








生物技术通报 ›› 2021, Vol. 37 ›› Issue (7): 88-97.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0508
李治文1( ), 刘培燕1, 陈建松1,2, 廖金铃1,2,3, 林柏荣1,2(
), 刘培燕1, 陈建松1,2, 廖金铃1,2,3, 林柏荣1,2( ), 卓侃1,2(
), 卓侃1,2( )
)
收稿日期:2021-04-18
出版日期:2021-07-26
发布日期:2021-08-13
作者简介:李治文,男,博士研究生,研究方向:植物与病原物相互作用;E-mail:基金资助:
LI Zhi-wen1( ), LIU Pei-yan1, CHEN Jian-song1,2, LIAO Jin-ling1,2,3, LIN Bo-rong1,2(
), LIU Pei-yan1, CHEN Jian-song1,2, LIAO Jin-ling1,2,3, LIN Bo-rong1,2( ), ZHUO Kan1,2(
), ZHUO Kan1,2( )
)
Received:2021-04-18
Published:2021-07-26
Online:2021-08-13
摘要:
前期研究表明拟禾本科根结线虫效应子MgMO237与水稻OsCRRSP55蛋白互作,抑制水稻对根结线虫的防卫反应。植物CRRSPs蛋白参与植物的生物和非生物胁迫响应,因此研究MgMO237与OsCRRSP55相互作用对下游基因表达的影响可为阐明水稻对根结线虫的防卫反应机制提供理论依据。生物信息学分析表明OsCRRSP55蛋白有N端信号肽,没有跨膜结构域,具两个DUF26结构域及一保守基序C-X8-C-X2-C。RT-qPCR分析显示OsCRRSP55在水稻根中表达,且在拟禾本科根结线虫侵染7 d后的根结中表达显著上调。水杨酸和茉莉酸显著提高水稻OsCRRSP55及其启动子结合转录因子OsWRKY47的表达。转录组测序结果显示茉莉酸响应基因OsERF87在MgMO237转基因植株中表达显著下调,而RT-qPCR检测发现OsERF87在OsCRRSP55转化水稻原生质体中表达显著上调,表明茉莉酸响应基因OsERF87是OsCRRSP55和MgMO237的共响应基因。该结果表明拟禾本科根结线虫效应子MgMO237可能通过水稻OsCRRSP55蛋白调控茉莉酸激素信号传导,从而抑制植物对线虫的防卫反应。
李治文, 刘培燕, 陈建松, 廖金铃, 林柏荣, 卓侃. 线虫效应子MgMO237及互作蛋白OsCRRSP55在水稻中的共响应基因鉴定[J]. 生物技术通报, 2021, 37(7): 88-97.
LI Zhi-wen, LIU Pei-yan, CHEN Jian-song, LIAO Jin-ling, LIN Bo-rong, ZHUO Kan. Identification of Rice Genes Responding to Both the Nematode Effector MgMO237 and Its Interacting Protein OsCRRSP55[J]. Biotechnology Bulletin, 2021, 37(7): 88-97.
| 引物 Primer | 引物序列 Sequence(5'-3') | 用途 Purpose |
|---|---|---|
| OsCRRSP55 F | ATGGCATTCAGTAGCAAAGC | 用于OsCRRSP55基因全长扩增 |
| OsCRRSP55 R | TTAGCGGTGCACAACGATCT | |
| qOsCRRSP55 F | AGGATCAGTACACGCCGTTC | 用于OsCRRSP55基因的RT-qPCR检测 |
| qOsCRRSP55 R | CGCAGTATTGACCGAACCTT | |
| OsUBQ-F | CCAGTAAGTCCTCAGCCATGGAG | 用于水稻看家基因OsUBQ(Os03g13170)的RT-qPCR检测[ |
| OsUBQ-R | GGACACAATGATTAGGGATC | |
| MgMO237-qPCR-F | TTGAGCGAAAGAGTCTAAATC | 用于MgMO237基因的RT-qPCR检测[ |
| MgMO237-qPCR-R | ACAGCAGGTCCATACATAA | |
| OsCRRSP55-Nco1 F | CGCGGATCCATGGCATTCAGTAGCAAAGC | 用于OsCRRSP55基因瞬时表达载体构建 |
| OsCRRSP55:FLAG-Pml1 R | CGACGCGTTTACTTATCGTCGTCATCCTTGT- AATCGCGGTGCACAACGATCTTGG | |
| OsWRKY47 F | ATGGACGACCTGCTCGAGAT | 用于OsWRKY47基因的RT-qPCR检测 |
| OsWRKY47 R | GGCGATGTCCCATGTAGCAT | |
| ERF87-qPCR-F | ACTTGTCTTCTTGTTGTTGTT | 用于植物激素通路相关基因的RT-qPCR检测 |
| ERF87-qPCR-R | TCCATCTATCTACTCCTCTATCTA | |
| PR-1b-qPCR-F | ACGGGCGTACGTACTGGCTA | |
| PR-1b-qPCR-R | CTCGGTATGGACCGTGAAG | |
| PR-1a- qPCR-F | CAGTGGTACGACCACGACAG | |
| PR-1a-qPCR-R | GGCGAGTAGTTGCAGGTGAT | |
| OsTIFY11e-qPCR-F | GTGAGGATGCTTATGCTTGC | |
| OsTIFY11e-qPCR-R | GATCCCTAGCATATGTACTAC | |
| IAA24-qPCR-F | AATGTTCATCTCTTCCTG | |
| IAA24-qPCR-R | ATTCATCATCCATCTTCTC | |
| PIL13-qPCR-F | TTCATAACCTGTCAGAGA | |
| PIL13-qPCR-R | CGATTGCTTCATCTAATATAG | |
| CYCLIN-D3-1-qPCR-F | ATTTCTCAACATTCCCTA | |
| CYCLIN-D3-1-qPCR-R | ATTTCGCTATGAACATTG |
表1 本研究所用的引物
Table 1 Primers used in this study
| 引物 Primer | 引物序列 Sequence(5'-3') | 用途 Purpose |
|---|---|---|
| OsCRRSP55 F | ATGGCATTCAGTAGCAAAGC | 用于OsCRRSP55基因全长扩增 |
| OsCRRSP55 R | TTAGCGGTGCACAACGATCT | |
| qOsCRRSP55 F | AGGATCAGTACACGCCGTTC | 用于OsCRRSP55基因的RT-qPCR检测 |
| qOsCRRSP55 R | CGCAGTATTGACCGAACCTT | |
| OsUBQ-F | CCAGTAAGTCCTCAGCCATGGAG | 用于水稻看家基因OsUBQ(Os03g13170)的RT-qPCR检测[ |
| OsUBQ-R | GGACACAATGATTAGGGATC | |
| MgMO237-qPCR-F | TTGAGCGAAAGAGTCTAAATC | 用于MgMO237基因的RT-qPCR检测[ |
| MgMO237-qPCR-R | ACAGCAGGTCCATACATAA | |
| OsCRRSP55-Nco1 F | CGCGGATCCATGGCATTCAGTAGCAAAGC | 用于OsCRRSP55基因瞬时表达载体构建 |
| OsCRRSP55:FLAG-Pml1 R | CGACGCGTTTACTTATCGTCGTCATCCTTGT- AATCGCGGTGCACAACGATCTTGG | |
| OsWRKY47 F | ATGGACGACCTGCTCGAGAT | 用于OsWRKY47基因的RT-qPCR检测 |
| OsWRKY47 R | GGCGATGTCCCATGTAGCAT | |
| ERF87-qPCR-F | ACTTGTCTTCTTGTTGTTGTT | 用于植物激素通路相关基因的RT-qPCR检测 |
| ERF87-qPCR-R | TCCATCTATCTACTCCTCTATCTA | |
| PR-1b-qPCR-F | ACGGGCGTACGTACTGGCTA | |
| PR-1b-qPCR-R | CTCGGTATGGACCGTGAAG | |
| PR-1a- qPCR-F | CAGTGGTACGACCACGACAG | |
| PR-1a-qPCR-R | GGCGAGTAGTTGCAGGTGAT | |
| OsTIFY11e-qPCR-F | GTGAGGATGCTTATGCTTGC | |
| OsTIFY11e-qPCR-R | GATCCCTAGCATATGTACTAC | |
| IAA24-qPCR-F | AATGTTCATCTCTTCCTG | |
| IAA24-qPCR-R | ATTCATCATCCATCTTCTC | |
| PIL13-qPCR-F | TTCATAACCTGTCAGAGA | |
| PIL13-qPCR-R | CGATTGCTTCATCTAATATAG | |
| CYCLIN-D3-1-qPCR-F | ATTTCTCAACATTCCCTA | |
| CYCLIN-D3-1-qPCR-R | ATTTCGCTATGAACATTG |

图1 植物CRRSP55蛋白多序列比对和保守结构域分析SbCRRSP55(XP_002465466.1):高粱CRRSP55蛋白;ZmCRRSP55(PWZ56059.1):玉米CRRSP55蛋白;DoCRRSP55(OEL36740.1):扫帚黍CRRSP55蛋白;OsCRRSP5:水稻OsCRRSP55蛋白;AtCRRSP55(AB020745.1 65306-64515):拟南芥CRRSP55蛋白。红色虚线框为水稻OsCRRSP55蛋白信号肽序列;红色下划线为Unknown Function 26(DUF26)结构域,绿色方框为保守基序
Fig. 1 Multiple sequence alignment and conserved motif analysis of plant CRRSP55 proteinsSbCRRSP55(XP_0024654665.1):Sorghum bicolor CRRSP55 protein. ZmCRRSP55(PWZ56059.1):Zea mays CRRSP55 protein. DoCRRSP55(OEL36740.1):Dichanthelium oligosanthes CRRSP55 protein. OsCRRSP5:Rice CRRSP55 protein. AtCRRSP55(AB020745.1 65306-64515):Arabidopsis thaliana CRRSP55 protein. The red dotted box refers to the signal peptide sequence of rice OsCRRSP55 protein. Underlined in red refers to Unknown Function 26(DUF26)domain,and the green box refers to a conserved motif
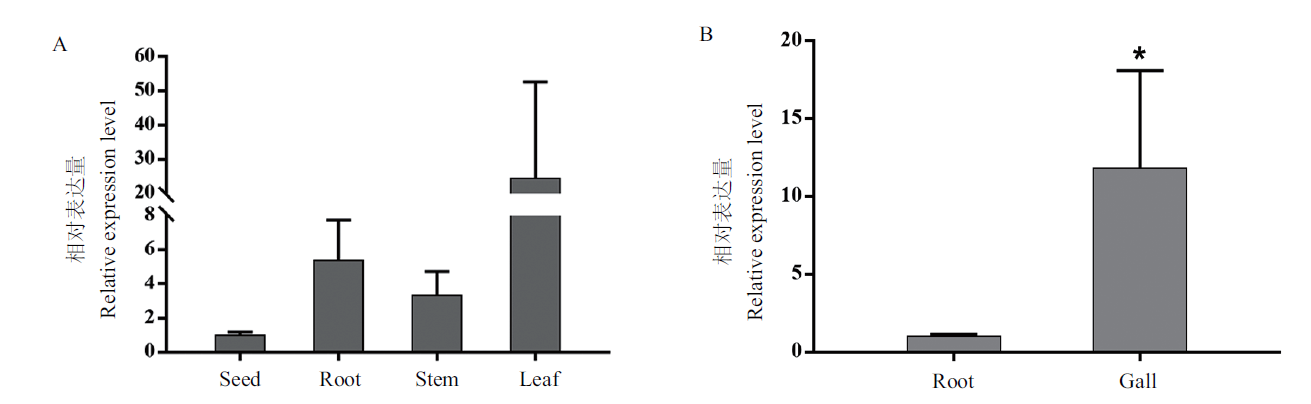
图2 水稻OsCRRSP55基因的表达 A:OsCRRSP55在水稻种子及14日龄水稻根、茎和叶中的表达水平。B:OsCRRSP55在拟禾本科根结线虫侵染7 d后的根结中的表达平。图中误差线表示标准偏差,*表示P < 0.05差异显著。下同
Fig. 2 Expression levels of OsCRRSP55 gene in riceA:Expression levels of OsCRRSP55 gene in the seeds,roots,stems and leaves of 14-day-old rice. B:Expression levels of OsCRRSP55 gene in the galls at 7 d after M. graminicola infection. The error line in the figure refers to the standard deviation,*:P<0.05 significant difference. The same below
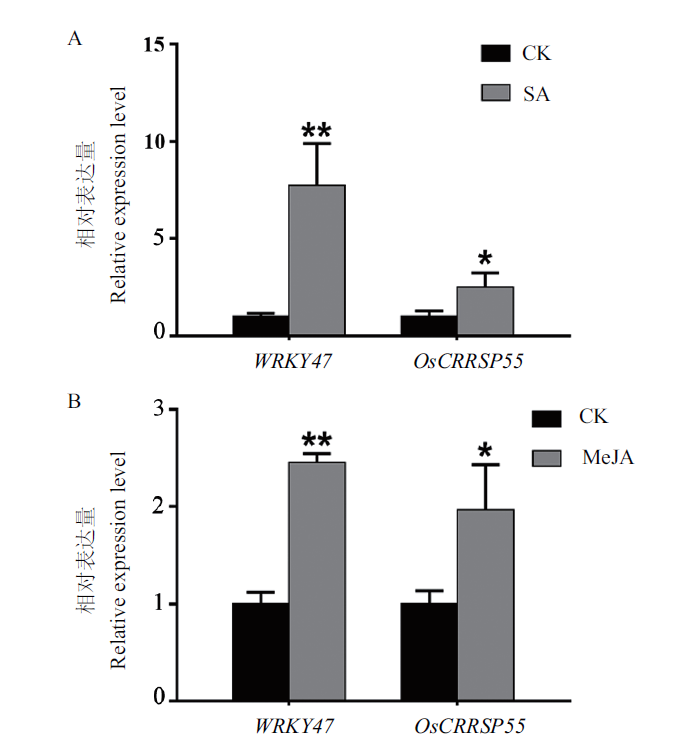
图3 水杨酸和茉莉酸甲酯处理水稻后OsCRRSP55和WRKY47基因的表达变化 A:水杨酸处理24 h后水稻WRKY47和OsCRRSP55基因的表达变化。B:茉莉酸甲酯处理24 h后水稻WRKY47和OsCRRSP55基因的表达变化。CK:清水处理;SA:水杨酸处理;MeJA:茉莉酸甲酯处理。**表示P < 0.01差异显著。下同
Fig. 3 Expression variations of OsCRRSP55 and WRKY47 genes in the rice treated with salicylic acid and methyl jasmonateA:Expression variations of WRKY47 and OsCRRSP55 genes in the rice treated with salicylic acid for 24 h. B:Expression variations of WRKY47 and OsCRRSP55 genes in the rice treated with methyl jasmonate for 24 h. CK:Water treatment. SA:Salicylic acid treatment. MeJA:Methyl jasmonate treatment. **:P<0.01 significant difference. The same below
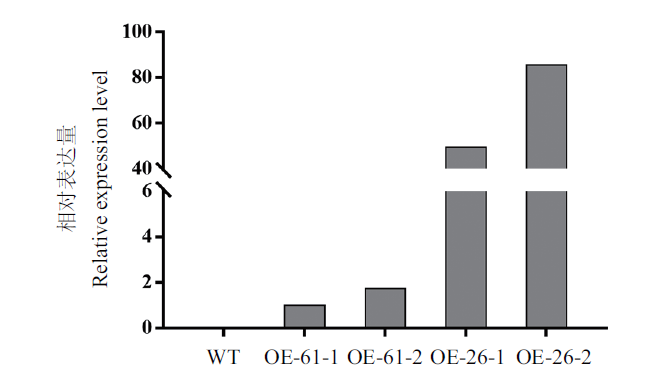
图4 MgMO237转基因水稻的鉴定 WT:野生型日本晴水稻。OE61-1、OE61-2:MgMO237转基因水稻OE61株系不同植株;OE26-1、OE26-2:MgMO237转基因水稻OE26株系不同植株。OE61和OE26检测到MgMO237表达,WT未检测到MgMO237基因表达
Fig. 4 Identification of MgMO237-transgenic rice WT:Wild-type rice. OE61-1 and OE61-2:Different plants of the OE61 line of MgMO237-transgenic rice. OE26-1 and OE26-2:Different plants of line the OE26 of MgMO237-transgenic rice. The transcript of MgMO237 was detected in lines OE61 and OE26 but not in WT
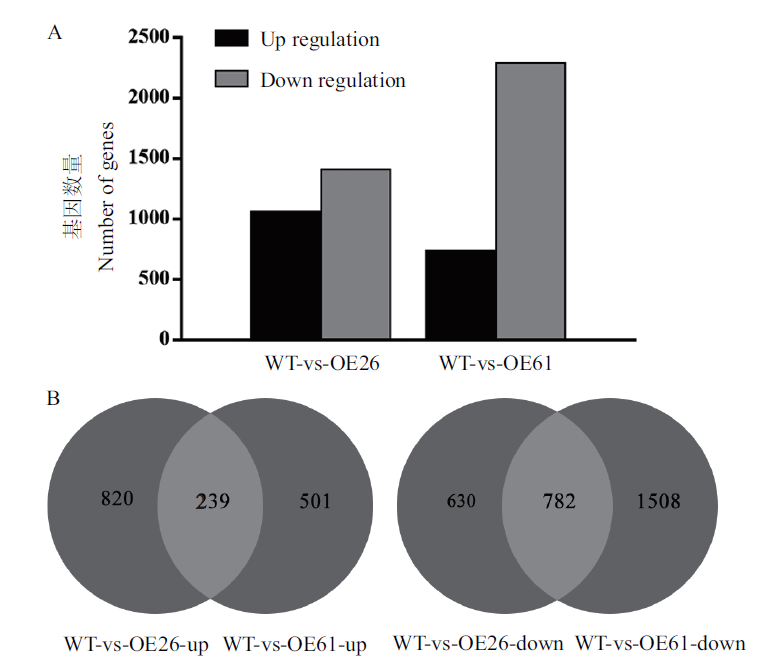
图5 MgMO237转基因水稻与野生型水稻差异表达基因统计 A:MgMO237转基因水稻OE26和OE61株系与野生型水稻差异表达基因数量统计图。B:MgMO237转基因水稻OE26和OE61植株相对野生型水稻植株共同上调基因数(左图)和共同下调基因数(右图)统计图。OE26和OE61:MgMO237转基因水稻OE26和OE61株系;WT:野生型水稻
Fig. 5 Statistics of differentially expressed genes between MgMO237-transgenic rice and wild-type rice A:Statistical diagram showing the number of differentially expressed genes in lines OE26 and OE61 of MgMO237-transgenic rice and wild-type rice lines. B:Number of up-regulated genes(left)and down-regulated genes(right)in line OE26 and OE61 of MgMO237-transgenic rice compared with wild-type rice plants. OE26 and OE61:Line OE26 and OE61 of MgMO237 transgenic rice. WT:Wild-type rice

图6 RT-qPCR验证MgMO237转基因水稻与野生型水稻差异表达的植物激素通路基因 OE26和OE61:MgMO237转基因水稻OE26和OE61株系;WT:野生型水稻
Fig. 6 Confirmation of differentially expressed genes within plant hormone pathways in MgMO237-transgenic rice and wild-type rice by RT-qPCR OE26 and OE61:Line OE26 and OE61 of MgMO237-transgenic rice. WT:Wild-type rice
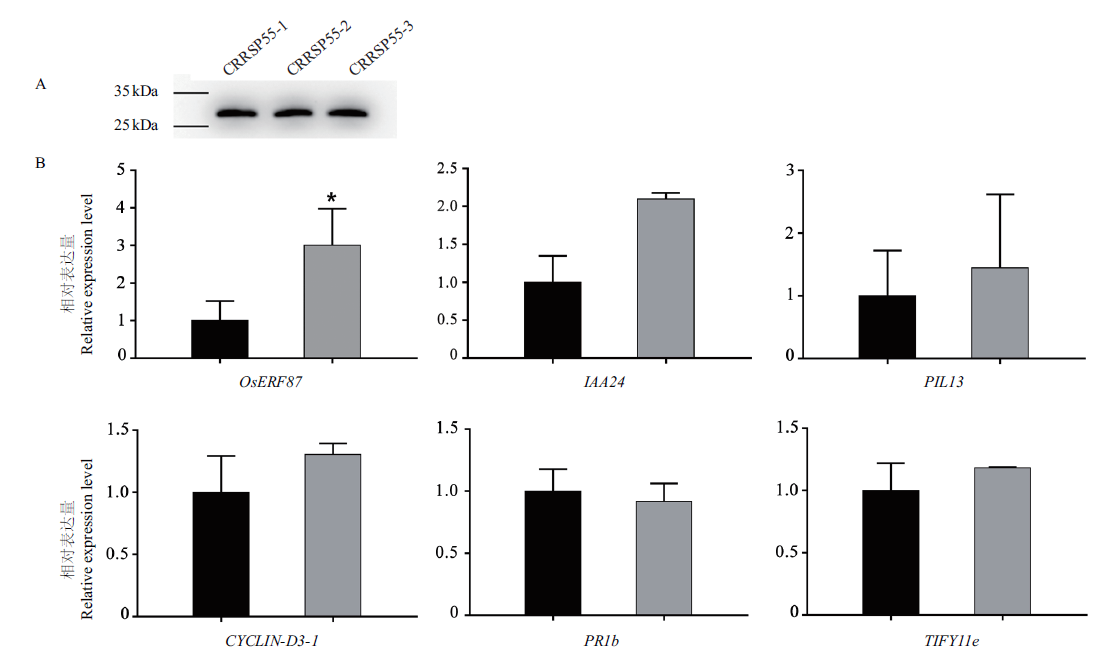
图7 OsCRRSP55影响水稻植物激素通路基因的表达 A:Western blot检测OsCRRSP55在水稻原生质体中的表达。CRRSP55-1,CRRSP55-2,CRRSP55-3为3次生物学重复;B:植物激素通路基因OsERF87、IAA24、PIL13、CYCLIN-D3-1、PR-1b和TIFY11e在水稻中的表达情况。黑色柱:野生型水稻原生质体;灰色柱:瞬时表达OsCRRSP55的水稻原生质体
Fig. 7 Expression levels of genes within rice hormone pathways responding to OsCRRSP55 A:The expression of OsCRRSP55 was detected in rice protoplasts by Western blot. CRRSP55-1,CRRSP55-2 and CRRSP55-3 refer to three biological replicates. B:Expression levels of genes OSERF87,IAA24,PIL13,Cyclin-D3-1,PR-1b and TIFY11e in rice hormone pathways. Black columns:Protoplasts of wild-type rice. Gray columns:Rice protoplasts overexpressing OsCRRSP55
| [1] | Singh P.The rice root-knot nematode, Meloidogyne graminicola:an emerging problem in rice-wheat cropping system[J].Indian Journal of Nematology,2010,40:1-11. |
| [2] |
Mantelin S,Bellafiore S,Kyndt T.Meloidogyne graminicola:a major threat to rice agriculture[J].Mol Plant Pathol,2017,18(1):3-15.
doi: 10.1111/mpp.12394 pmid: 26950515 |
| [3] | 赵洪海,刘维志,梁晨,等.根结线虫在中国的一新纪录种——拟禾本科根结线虫Meloidogyne graminicol[J].植物病理学报,2001,31(2):184-188. |
| Zhao HH,Liu WZ,Liang C,et al.Meloidogyne graminicola, A new record species from China[J].Acta Phytopathol Sin,2001,31(2):184-188. | |
| [4] | 黄文坤,向超,刘莹,等.水稻拟禾本科根结线虫发生与防治[J].植物病理学报,2018,48(3):289-296. |
| Huang WK,Xiang C,Liu Y,et al.Rearch progress on the occurrence and controlling of root-knot nematode Meloidogyne graminicola in rice[J].Acta Phytopathol Sin,2018,48(3):289-296. | |
| [5] |
Ju YL,Wu X,Tan GJ,et al.First report of Meloidogyne graminicola on rice in Anhui Province, China[J].Plant Dis,2021,105(2):512.
doi: 10.1094/PDIS-06-20-1319-PDN URL |
| [6] |
Plowright R,Bridge J.Effect of Meloidogyne graminicola(Nemat-oda)on the establishment, growth and yield of rice cv Ir36[J].Nematologica,1990,36(1/2/3/4):81-89.
doi: 10.1163/002925990X00059 URL |
| [7] |
Chen J,Lin B,Huang Q,et al.A novel Meloidogyne Graminicola effector, MgGPP, is secreted into host cells and undergoes glycosylation in concert with proteolysis to suppress plant defenses and promote parasitism[J].PLoS Pathog,2017,13(4):e1006301.
doi: 10.1371/journal.ppat.1006301 URL |
| [8] |
Naalden D,Haegeman A,de Almeida-Engler J,et al.The Meloidogyne Graminicola effector Mg16820 is secreted in the apoplast and cytoplasm to suppress plant host defense responses[J].Mol Plant Pathol,2018,19(11):2416-2430.
doi: 10.1111/mpp.12719 pmid: 30011122 |
| [9] |
Zhuo K,Naalden D,Nowak S,et al.A Meloidogyne graminicola C-type lectin, Mg01965, is secreted into the host apoplast to suppress plant defence and promote parasitism[J].Mol Plant Pathol,2019,20(3):346-355.
doi: 10.1111/mpp.12759 pmid: 30315612 |
| [10] |
Chen JS,Hu LL,Sun LH,et al.A novel Meloidogyne graminicola effector, MgMO237, interacts with multiple host defence-related proteins to manipulate plant basal immunity and promote parasitism[J].Mol Plant Pathol,2018,19(8):1942-1955.
doi: 10.1111/mpp.2018.19.issue-8 URL |
| [11] |
Raineri J,Wang S,Peleg Z,et al.The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress[J].Plant Mol Biol,2015,88(4/5):401-413.
doi: 10.1007/s11103-015-0329-7 URL |
| [12] |
Yu D,Chen C,Chen Z.Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression[J].Plant Cell,2001,13(7):1527-1540.
pmid: 11449049 |
| [13] | 彭喜旭,胡耀军,唐新科,等.茉莉酸和真菌病原诱导的水稻WRKY30转录因子基因的分离及表达特征[J].中国农业科学,2011,44(12):2454-2461. |
| Peng XX,Hu YJ,Tang XK,et al.Isolation and expression profiles of rice WRKY30 induced by jasmonic acid application and fungal pathogen infection[J].Sci Agric Sin,2011,44(12):2454-2461. | |
| [14] |
Guo L,Li CF,Jiang YZ,et al.Heterologous expression of poplar WRKY18/35 paralogs in Arabidopsis reveals their antagonistic regulation on pathogen resistance and abiotic stress tolerance via variable hormonal pathways[J].Int J Mol Sci,2020,21(15):5440.
doi: 10.3390/ijms21155440 URL |
| [15] | 王瑞霞,王振中,纪春艳,等.水杨酸诱导水稻抗菌物质对稻瘟病菌的抑制作用[J].华中农业大学学报,2011,30(2):193-196. |
| Wang RX,Wang ZZ,Ji CY,et al.Inhibitory activity of antibiotic substances extraction induced by salicylic acid in rice leaves against Magnaporthe grisea[J].J Huazhong Agric Univ,2011,30(2):193-196. | |
| [16] |
Nahar K,Kyndt T,De Vleesschauwer D,et al.The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice[J].Plant Physiol,2011,157(1):305-316.
doi: 10.1104/pp.111.177576 URL |
| [17] |
Yoo SD,Cho YH,Sheen J.Arabidopsis mesophyll protoplasts:a versatile cell system for transient gene expression analysis[J].Nat Protoc,2007,2(7):1565-1572.
doi: 10.1038/nprot.2007.199 URL |
| [18] |
Vaattovaara A,Brandt B,Rajaraman S,et al.Mechanistic insights into the evolution of DUF26-containing proteins in land plants[J].Commun Biol,2019,2:56.
doi: 10.1038/s42003-019-0306-9 pmid: 31924924 |
| [19] | Yeh YH,Chang YH,Huang PY,et al.Enhanced Arabidopsis pattern-triggered immunity by overexpression of cysteine-rich receptor-like kinases[J].Front Plant Sci,2015,6:322. |
| [20] |
Bourdais G,Burdiak P,Gauthier A,et al.Large-scale phenomics identifies primary and fine-tuning roles for CRKs in responses related to oxidative stress[J].PLoS Genet,2015,11(7):e1005373.
doi: 10.1371/journal.pgen.1005373 URL |
| [21] |
Miyakawa T,Miyazono K,Sawano Y,et al.Crystal structure of ginkbilobin-2 with homology to the extracellular domain of plant cysteine-rich receptor-like kinases[J].Proteins,2009,77(1):247-251.
doi: 10.1002/prot.v77:1 URL |
| [22] | Sawano Y,Miyakawa T,Yamazaki H,et al.Purification, characterization, and molecular gene cloning of an antifungal protein from Ginkgo biloba seeds[J].Biol Chem,2007,388(3):273-280. |
| [23] |
Miyakawa T,Hatano K,Miyauchi Y,et al.A secreted protein with plant-specific cysteine-rich motif functions as a mannose-binding lectin that exhibits antifungal activity[J].Plant Physiol,2014,166(2):766-778.
doi: 10.1104/pp.114.242636 pmid: 25139159 |
| [24] |
Ma LS,Wang L,Trippel C,et al.The Ustilago maydis repetitive effector Rsp3 blocks the antifungal activity of mannose-binding maize proteins[J].Nat Commun,2018,9(1):1711.
doi: 10.1038/s41467-018-04149-0 URL |
| [25] |
Yamamoto T,Yoshida Y,Nakajima K,et al.Expression of RSOsPR10 in rice roots is antagonistically regulated by jasmonate/ethylene and salicylic acid via the activator OsERF87 and the repressor OsWRKY76, respectively[J].Plant Direct,2018,2(3):e00049.
doi: 10.1002/pld3.49 URL |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||