mg/L | 摇瓶 | 酿酒酵母:异源表达欧芹来源的AAS和短双歧杆菌来源的BbXFPKopt,过表达ARO4K229L 、 ARO7G141S;大肠杆菌:过表达HPABC | [ |
地衣芽胞杆菌 B. licheniformis | 葡萄糖 | 9.48 g/L | 5-L发酵罐 | 异源表达大肠杆菌来源的HPABC和乳酸杆菌来源的KIVD,过表达GLCU、 GLCK、 ZWF、 TKT、 AROGfbr 、 AROK、 TYRAfbr、 YUGJ,敲除PYK、 DHBC、 PHEA、 HISG、 DHAS和ADHA | [ |
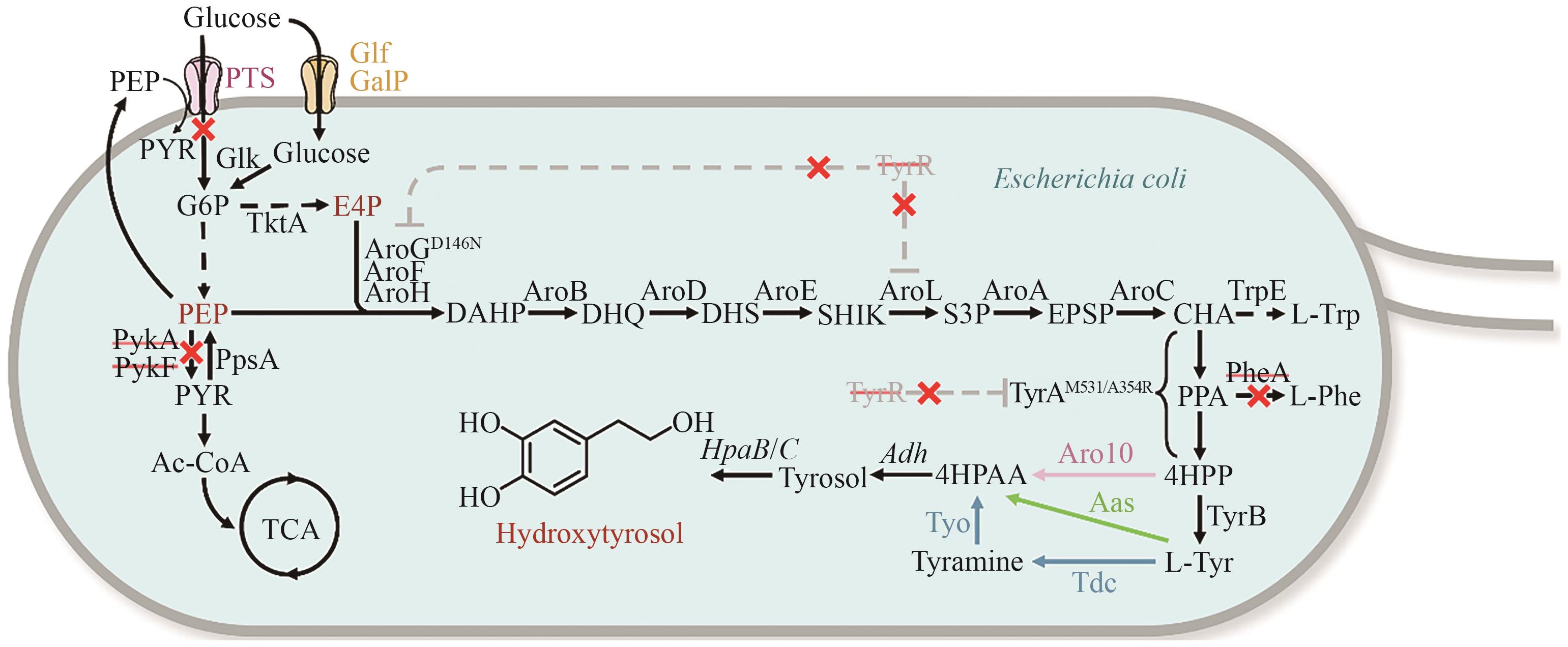
图4 大肠杆菌中羟基酪醇的合成途径黑色虚线代表多步反应;灰色虚线代表反馈抑制;彩色实线代表异源酶催化步骤;红×代表敲除。Ac-CoA:乙酰辅酶A;CHA:分支酸;DAHP:3-脱氧-D-阿拉伯庚酮糖-7-磷酸;DHQ:3-脱氢奎尼酸;DHS:3-脱氢莽草酸;EPSP:5-烯醇式丙酮酸莽草酸-3-磷酸;E4P:赤藓糖-4磷酸;Glucose:葡萄糖;G6P:6-磷酸葡萄糖;Hydroxytyrosol:羟基酪醇;L-Trp:L-色氨酸;L-Tyr:L-酪氨酸;L-Phe:L-苯丙氨酸;PEP:磷酸烯醇式丙酮酸;PPA:预苯酸;PYR:丙酮酸;SHIK:莽草酸;S3P:莽草酸-3-磷酸;Tyramine:酪胺;Tyrosol:酪醇;4HPAA:4-羟基苯乙醛;4HPP:4-羟基苯丙酮酸
Fig. 4 Synthetic pathway for hydroxytyrosol production in Escherichia coliBlack dashed lines indicate multi-step reactions. Gray dashed lines indicate feedback inhibition. Colored solid lines denote heterologous enzyme-catalyzed steps. Red × indicates gene knockouts. DAHP: 3-deoxy-D-arabinoheptulosonate-7-phosphate, DHQ: 3-dehydroquinate, DHS: 3-dehydroshikimate, EPSP: 5-enolpyruvylshikimate-3-phosphate, E4P: erythrose-4-phosphate, G6P: glucose-6-phosphate, L-Trp: L-tryptophan, L-Tyr: L-Tyrosine, L-Phe: L-phenylalanine, PEP: phosphoenolpyruvate, PPA: prephenate, PYR: pyruvate, SHIK: shikimate,S3P: shikimate-3-phosphate, 4HPAA: 2-(4-hydroxyphenyl) acetaldehyde, 4HPP: 4-hydroxyphenylpyruvic acid
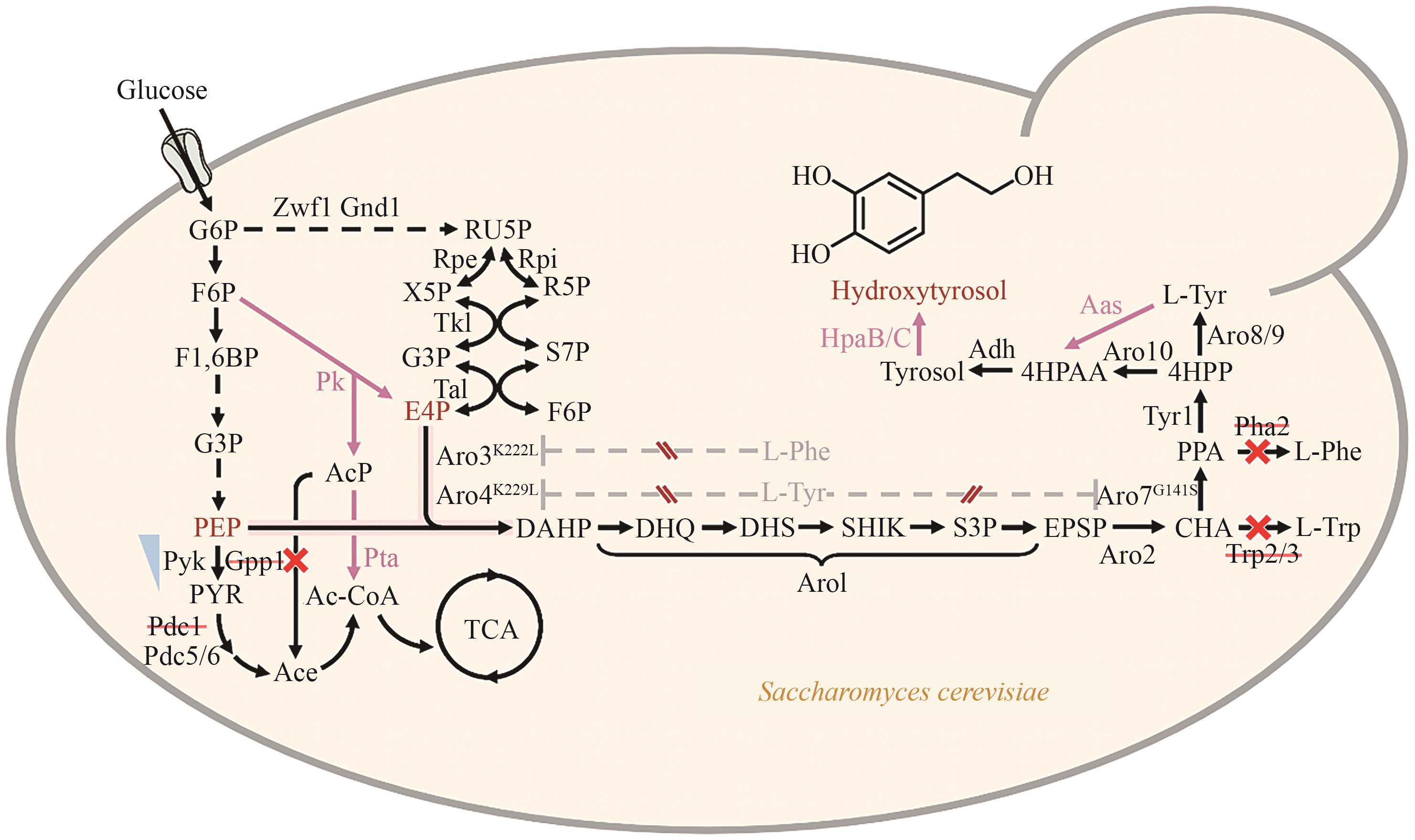
图5 酿酒酵母中羟基酪醇的合成途径黑色虚线代表多步反应;灰色虚线代表反馈抑制;红色实线代表异源酶催化步骤;蓝色倒三角代表下调表达;红×代表敲除。Ac-CoA:乙酰辅酶A;Ace:乙酸盐;AcP:乙酰磷酸;CHA:分支酸;DAHP:3-脱氧-D-阿拉伯庚酮糖-7-磷酸;DHQ:3-脱氢奎宁;DHS:3-脱氢莽草酸盐;EPSP:5-烯醇式丙酮酸莽草酸-3-磷酸;E4P:赤藓糖-4磷酸;F1,6BP:果糖-1,6-二磷酸;F6P:果糖-6-磷酸;Glucose:葡萄糖;G3P:甘油醛-3-磷酸;G6P:6-磷酸葡萄糖;Hydroxytyrosol:羟基酪醇;L-Trp:L-色氨酸;L-Tyr:L-酪氨酸;L-Phe:L-苯丙氨酸;PEP:磷酸烯醇式丙酮酸;PPA:预苯酸;PYR:丙酮酸;RU5P:核酮糖-5-磷酸;R5P:核糖-5-磷酸;SHIK:莽草酸;S3P:莽草酸-3-磷酸;S7P:景天庚酮糖-7-磷酸;Tyramine:酪胺;Tyrosol:酪醇;X5P:木酮糖-5-磷酸;4HPAA:4-羟基苯乙醛;4HPP:4-羟基苯丙酮酸
Fig. 5 Synthetic pathway for hydroxytyrosol production in Saccharomyces cerevisiaeBlack dashed lines indicate multi-step reactions. Gray dashed lines indicate feedback inhibition. Red solid lines denote heterologous enzyme-catalyzed steps. Blue inverted triangles indicate downregulation of gene expression. Red × indicates gene knockouts. Ace: acetate, AcP: acetyl phosphate, CHA: chorismate, DAHP: 3-deoxy-D-arabinoheptulosonate-7-phosphate, DHQ: 3-dehydroquinate, DHS: 3-dehydroshikimate, EPSP: 5-enolpyruvylshikimate-3-phosphate, E4P: erythrose-4-phosphate, F1,6BP: fructose-1,6-bisphosphate, F6P: fructose-6-phosphate. G3P: glyceraldehyde-3-phosphate, G6P: glucose-6-phosphate, L-Trp: L-Tryptophan, L-Tyr: L-Tyrosine, L-Phe: L-Phenylalanine, PEP: phosphoenolpyruvate, PPA: prephenate, PYR: pyruvate, RU5P: ribulose-5-phosphate, R5P: ribose-5-phosphate, SHIK: shikimate, S3P: shikimate-3-phosphate, S7P: sedoheptulose-7-phosphate, X5P: xylulose-5-phosphate, 4HPAA: 2-(4-hydroxyphenyl) acetaldehyde, 4HPP: 4-hydroxyphenylpyruvic acid
| [1] | Gallardo-Fernández M, Gonzalez-Ramirez M, Cerezo AB, et al. Hydroxytyrosol in foods: analysis, food sources, EU dietary intake, and potential uses [J]. Foods, 2022, 11(15): 2355. |
| [2] | Gonzalez-Ramirez M, Gallardo-Fernandez M, Cerezo AB, et al. The production of bioactive hydroxytyrosol in fermented beverages: the role of must composition and a genetically modified yeast strain [J]. Fermentation, 2024, 10(4): 198. |
| [3] | Mastralexi A, Nenadis N, Tsimidou MZ. Addressing analytical requirements to support health claims on “olive oil polyphenols” (EC Regulation 432/2012) [J]. J Agric Food Chem, 2014, 62(12): 2459-2461. |
| [4] | Visioli F, Poli A, Gall C. Antioxidant and other biological activities of phenols from olives and olive oil [J]. Med Res Rev, 2002, 22(1): 65-75. |
| [5] | Servili M, Rizzello CG, Taticchi A, et al. Functional milk beverage fortified with phenolic compounds extracted from olive vegetation water, and fermented with functional lactic acid bacteria [J]. Int J Food Microbiol, 2011, 147(1): 45-52. |
| [6] | Bañares C, Martin D, Reglero G, et al. Protective effect of hydroxytyrosol and rosemary extract in a comparative study of the oxidative stability of Echium oil [J]. Food Chem, 2019, 290: 316-323. |
| [7] | Raposo R, Ruiz-Moreno MJ, Garde-Cerdán T, et al. Effect of hydroxytyrosol on quality of sulfur dioxide-free red wine [J]. Food Chem, 2016, 192: 25-33. |
| [8] | Medina-Martínez MS, Truchado P, Castro-Ibáñez I, et al. Antimicrobial activity of hydroxytyrosol: a current controversy [J]. Biosci Biotechnol Biochem, 2016, 80(4): 801-810. |
| [9] | Bedoya LM, Beltrán M, Obregón-Calderón P, et al. Hydroxytyrosol: a new class of microbicide displaying broad anti-HIV-1 activity [J]. AIDS, 2016, 30(18): 2767-2776. |
| [10] | Chaves-López C, Serio A, Mazzarrino G, et al. Control of household mycoflora in fermented sausages using phenolic fractions from olive mill wastewaters [J]. Int J Food Microbiol, 2015, 207: 49-56. |
| [11] | Bertelli M, Kiani AK, Paolacci S, et al. Hydroxytyrosol: a natural compound with promising pharmacological activities [J]. J Biotechnol, 2020, 309: 29-33. |
| [12] | Kalogerakis N, Politi M, Foteinis S, et al. Recovery of antioxidants from olive mill wastewaters: a viable solution that promotes their overall sustainable management [J]. J Environ Manag, 2013, 128: 749-758. |
| [13] | Ziosi P, Paolucci C, Santarelli F, et al. A two-step process for the synthesis of hydroxytyrosol [J]. ChemSusChem, 2018, 11(13): 2202-2210. |
| [14] | Bovicelli P, Antonioletti R, Mancini S, et al. Expedient synthesis of hydroxytyrosol and its esters [J]. Synth Commun, 2007, 37(23): 4245-4252. |
| [15] | Tang JL, Wang JY, Gong PF, et al. Biosynthesis and biotechnological synthesis of hydroxytyrosol [J]. Foods, 2024, 13(11): 1694. |
| [16] | Jiang M, Zhang HR. Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli [J]. Curr Opin Biotechnol, 2016, 42: 1-6. |
| [17] | Schenck CA, Maeda HA. Tyrosine biosynthesis, metabolism, and catabolism in plants [J]. Phytochemistry, 2018, 149: 82-102. |
| [18] | Cordente AG, Schmidt S, Beltran G, et al. Harnessing yeast metabolism of aromatic amino acids for fermented beverage bioflavouring and bioproduction [J]. Appl Microbiol Biotechnol, 2019, 103(11): 4325-4336. |
| [19] | Hazelwood LA, Daran JM, van Maris AJA, et al. The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism [J]. Appl Environ Microbiol, 2008, 74(8): 2259-2266. |
| [20] | 孙中贯, 刘琳, 王亚平, 等. 酿酒酵母高级醇代谢研究进展 [J]. 生物工程学报, 2021, 37(2): 429-447. |
| Sun ZG, Liu L, Wang YP, et al. Higher alcohols metabolism by Saccharomyces cerevisiae: a mini review [J]. Chin J Biotechnol, 2021, 37(2): 429-447. | |
| [21] | Nisbet MA, Tobias HJ, Brenna JT, et al. Quantifying the contribution of grape hexoses to wine volatiles by high-precision [U¹³C]-glucose tracer studies [J]. J Agric Food Chem, 2014, 62(28): 6820-6827. |
| [22] | Claus H, Decker H. Bacterial tyrosinases [J]. Syst Appl Microbiol, 2006, 29(1): 3-14. |
| [23] | Olivares C, Solano F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins [J]. Pigment Cell Melanoma Res, 2009, 22(6): 750-760. |
| [24] | Solomon EI, Sundaram UM, Machonkin TE. Multicopper oxidases and oxygenases [J]. Chem Rev, 1996, 96(7): 2563-2606. |
| [25] | Deri-Zenaty B, Bachar S, Rebroš M, et al. A coupled enzymatic reaction of tyrosinase and glucose dehydrogenase for the production of hydroxytyrosol [J]. Appl Microbiol Biotechnol, 2020, 104(11): 4945-4955. |
| [26] | Rodríguez-Morató J, Robledo P, Tanner JA, et al. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol [J]. Food Chem, 2017, 217: 716-725. |
| [27] | Satoh Y, Tajima K, Munekata M, et al. Engineering of l-tyrosine oxidation in Escherichia coli and microbial production of hydroxytyrosol [J]. Metab Eng, 2012, 14(6): 603-610. |
| [28] | Chung D, Kim SY, Ahn JH. Production of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside, using plant genes expressing in Escherichia coli [J]. Sci Rep, 2017, 7(1): 2578. |
| [29] | Choo HJ, Kim EJ, Kim SY, et al. Microbial synthesis of hydroxytyrosol and hydroxysalidroside [J]. Appl Biol Chem, 2018, 61(3): 295-301. |
| [30] | Li XL, Chen ZY, Wu YF, et al. Establishing an artificial pathway for efficient biosynthesis of hydroxytyrosol [J]. ACS Synth Biol, 2018, 7(2): 647-654. |
| [31] | Trantas E, Navakoudis E, Pavlidis T, et al. Dual pathway for metabolic engineering of Escherichia coli to produce the highly valuable hydroxytyrosol [J]. PLoS One, 2019, 14(11): e0212243. |
| [32] | Xia YY, Qi LN, Shi XL, et al. Construction of an Escherichia coli cell factory for de novo synthesis of tyrosol through semi-rational design based on phenylpyruvate decarboxylase ARO10 engineering [J]. Int J Biol Macromol, 2023, 253: 127385. |
| [33] | Koma D, Fujisawa M, Ohashi H, et al. Production of 3-hydroxytyrosol from glucose by chromosomally engineered Escherichia coli by fed-batch cultivation in a jar fermenter [J]. J Agric Food Chem, 2023, 71(24): 9451-9459. |
| [34] | Wang HJ, Wang L, Chen JB, et al. Promoting FADH2 regeneration of hydroxylation for high-level production of hydroxytyrosol from glycerol in Escherichia coli [J]. J Agric Food Chem, 2023, 71(44): 16681-16690. |
| [35] | Chen XC, Qian T, Wei WP, et al. De novo synthesis of tyrosol and hydroxytyrosol through temperature-inducible systems and metabolic engineering [J]. ACS Synth Biol, 2025, 14(6): 2294-2304. |
| [36] | Liu YJ, Liu H, Hu HT, et al. De novo production of hydroxytyrosol by metabolic engineering of Saccharomyces cerevisiae [J]. J Agric Food Chem, 2022, 70(24): 7490-7499. |
| [37] | Bisquert R, Planells-Cárcel A, Valera-García E, et al. Metabolic engineering of Saccharomyces cerevisiae for hydroxytyrosol overproduction directly from glucose [J]. Microb Biotechnol, 2022, 15(5): 1499-1510. |
| [38] | Liu HY, Wu XX, Ma H, et al. High-level production of hydroxytyrosol in engineered Saccharomyces cerevisiae [J]. ACS Synth Biol, 2022, 11(11): 3706-3713. |
| [39] | Liu YJ, Gu BX, Shi JH, et al. Inverse metabolic engineering based on metabonomics for efficient production of hydroxytyrosol by Saccharomyces cerevisiae [J]. Bioresour Technol, 2024, 409: 131187. |
| [40] | Liu YJ, Song D, Hu HT, et al. De novo production of hydroxytyrosol by Saccharomyces cerevisiae-Escherichia coli coculture engineering [J]. ACS Synth Biol, 2022, 11(9): 3067-3077. |
| [41] | Zhan YY, Zhou F, Ruan WQ, et al. Systematic metabolic engineering of Bacillus licheniformis for hyperproduction of the antioxidant hydroxytyrosol [J]. Green Chem, 2023, 25(21): 8718-8729. |
| [42] | Yang D, Park SY, Park YS, et al. Metabolic engineering of Escherichia coli for natural product biosynthesis [J]. Trends Biotechnol, 2020, 38(7): 745-765. |
| [43] | Iacometti C, Marx K, Hönick M, et al. Activating silent glycolysis bypasses in Escherichia coli [J]. Biodes Res, 2022, 2022: 9859643. |
| [44] | Liu XZ, Niu H, Li Q, et al. Metabolic engineering for the production of l-phenylalanine in Escherichia coli [J]. 3 Biotech, 2019, 9(3): 85. |
| [45] | Siedler S, Bringer S, Blank LM, et al. Engineering yield and rate of reductive biotransformation in Escherichia coli by partial cyclization of the pentose phosphate pathway and PTS-independent glucose transport [J]. Appl Microbiol Biotechnol, 2012, 93(4): 1459-1467. |
| [46] | Liu YF, Xu YR, Ding DQ, et al. Genetic engineering of Escherichia coli to improve L-phenylalanine production [J]. BMC Biotechnol, 2018, 18(1): 5. |
| [47] | Chandran SS, Yi J, Draths KM, et al. Phosphoenolpyruvate availability and the biosynthesis of shikimic acid [J]. Biotechnol Prog, 2003, 19(3): 808-814. |
| [48] | 盛华康, 张博, 申晓林, 等. 微生物合成白藜芦醇及其衍生物[J]. 化工进展, 2025, 44(5): 2463-2474. |
| Sheng HK, Zhang B, Shen XL, et al. Microbial synthesis of resveratrol and its derivatives [J]. Chem Ind Eng Prog, 2025, 44(5): 2463-2474. | |
| [49] | Koma D, Kishida T, Yoshida E, et al. Chromosome engineering to generate plasmid-free phenylalanine- and tyrosine-overproducing Escherichia coli strains that can be applied in the generation of aromatic-compound-producing bacteria [J]. Appl Environ Microbiol, 2020, 86(14): e00525-20. |
| [50] | 江晶洁, 刘涛, 林双君. 基于莽草酸途径微生物合成芳香族化合物及其衍生物的研究进展 [J]. 生命科学, 2019, 31(5): 430-448. |
| Jiang JJ, Liu T, Lin SJ. Research progress on the biosynthesis of aromatic compounds by microorganisms [J]. Chin Bull Life Sci, 2019, 31(5): 430-448. | |
| [51] | Cui D, Deng AH, Bai H, et al. Molecular basis for feedback inhibition of tyrosine-regulated 3-deoxy-d-arabino-heptulosonate-7-phosphate synthase from Escherichia coli [J]. J Struct Biol, 2019, 206(3): 322-334. |
| [52] | Rodriguez A, Martínez JA, Flores N, et al. Engineering Escherichia coli to overproduce aromatic amino acids and derived compounds [J]. Microb Cell Fact, 2014, 13(1): 126. |
| [53] | Pittard J, Camakaris H, Yang J. The TyrR regulon [J]. Mol Microbiol, 2005, 55(1): 16-26. |
| [54] | Bai YF, Bi HP, Zhuang YB, et al. Production of salidroside in metabolically engineered Escherichia coli [J]. Sci Rep, 2014, 4: 6640. |
| [55] | Satoh Y, Tajima K, Munekata M, et al. Engineering of a tyrosol-producing pathway, utilizing simple sugar and the central metabolic tyrosine, in Escherichia coli [J]. J Agric Food Chem, 2012, 60(4): 979-984. |
| [56] | Chen W, Yao J, Meng J, et al. Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis [J]. Nat Commun, 2019, 10(1): 960. |
| [57] | Kaminaga Y, Schnepp J, Peel G, et al. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation [J]. J Biol Chem, 2006, 281(33): 23357-23366. |
| [58] | Mermigka G, Vavouraki AI, Nikolaou C, et al. An engineered plant metabolic pathway results in high yields of hydroxytyrosol due to a modified whole-cell biocatalysis in bioreactor [J]. Metabolites, 2023, 13(11): 1126. |
| [59] | Zhao Y, Coelho C, Lauer S, et al. CREEPY: CRISPR-mediated editing of synthetic episomes in yeast [J]. Nucleic Acids Res, 2023, 51(13): e72. |
| [60] | Lian JZ, Mishra S, Zhao HM. Recent advances in metabolic engineering of Saccharomyces cerevisiae: New tools and their applications [J]. Metab Eng, 2018, 50: 85-108. |
| [61] | Borodina I, Nielsen J. Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals [J]. Biotechnol J, 2014, 9(5): 609-620. |
| [62] | Suástegui M, Guo WH, Feng XY, et al. Investigating strain dependency in the production of aromatic compounds in Saccharomyces cerevisiae [J]. Biotechnol Bioeng, 2016, 113(12): 2676-2685. |
| [63] | Paravicini G, Schmidheini T, Braus G. Purification and properties of the 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase (phenylalanine-inhibitable) of Saccharomyces cerevisiae [J]. Eur J Biochem, 1989, 186(1-2): 361-366. |
| [64] | Schnappauf G, Hartmann M, Künzler M, et al. The two 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase isoenzymes from Saccharomyces cerevisiae show different kinetic modes of inhibition [J]. Arch Microbiol, 1998, 169(6): 517-524. |
| [65] | Yang SL, Pan XW, You JJ, et al. Systematic metabolic engineering of Yarrowia lipolytica for the enhanced production of erythritol [J]. Bioresour Technol, 2024, 391: 129918. |
| [66] | Suástegui M, Yu Ng C, Chowdhury A, et al. Multilevel engineering of the upstream module of aromatic amino acid biosynthesis in Saccharomyces cerevisiae for high production of polymer and drug precursors [J]. Metab Eng, 2017, 42: 134-144. |
| [67] | Deaner M, Alper HS. Systematic testing of enzyme perturbation sensitivities via graded dCas9 modulation in Saccharomyces cerevisiae [J]. Metab Eng, 2017, 40: 14-22. |
| [68] | Liu QL, Yu T, Li XW, et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals [J]. Nat Commun, 2019, 10(1): 4976. |
| [69] | Castaño-Cerezo S, Pastor JM, Renilla S, et al. An insight into the role of phosphotransacetylase (pta) and the acetate/acetyl-CoA node in Escherichia coli [J]. Microb Cell Fact, 2009, 8: 54. |
| [70] | Guo W, Huang QL, Feng YH, et al. Rewiring central carbon metabolism for tyrosol and salidroside production in Saccharomyces cerevisiae [J]. Biotechnol Bioeng, 2020, 117(8): 2410-2419. |
| [71] | Song N, Xia HL, Xie YR, et al. Semi-rational design and modification of phosphoketolase to improve the yield of tyrosol in Saccharomyces cerevisiae [J]. Synth Syst Biotechnol, 2025, 10(1): 294-306. |
| [72] | Gold ND, Gowen CM, Lussier FX, et al. Metabolic engineering of a tyrosine-overproducing yeast platform using targeted metabolomics [J]. Microb Cell Fact, 2015, 14: 73. |
| [73] | Brochado AR, Matos C, Møller BL, et al. Improved vanillin production in baker’s yeast through in silico design [J]. Microb Cell Fact, 2010, 9: 84. |
| [74] | Remize F, Andrieu E, Dequin S. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation [J]. Appl Environ Microbiol, 2000, 66(8): 3151-3159. |
| [75] | Guo W, Huang QL, Liu H, et al. Rational engineering of chorismate-related pathways in Saccharomyces cerevisiae for improving tyrosol production [J]. Front Bioeng Biotechnol, 2019, 7: 152. |
| [76] | Sträter N, Schnappauf G, Braus G, et al. Mechanisms of catalysis and allosteric regulation of yeast chorismate mutase from crystal structures [J]. Structure, 1997, 5(11): 1437-1452. |
| [77] | Brückner C, Oreb M, Kunze G, et al. An expanded enzyme toolbox for production of cis, cis-muconic acid and other shikimate pathway derivatives in Saccharomyces cerevisiae [J]. FEMS Yeast Res, 2018, 18(2). DOI: 10.1093/femsyr/foy017 . |
| [78] | Strater N, Hakansson K, Schnappauf G, et al. Crystal structure of the T state of allosteric yeast chorismate mutase and comparison with the R state [J]. Proc Natl Acad Sci USA, 1996, 93(8): 3330-3334. |
| [79] | Luttik MAH, Vuralhan Z, Suir E, et al. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: Quantification of metabolic impact [J]. Metab Eng, 2008, 10(3/4): 141-153. |
| [80] | Liu HY, Xiao QJ, Wu XX, et al. Mechanistic investigation of a D to N mutation in DAHP synthase that dictates carbon flux into the shikimate pathway in yeast [J]. Commun Chem, 2023, 6(1): 152. |
| [81] | Kallscheuer N, Menezes R, Foito A, et al. Identification and microbial production of the raspberry phenol salidroside that is active against Huntington’s disease [J]. Plant Physiol, 2019, 179(3): 969-985. |
| [82] | Rodriguez A, Kildegaard KR, Li MJ, et al. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis [J]. Metab Eng, 2015, 31: 181-188. |
| [83] | Anissi J, Sendide K, Ouardaoui A, et al. Production of hydroxytyrosol from hydroxylation of tyrosol by Rhodococcus pyridinivorans 3HYL DSM109178 [J]. Biocatal Biotransform, 2021, 39(6): 418-428. |
| [84] | Gu Y, Ma JB, Zhu YL, et al. Engineering Yarrowia lipolytica as a chassis for De novo synthesis of five aromatic-derived natural products and chemicals [J]. ACS Synth Biol, 2020, 9(8): 2096-2106. |
| [85] | Gu Y, Ma JB, Zhu YL, et al. Refactoring Ehrlich pathway for high-yield 2-phenylethanol production in Yarrowia lipolytica [J]. ACS Synth Biol, 2020, 9(3): 623-633. |
| [86] | Guan AL, He ZX, Wang X, et al. Engineering the next-generation synthetic cell factory driven by protein engineering [J]. Biotechnol Adv, 2024, 73: 108366. |
| [87] | Chen ZY, Wu T, Yu SZ, et al. Self-assembly systems to troubleshoot metabolic engineering challenges [J]. Trends Biotechnol, 2024, 42(1): 43-60. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||