








生物技术通报 ›› 2026, Vol. 42 ›› Issue (1): 271-278.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0944
张悦1,2( ), 任倩1,2(
), 任倩1,2( ), 栾雅梦3, 李翌琛1, 孙无瑕1, 任姝颖1, 许小洁1,2(
), 栾雅梦3, 李翌琛1, 孙无瑕1, 任姝颖1, 许小洁1,2( ), 孙晓辉3(
), 孙晓辉3( )
)
收稿日期:2025-09-03
出版日期:2026-01-26
发布日期:2026-02-04
通讯作者:
许小洁,讲师,研究方向 :植物病毒学;E-mail: xiaojiexua105@163.com;作者简介:张悦,硕士研究生,研究方向 :植物病毒学;E-mail: z17763388238@163.com基金资助:
ZHANG Yue1,2( ), REN Qian1,2(
), REN Qian1,2( ), LUAN Ya-meng3, LI Yi-chen1, SUN Wu-xia1, REN Shu-ying1, XU Xiao-jie1,2(
), LUAN Ya-meng3, LI Yi-chen1, SUN Wu-xia1, REN Shu-ying1, XU Xiao-jie1,2( ), SUN Xiao-hui3(
), SUN Xiao-hui3( )
)
Received:2025-09-03
Published:2026-01-26
Online:2026-02-04
摘要:
目的 通过研究辅助成分-蛋白酶(helper component-proteinase, HC-Pro)中第364位保守的赖氨酸(lysine, K364)突变对小西葫芦黄花叶病毒(zucchini yellow mosaic virus, ZYMV)致病力的影响,构建ZYMV弱毒突变体,并评估其遗传稳定性和交叉保护防治效果,为利用弱毒株开发针对ZYMV的绿色防治策略提供理论依据。 方法 利用定点突变的方法,将HC-Pro中K364位点替换为天冬氨酸(aspartic acid, D),研究K364D突变对ZYMV致病力和HC-Pro RNA沉默抑制活性的影响,构建弱毒突变体ZYMV-HC-ProK364D。通过继代接种实验,测试弱毒突变体ZYMV-HC-ProK364D的遗传稳定性;通过交叉保护实验,评估弱毒突变体ZYMV-HC-ProK364D的交叉保护防治作用。 结果 HC-Pro中K364D突变影响了ZYMV致病力,减轻了病毒在甜瓜(Cucumis melo)植株上引起的典型黄化症状,显著降低了病毒积累水平,同时,该突变削弱了ZYMV HC-Pro的RNA沉默抑制活性。ZYMV-HC-ProK364D-GFP突变体在甜瓜植株中连续3次继代接种后未发生回复突变,保持遗传稳定性。在交叉保护实验中,ZYMV-HC-ProK364D突变体在保护间隔期11 d时,可诱导甜瓜植株产生对野生型ZYMV的显著抗性。 结论 HC-Pro中保守的K364残基是影响ZYMV致病力和HC-Pro RNA沉默抑制活性的关键位点,ZYMV-HC-ProK364D突变体在甜瓜中具有良好的交叉保护作用,可作为弱毒疫苗防治甜瓜ZYMV侵染。
张悦, 任倩, 栾雅梦, 李翌琛, 孙无瑕, 任姝颖, 许小洁, 孙晓辉. 小西葫芦黄花叶病毒弱毒疫苗的构建及其交叉保护防治作用[J]. 生物技术通报, 2026, 42(1): 271-278.
ZHANG Yue, REN Qian, LUAN Ya-meng, LI Yi-chen, SUN Wu-xia, REN Shu-ying, XU Xiao-jie, SUN Xiao-hui. Construction of a Mild Vaccine of Zucchini Yellow Mosaic Virus and Its Cross-protective Efficacy[J]. Biotechnology Bulletin, 2026, 42(1): 271-278.
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|
| ZYMV-HC-ProK364D-F | ATTTCACC |
| ZYMV-HC-ProK364D-R | CGAATCAT |
| ZYMV-HC-Pro-F | GCGCTCTAGCAAGGCTATG |
| ZYMV-HC-Pro-R | CACCTAGTATGTATGCTGCAGT |
| GFP-F | ATGAGTAAAGGAGAAGAAC |
| GFP-R | TTTGTAGAGCTCATCCATG |
| GFP-qRT-F | GTGGAGAGGGTGAAGGTGAT |
| GFP-qRT-R | CGGATAACGGGAAAAGCATTGA |
| actin-qRT-F | CTGATGAAGATACTCACAGAAAGAG |
| actin-qRT-R | CAGGATACGGGGAGCTAATG |
| EF1α-qRT-F | CCACGAGTCTCTCCCAGAAG |
| EF1α-qRT-R | CACGCTTGAGATCCTTGACA |
表1 本实验中用到的引物名称和序列
Table 1 Primer names and sequences used in this study
| 引物名称 Primer name | 引物序列 Primer sequence (5'-3') |
|---|---|
| ZYMV-HC-ProK364D-F | ATTTCACC |
| ZYMV-HC-ProK364D-R | CGAATCAT |
| ZYMV-HC-Pro-F | GCGCTCTAGCAAGGCTATG |
| ZYMV-HC-Pro-R | CACCTAGTATGTATGCTGCAGT |
| GFP-F | ATGAGTAAAGGAGAAGAAC |
| GFP-R | TTTGTAGAGCTCATCCATG |
| GFP-qRT-F | GTGGAGAGGGTGAAGGTGAT |
| GFP-qRT-R | CGGATAACGGGAAAAGCATTGA |
| actin-qRT-F | CTGATGAAGATACTCACAGAAAGAG |
| actin-qRT-R | CAGGATACGGGGAGCTAATG |
| EF1α-qRT-F | CCACGAGTCTCTCCCAGAAG |
| EF1α-qRT-R | CACGCTTGAGATCCTTGACA |
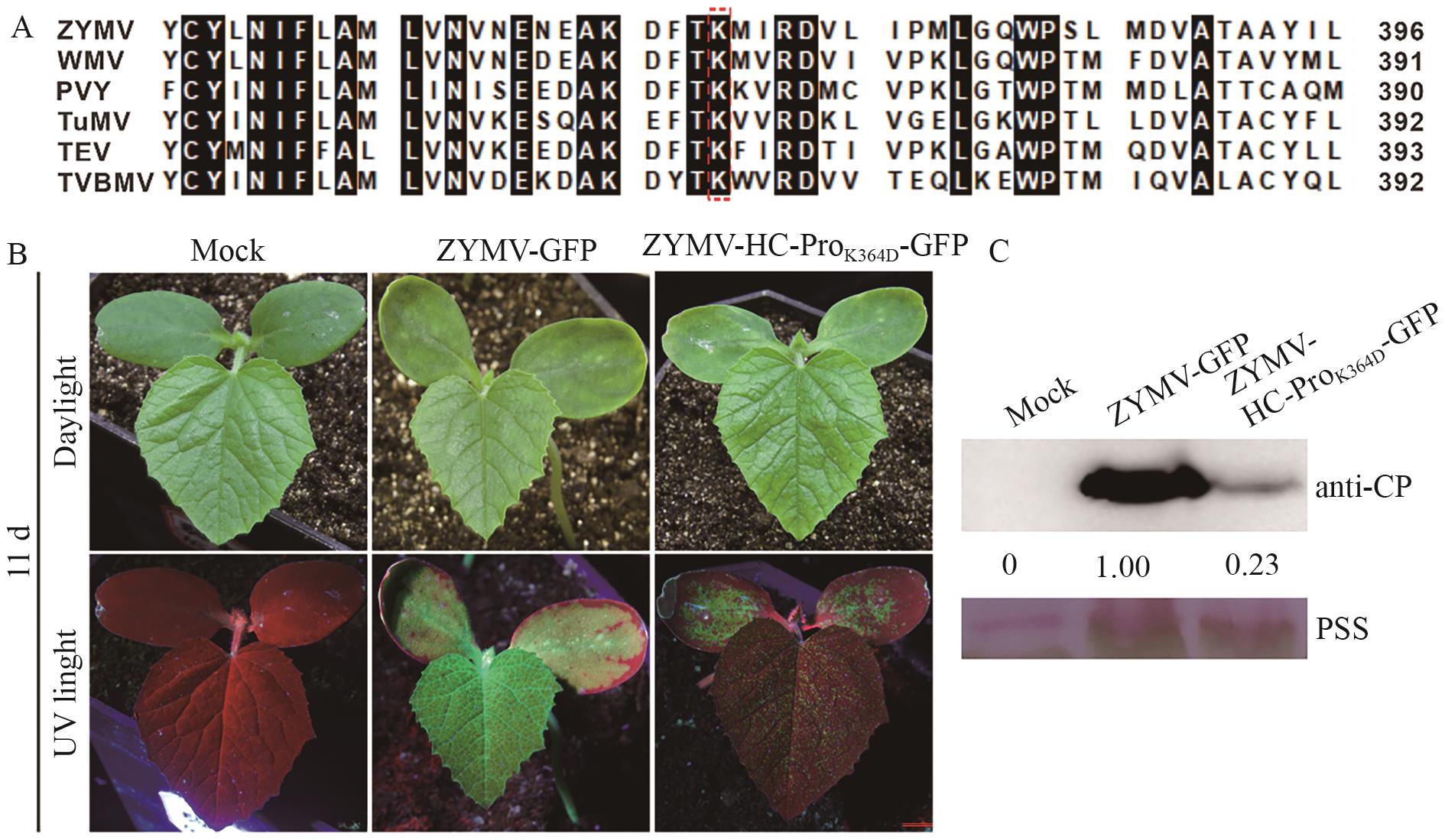
图1 K364D突变降低了ZYMV在甜瓜植株中的致病力A:比对马铃薯病毒属6种病毒部分HC-Pro的氨基酸序列。ZYMV HC-Pro中K364残基由红色虚线框标注。B:甜瓜植株在野生型ZYMV及ZYMV-HC-ProK364D-GFP突变体侵染后第11天出现的症状。C:Western blotting检测甜瓜上部叶片中ZYMV CP积累量。内参为丽春红染色条带(Ponceau S staining, PSS),条带灰度值使用ImageJ软件测定。ZYMV CP积累量以PSS归一化后的数据表示
Fig. 1 Mutation of K364D reduced ZYMV virulence in C. melo plantsA: Alignment of the partial amino acid sequences of 6 potyvirus HC-Pros. The K364 residue in ZYMV HC-Pro were pointed in the red box. B: The symptoms observed on C. melo plants at day 11 after infection with the wild-type ZYMV and ZYMV-HC-ProK364D-GFP mutant. C: Western blotting was used to detect the accumulation of ZYMV CP in the upper leaves of C. melo plants. The loading controls were the Ponceau S staining (PSS) bands, and the band gray values were measured using ImageJ software. The accumulation of ZYMV CP is represented by the data normalized to PSS
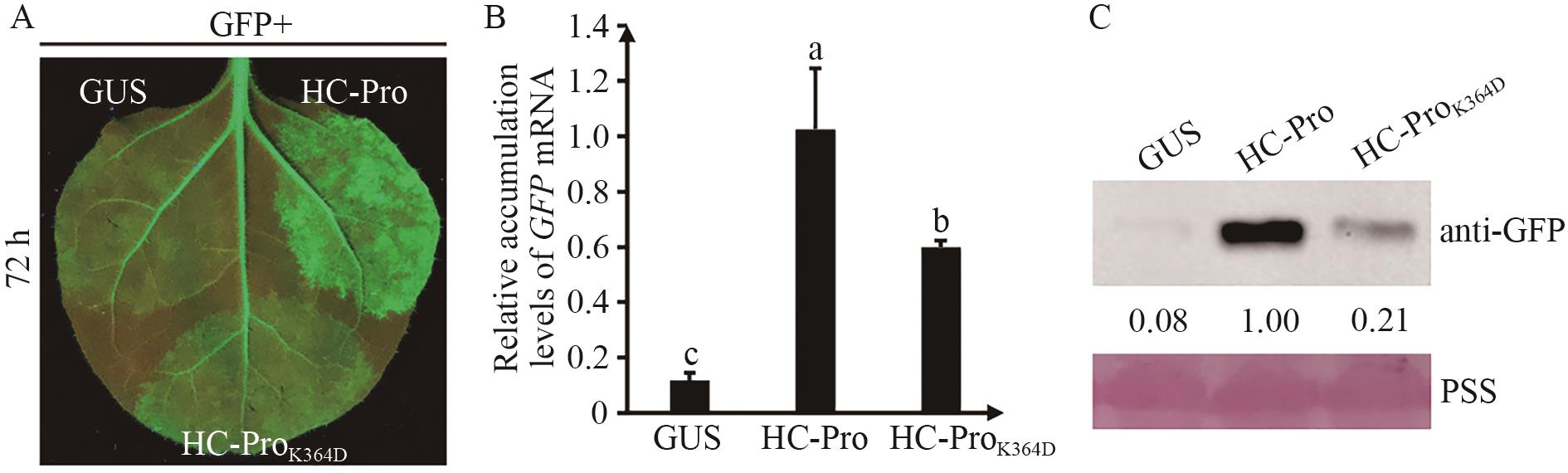
图2 ZYMV HC-Pro中K364D突变影响其抑制RNA沉默的活性A:野生型ZYMV HC-Pro及HC-ProK364D突变体抑制RNA沉默的活性;B:GFP mRNA在16c叶片不同区域的积累量。Tukey多重检验进行组间统计学分析,显著性差异用不同字母来表示(P<0.05);C:GFP蛋白在16c叶片不同区域的积累量
Fig. 2 Mutation of K364D affected the activity of RNA silencing of ZYMV HC-ProA: Suppression activity of RNA silencing of wild-type ZYMV HC-Pro and HC-ProK364D mutant. B: The accumulation of GFP mRNA in different regions of 16c leaves. Tukey multiple range test was used for statistical analysis between groups. Significant differences are indicated by different letters (P<0.05). C: Accumulation of GFP protein in different regions of 16c leaves
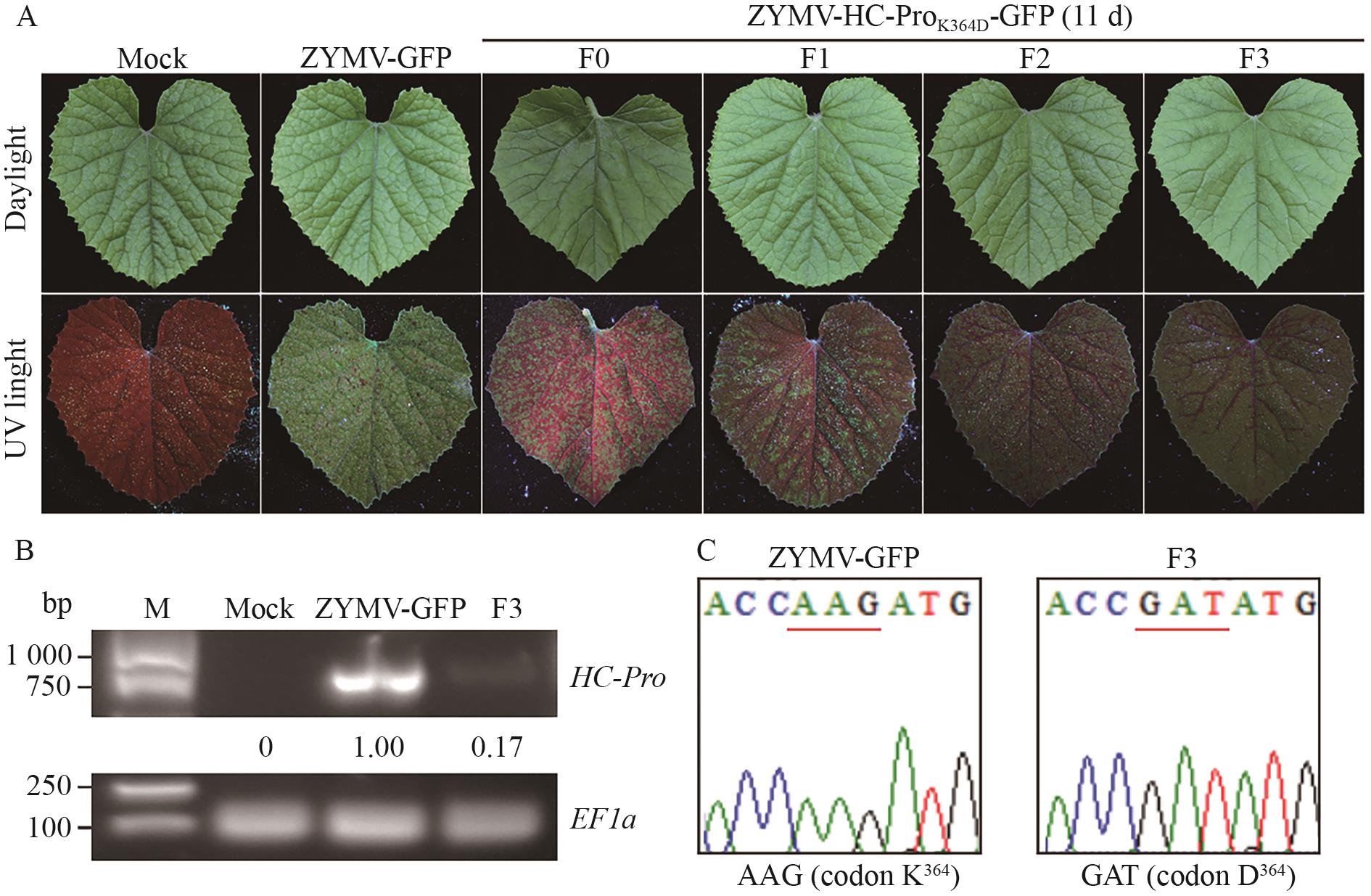
图3 ZYMV-HC-ProK364D-GFP突变体的遗传稳定性A:在连续3次继代中,ZYMV-HC-ProK364D-GFP突变体在甜瓜植株上引起的症状;B: ZYMV-HC-ProK364D-GFP突变体F3代在甜瓜植株中的HC-ProRNA积累量;C: ZYMV-HC-ProK364D-GFP突变体F3代在甜瓜植株中HC-Pro序列分析。ZYMV HC-Pro中编码第364位氨基酸残基的密码子用下划线标注
Fig. 3 Genetic stability of ZYMV-HC-ProK364D-GFP mutantA: During three consecutive passages, the symptoms caused by the ZYMV-HC-ProK364D-GFP mutant on C. melo plants. B: The HC-Pro RNA accumulations of the ZYMV-HC-ProK364D-GFP mutant F3 generation in C. melo plants. C: Analysis of HC-Pro sequence in the F3 generation of ZYMV-HC-ProK364D-GFP mutant in C. melo plants. The codon encoding the 364th amino acid residues in ZYMV HC-Pro was underlined
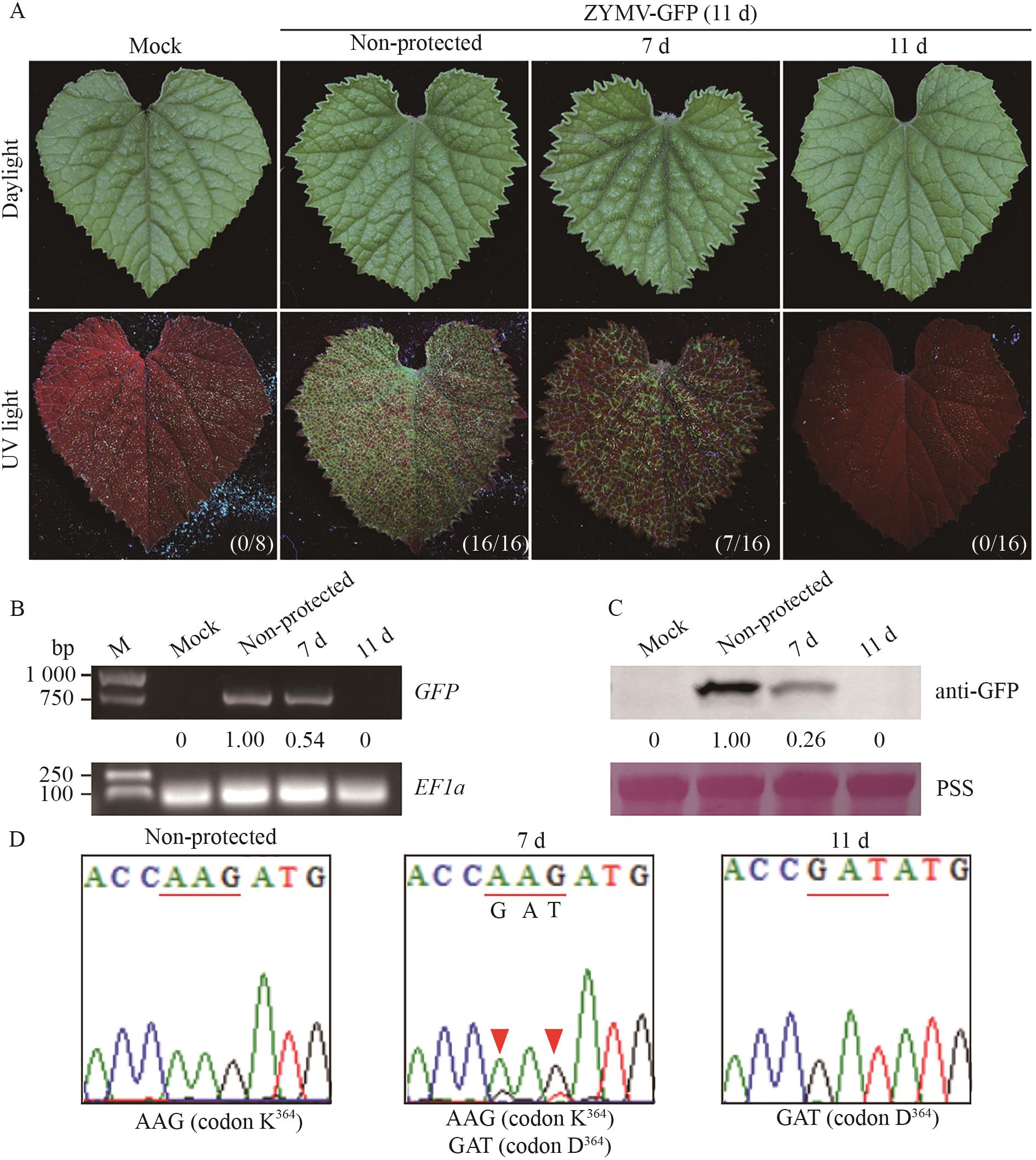
图4 ZYMV-HC-ProK364D突变体介导的交叉保护作用A:间隔期为7或11 d的甜瓜植株在ZYMV-GFP挑战后第11天的症状表现。括号里列出的为有GFP荧光的甜瓜植株数/接种的总甜瓜植株数;B: GFP mRNA在ZYMV-GFP挑战后第11天的甜瓜植株中的积累量;C: GFP蛋白在ZYMV-GFP挑战后第11天的甜瓜植株中的积累量;D: 在ZYMV-GFP挑战后第11天的甜瓜植株中病毒HC-Pro的序列分析。ZYMV HC-Pro中编码第364位氨基酸残基的密码子用下划线标注。每株甜瓜中ZYMV后代HC-Pro编码序列单独测序3次
Fig. 4 Cross protection efficacy mediated by ZYMV-HC-ProK364D mutantA: Symptoms of C. melo plants with an interval period of 7 or 11 d at the 11th day post ZYMV-GFP challenge. The numbers in parentheses indicate the number of C. melo plants with GFP fluorescence/the total number of inoculated C. melo plants. B: The accumulation of GFP mRNA in C. melo plants on the 11th day after ZYMV-GFP challenge. C: The accumulation of GFP protein in C. melo plants on the 11th day after ZYMV-GFP challenge. D: Analysis of HC-Pro sequence in C. melo plants on the 11th day after ZYMV-GFP challenge. The codon encoding the 364th amino acid residue in ZYMV HC-Pro was underlined. The HC-Pro coding sequence from the ZYMV progeny in each C. melo plant was individually sequenced three times
| [1] | 韦盈, 康蕊, 叶乃豪, 等. 海南地区不同品种甜瓜营养成分比较分析 [J]. 中国食物与营养, 2023, 29(7): 36-42. |
| Wei Y, Kang R, Ye NH, et al. Comparative analysis on nutritional components of different varieties of muskmelons in Hainan [J]. Food Nutr China, 2023, 29(7): 36-42. | |
| [2] | 王东, 麦麦提艾则孜·穆合塔尔, 刘艳全, 等. 新疆伽师县甜瓜病毒病种类鉴定 [J]. 园艺学报, 2023, 50(8): 1793-1802. |
| Wang D, Maimaitiaizezi·MHTE, Liu YQ, et al. Identification of virus disease types of melon planting in Jiashi County of Xinjiang [J]. Acta Hortic Sin, 2023, 50(8): 1793-1802. | |
| [3] | 王敏, 邱艳红, 古勤生, 等. 海南甜瓜的主要病毒病及其防控措施 [J]. 中国瓜菜, 2023, 36(3): 15-20. |
| Wang M, Qiu YH, Gu QS, et al. Main virus diseases and the control measures of melon in Hainan Province [J]. China Cucurbits Veg, 2023, 36(3): 15-20. | |
| [4] | 阿斯亚姆·阿布都克依木. 喀什地区甜瓜主要病毒病发生规律的初步研究 [D]. 乌鲁木齐: 新疆农业大学, 2024. |
| Asiyamu·ABDKYM. Preliminary study on the occurrence patterns of major viral diseases in melons growing areas of Kashi prefecture [D]. Urumqi: Xinjiang Agricultural University, 2024. | |
| [5] | 张磊, 任婷璐, 刘艳, 等. 甜瓜病毒研究进展 [J]. 中国果树, 2023(4): 24-30. |
| Zhang L, Ren TL, Liu Y, et al. Research progress of melon virus [J]. China Fruits, 2023(4): 24-30. | |
| [6] | Castle SJ. Field and laboratory transmission of watermelon mosaic virus 2 and zucchini yellow mosaic virus by various aphid species [J]. Phytopathology, 1992, 82(2): 235. |
| [7] | Chung BY, Miller WA, Atkins JF, et al. An overlapping essential gene in the Potyviridae [J]. Proc Natl Acad Sci U S A, 2008, 105(15): 5897-5902. |
| [8] | Olspert A, Chung BY, Atkins JF, et al. Transcriptional slippage in the positive-sense RNA virus family Potyviridae [J]. EMBO Rep, 2015, 16(8): 995-1004. |
| [9] | Huet H, Gal-On A, Meir E, et al. Mutations in the helper component protease gene of zucchini yellow mosaic virus affect its ability to mediate aphid transmissibility [J]. J Gen Virol, 1994, 75(6): 1407-1414. |
| [10] | Kimalov B, Gal-On A, Stav R, et al. Maintenance of coat protein N-terminal net charge and not primary sequence is essential for zucchini yellow mosaic virus systemic infectivity [J]. J Gen Virol, 2004, 85(Pt 11): 3421-3430. |
| [11] | Shiboleth YM, Haronsky E, Leibman D, et al. The conserved FRNK box in HC-pro, a plant viral suppressor of gene silencing, is required for small RNA binding and mediates symptom development [J]. J Virol, 2007, 81(23): 13135-13148. |
| [12] | Lin SS, Wu H-W, Jan FJ, et al. Modifications of the helper component-protease of zucchini yellow mosaic virus for generation of attenuated mutants for cross protection against severe infection [J]. Phytopathology® , 2007, 97(3): 287-296. |
| [13] | Desbiez C, Girard M, Lecoq H. A novel natural mutation in HC-Pro responsible for mild symptomatology of Zucchini yellow mosaic virus (ZYMV, Potyvirus) in cucurbits [J]. Arch Virol, 2010, 155(3): 397-401. |
| [14] | Huang XD, Fang L, Gu QS, et al. Cross protection against the watermelon strain of papaya ringspotvirus through modification of viral RNA silencing suppressor [J]. Virus Res, 2019, 265: 166-171. |
| [15] | Xu XJ, Zhu Q, Jiang SY, et al. Corrigendum: development and evaluation of stable sugarcane mosaic virus mild mutants for cross-protection against infection by severe strain [J]. Front Plant Sci, 2022, 13: 956567. |
| [16] | Kurihara Y, Watanabe Y. Cross-protection in Arabidopsis against crucifer tobamovirus Cg by an attenuated strain of the virus [J]. Mol Plant Pathol, 2003, 4(4): 259-269. |
| [17] | Folimonova SY. Developing an understanding of cross-protection by Citrus tristeza virus [J]. Front Microbiol, 2013, 4: 76. |
| [18] | You BJ, Chiang CH, Chen LF, et al. Engineered mild strains of Papaya ringspot virus for broader cross protection in cucurbits [J]. Phytopathology, 2005, 95(5): 533-540. |
| [19] | Wang LX, Shi W, Aziz A, et al. Mutating the arginine residue within the FRNK motif of telosma mosaic virus (TelMV) HC-Pro protein attenuates viral infection and confers effective protection against TelMV in passion fruit (Passiflora edulis) [J]. Pest Manag Sci, 2024, 80(10): 5256-5265. |
| [20] | Tuo DC, Zhou P, Zhao GY, et al. A double mutation in the conserved motifs of the helper component protease of papaya leaf distortion mosaic virus for the generation of a cross-protective attenuated strain [J]. Phytopathology® , 2020, 110(1): 187-193. |
| [21] | 王健. 小西葫芦黄花叶病毒遗传多样性及致病力分析 [D]. 泰安: 山东农业大学, 2019. |
| Wang J. Genetic diversity and pathogenicity analysis of zucchini yellow mosaic virus [D]. Taian: Shandong Agricultural University, 2019. | |
| [22] | Ismayil A, Haxim Y, Wang YJ, et al. Cotton Leaf Curl Multan virus C4 protein suppresses both transcriptional and post-transcriptional gene silencing by interacting with SAM synthetase [J]. PLoS Pathog, 2018, 14(8): e1007282. |
| [23] | Voinnet O. RNA silencing as a plant immune system against viruses [J]. Trends Genet, 2001, 17(8): 449-459. |
| [24] | Lopez-Gomollon S, Baulcombe DC. Roles of RNA silencing in viral and non-viral plant immunity and in the crosstalk between disease resistance systems [J]. Nat Rev Mol Cell Biol, 2022, 23(10): 645-662. |
| [25] | Radhamani Anandalakshmi GJP. A viral suppressor of gene silencing in plants [J]. Proc Natl Acad Sci U S A, 1998, 95(22): 13079-13084. |
| [26] | Kasschau KD, Carrington JC. A counterdefensive strategy of plant viruses [J]. Cell, 1998, 95(4): 461-470. |
| [27] | del Toro F, Sun H, Robinson C, et al. In planta vs viral expression of HCPro affects its binding of nonplant 21-22 nucleotide small RNAs, but not its preference for 5'-terminal adenines, or its effects on small RNA methylation [J]. New Phytol, 2022, 233(5): 2266-2281. |
| [28] | Yambao MLM, Yagihashi H, Sekiguchi H, et al. Point mutations in helper component protease of clover yellow vein virus are associated with the attenuation of RNA-silencing suppression activity and symptom expression in broad bean [J]. Arch Virol, 2008, 153(1): 105-115. |
| [29] | Raja JAJ, Huang CH, Chen CC, et al. Modification of the N-terminal FWKG-αH1 element of potyviral HC-Pro affects its multiple functions and generates effective attenuated mutants for cross-protection [J]. Mol Plant Pathol, 2022, 23(7): 947-965. |
| [30] | Lecoq H. Control of zucchini yellow mosaic virus in squash by cross protection [J]. Plant Dis, 1991, 75(2): 208. |
| [31] | Rast ATB. M II-16, an artificial symptomless mutant of tobacco mosaic virus for seedling inoculation of tomato crops [J]. Neth J Plant Pathol, 1972, 78(3): 110-112. |
| [32] | Cook G, van Vuuren SP, Breytenbach JHJ, et al. Expanded strain-specific RT-PCR assay for differential detection of currently known Citrus tristeza virus strains: a useful screening tool [J]. J Phytopathol, 2016, 164(10): 847-851. |
| [33] | Tran TTY, Cheng H-W, Nguyen VH, et al. Modification of the helper component proteinase of papaya ringspot virus Vietnam isolate to generate attenuated mutants for disease management by cross protection [J]. Phytopathology® , 2023, 113(2): 334-344. |
| [34] | Chewachong GM, Miller SA, Blakeslee JJ, et al. Generation of an attenuated, cross-protective pepino mosaic virus variant through alignment-guided mutagenesis of the viral capsid protein [J]. Phytopathology® , 2015, 105(1): 126-134. |
| [35] | Deng CH, Hu WY, Shen WT, et al. A point mutation in the pepper veinal mottle virus 6K1 protein yields a stable attenuated strain for engineering virus resistance in pepper plants [J]. Plant Dis, 2025, 109(7): 1459-1469. |
| [36] | Cheng H-W, Lin TT, Huang CH, et al. Modification of papaya ringspot virus HC-pro to generate effective attenuated mutants for overcoming the problem of strain-specific cross protection [J]. Plant Dis, 2023, 107(6): 1757-1768. |
| [37] | Chong YH, Do DH, Cheng H-W, et al. Generation of attenuated mutants of east asian passiflora virus for disease management by cross protection [J]. Mol Plant Microbe Interactions, 2023, 36(6): 345-358. |
| [38] | Xu XJ, Li HG, Cheng DJ, et al. A spontaneous complementary mutation restores the RNA silencing suppression activity of HC-pro and the virulence of sugarcane mosaic virus [J]. Front Plant Sci, 2020, 11: 1279. |
| [39] | Zhou CY, Zhou Y. Strategies for viral cross protection in plants [M]//Antiviral Resistance in Plants. Totowa, NJ: Humana Press, 2012: 69-81. |
| [1] | 李圣彦, 李香银, 李鹏程, 张明俊, 张杰, 郎志宏. 转基因玉米2HVB5的性状鉴定及遗传稳定性分析[J]. 生物技术通报, 2023, 39(1): 21-30. |
| [2] | 梁海生, 李梦桃, 李圣彦, 汪海, 张杰, 郎志宏. 转Bt基因抗虫玉米HGK60的农艺性状分析[J]. 生物技术通报, 2018, 34(7): 92-100. |
| [3] | 马艳玲,刘富来,张敏,孙宇辉,洪葵. 阿扎霉素F产生菌链霉菌211726基因转移系统的建立[J]. 生物技术通报, 2016, 32(4): 198-202. |
| [4] | 高红伟;李素波;艾雪;李妙;王颖丽;让文亮;季守平;宫锋;. 新型基因重组α-半乳糖苷酶工程菌菌种稳定性研究[J]. , 2011, 0(07): 201-204. |
| [5] | 梁晶晶;陆开宏;凌红丽;王宏华;. 斑马鱼干扰素基因工程菌发酵条件及稳定性研究[J]. , 2009, 0(02): 107-111. |
| [6] | . 生物防治[J]. , 1997, 0(04): 55-56. |
| [7] | 朱遐. FDA提出转基因动物生产“注意事项”[J]. , 1996, 0(02): 15-16. |
| [8] | . 生物防治[J]. , 1994, 0(05): 82-84. |
| [9] | . 细胞工程[J]. , 1991, 0(07): 39-43. |
| [10] | 李思经;. 未分化离体培养物的遗传稳定性[J]. , 1990, 0(10): 6-7. |
| [11] | 李思经;. 为作物改良进行体外选择时的注意事项[J]. , 1989, 0(10): 17-18. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||