








生物技术通报 ›› 2022, Vol. 38 ›› Issue (10): 106-114.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1172
刘宁宁1,2( ), 王鑫昕3, 兰欣悦2, 褚华硕2, 陈旭2, 常世敏1, 李腾飞1(
), 王鑫昕3, 兰欣悦2, 褚华硕2, 陈旭2, 常世敏1, 李腾飞1( ), 许文涛2(
), 许文涛2( )
)
收稿日期:2021-09-13
出版日期:2022-10-26
发布日期:2022-11-11
作者简介:刘宁宁,男,硕士研究生,研究方向:食品加工与安全;E-mail:基金资助:
LIU Ning-ning1,2( ), WANG Xin-xin3, LAN Xin-yue2, CHU Hua-shuo2, CHEN Xu2, CHANG Shi-min1, LI Teng-fei1(
), WANG Xin-xin3, LAN Xin-yue2, CHU Hua-shuo2, CHEN Xu2, CHANG Shi-min1, LI Teng-fei1( ), XU Wen-tao2(
), XU Wen-tao2( )
)
Received:2021-09-13
Published:2022-10-26
Online:2022-11-11
摘要:
四环素因其广谱活性和低成本,在畜牧业和水产养殖中被广泛用于预防细菌感染和提高生长速度,四环素的过度使用导致抗生素在食品中残留,严重威胁人类的健康。硫代黄素T(ThT)可以嵌入到特殊核酸结构中,从而荧光强度得到强激发实现信号输出。本文中四环素适配体可与富G序列结合形成双链,但四环素存在时优先与适配体结合,经解除结合后的富G序列可自身折叠形成G-三链体结构,增强了ThT的荧光强度,依据靶标浓度与ThT荧光强度良好的线性关系,实现对四环素的定量检测。本方法在四环素浓度为1 nmol/L-1 μmol/L范围内有良好线性,R2高达0.99,检测限为0.07 nmol/L,并且在25 min内可以完成检测。因此,一种基于G-三链体的双链竞争内劈裂可视化方法被开发出来并实现了四环素的简便、快速、灵敏、特异、低成本的检测。
刘宁宁, 王鑫昕, 兰欣悦, 褚华硕, 陈旭, 常世敏, 李腾飞, 许文涛. G-三链体可视化核酸传感器用于四环素的检测[J]. 生物技术通报, 2022, 38(10): 106-114.
LIU Ning-ning, WANG Xin-xin, LAN Xin-yue, CHU Hua-shuo, CHEN Xu, CHANG Shi-min, LI Teng-fei, XU Wen-tao. G-Triplex Visualization Nucleic Acid Sensor for the Detection of Tetracycline[J]. Biotechnology Bulletin, 2022, 38(10): 106-114.
| 名称 Name | 序列Sequence | 碱基数 Number of bases/nt |
|---|---|---|
| G01 | 5'-GGGCACCACCAGGGTTAGGG-3' | 20 |
| G02 | 5'-GGGCACCACAGGGTTAGGG-3' | 19 |
| G03 | 5'-GGGCACCAAGGGTTAGGG-3' | 18 |
| G04 | 5'-GGGCACCAGGGTTAGGG-3' | 17 |
| G05 | 5'-GGGCACAGGGTTAGGG-3' | 16 |
| G06 | 5'-GGGCAAGGGTTAGGG-3' | 15 |
| G07 | 5'-GGGCAGGGTTAGGG-3' | 14 |
| Apt1 | 5'-CGGTGGTGCCC-3' | 11 |
表1 适配体及G-三链体序列表
Table 1 Sequence list of aptamers and G-triplex
| 名称 Name | 序列Sequence | 碱基数 Number of bases/nt |
|---|---|---|
| G01 | 5'-GGGCACCACCAGGGTTAGGG-3' | 20 |
| G02 | 5'-GGGCACCACAGGGTTAGGG-3' | 19 |
| G03 | 5'-GGGCACCAAGGGTTAGGG-3' | 18 |
| G04 | 5'-GGGCACCAGGGTTAGGG-3' | 17 |
| G05 | 5'-GGGCACAGGGTTAGGG-3' | 16 |
| G06 | 5'-GGGCAAGGGTTAGGG-3' | 15 |
| G07 | 5'-GGGCAGGGTTAGGG-3' | 14 |
| Apt1 | 5'-CGGTGGTGCCC-3' | 11 |

图2 适配体及序列优化图 A:不同条件下胶体金的颜色图,1:阴性对照,2 :阳性对照,3-5: 分别加入四环素1、10和100 μmol/L;B:G01-G07序列与适配体结合加入靶标前后ThT荧光强度
Fig. 2 Aptamer and sequence optimization diagram A:Color diagram of colloidal gold under different conditions,1 is negative control,2 is positive control,and 3-5 refers to adding tetracycline 1,10,and 100 μmol/L respectively. B:ThT fluorescence intensities before and after adding target while G01-G07 sequences bound with aptamers
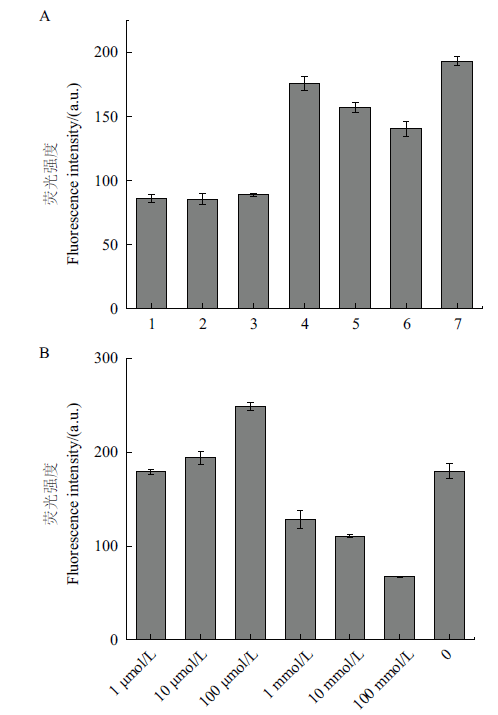
图3 不同条件下ThT荧光强度 A:不同离子存在下ThT荧光强度(1:Tris-HCl+CaCl2,2:Tris-HCl+MgCl2,3:Tris-HCl+KCl,4:H2O,5:CaCl2,6:MgCl2,7:KCl;pH=7.4);B:不同浓度KCl溶液对ThT荧光强度的影响
Fig. 3 ThT fluorescence intensities under different conditions A: ThT fluorescence intensity in the presence of different ions(1: Tris-HCl+CaCl2,2: Tris-HCl+MgCl2,3: Tris-HCl+KCl,4: H2O,5: CaCl2,6: MgCl2,and 7: KCl;PH=7.4);B: effect of KCl solution with different concentration on the ThT fluorescence intensity

图4 时间的优化图 A:靶标与适配体孵育0-30 min内ThT荧光强度变化;B:0-30 min内ThT荧光强度的变化
Fig. 4 Time optimization A: the change of ThT fluorescence intensity during 0-30 min incubation between target and aptamer;B: changes of ThT fluorescence intensity within 0-30 min
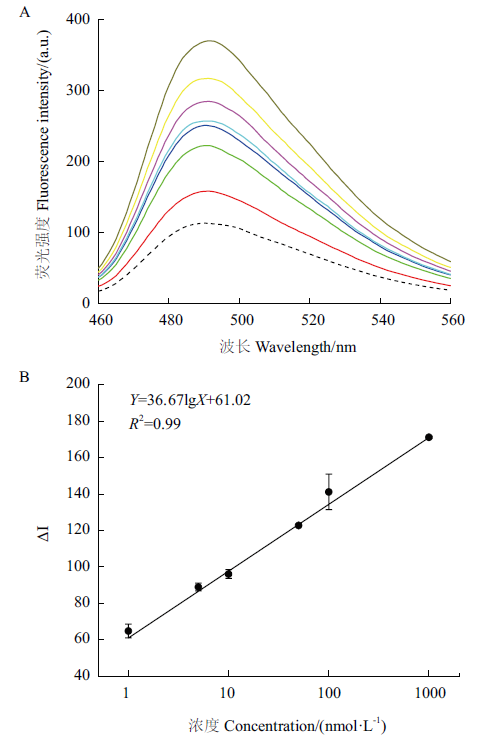
图5 G3-Apt1传感器的建立图 A:不同浓度四环素存在下ThT荧光强度;B:标准曲线
Fig. 5 Establishment of G3-Apt1 sensor A:ThT fluorescence intensity in the presence of tetracycline with different concentrations;B:standard curve
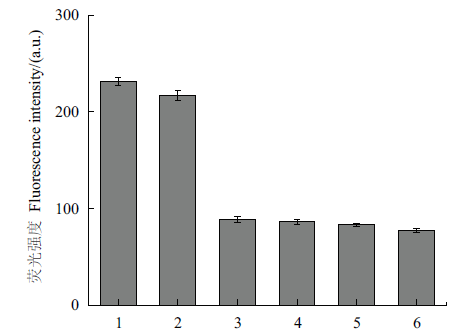
图6 特异性验证图 1:四环素;2:盐酸四环素;3:土霉素;4:氯霉素;5:链霉亲和素;6:林可霉素
Fig. 6 Specificity verification chart 1:Tetracycline. 2:Tetracycline hydrochloride. 3:Oxytetracycline. 4:Chloramphenicol. 5:Streptavidin. 6:Lincomycin
| 添加浓度Adding concentration/(nmol·L-1) | 荧光强度 Fluorescence intensity/(a.u.) | 回收浓度 Recovery concentration/(nmol·L-1) | 平均浓度 Mean concentration/(nmol·L-1) | 回收率 Recovery rate/% | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 115.2 | 114.1 | 115.1 | 0.01 | 0 | 0.03 | 0.01 | 101.33 |
| 1 | 17.0 | 173.2 | 172.5 | 1.08 | 1.10 | 1.05 | 1.08 | 107.67 |
| 10 | 210 | 208 | 209 | 11.09 | 9.78 | 10.42 | 10.43 | 104.3 |
| 100 | 245 | 245.6 | 246 | 99.89 | 104.41 | 106.35 | 103.55 | 103.55 |
| 1 000 | 282 | 281.1 | 281.5 | 1 019.66 | 1 120.36 | 988.14 | 1 042.72 | 104.27 |
表2 牛奶样品中四环素添加回收表
Table 2 Addition and recovery table of tetracycline in milk samples
| 添加浓度Adding concentration/(nmol·L-1) | 荧光强度 Fluorescence intensity/(a.u.) | 回收浓度 Recovery concentration/(nmol·L-1) | 平均浓度 Mean concentration/(nmol·L-1) | 回收率 Recovery rate/% | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 115.2 | 114.1 | 115.1 | 0.01 | 0 | 0.03 | 0.01 | 101.33 |
| 1 | 17.0 | 173.2 | 172.5 | 1.08 | 1.10 | 1.05 | 1.08 | 107.67 |
| 10 | 210 | 208 | 209 | 11.09 | 9.78 | 10.42 | 10.43 | 104.3 |
| 100 | 245 | 245.6 | 246 | 99.89 | 104.41 | 106.35 | 103.55 | 103.55 |
| 1 000 | 282 | 281.1 | 281.5 | 1 019.66 | 1 120.36 | 988.14 | 1 042.72 | 104.27 |
| [1] |
Anderson CR, Rupp HS, Wu WH. Complexities in tetracycline analysis-chemistry, matrix extraction, cleanup, and liquid chromatography[J]. J Chromatogr A, 2005, 1075(1/2):23-32.
doi: 10.1016/j.chroma.2005.04.013 URL |
| [2] |
Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture:a review[J]. Environ Pollut, 2017, 223:161-169.
doi: 10.1016/j.envpol.2017.01.003 URL |
| [3] |
Liu Y, Yang HL, Yang S, et al. High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2013, 917/918:11-17.
doi: 10.1016/j.jchromb.2012.12.036 URL |
| [4] |
Visek WJ. The mode of growth promotion by antibiotics[J]. J Animal Sci, 1978, 46(5):1447-1469.
doi: 10.2527/jas1978.4651447x URL |
| [5] |
Pastor-Navarro N, Morais S, Maquieira A, et al. Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues. Application to honey samples[J]. Anal Chim Acta, 2007, 594(2):211-218.
pmid: 17586117 |
| [6] |
Moats WA. Determination of tetracycline antibiotics in beef and pork tissues using ion-paired liquid chromatography[J]. J Agric Food Chem, 2000, 48(6):2244-2248.
doi: 10.1021/jf990649r URL |
| [7] |
Kowalski P. Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples[J]. J Pharm Biomed Anal, 2008, 47(3):487-493.
doi: 10.1016/j.jpba.2008.01.036 pmid: 18325708 |
| [8] |
Fritz JW, Zuo YG. Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography[J]. Food Chem, 2007, 105(3):1297-1301.
doi: 10.1016/j.foodchem.2007.03.047 URL |
| [9] |
Zhang YL, Lu SX, Liu W, et al. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk[J]. J Agric Food Chem, 2007, 55(2):211-218.
doi: 10.1021/jf062627s URL |
| [10] |
Okerman L, Croubels S, de Baere S, et al. Inhibition tests for detection and presumptive identification of tetracyclines, beta-lactam antibiotics and quinolones in poultry meat[J]. Food Addit Contam, 2001, 18(5):385-393.
pmid: 11358180 |
| [11] |
Aga DS, O’Connor S, Ensley S, et al. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry[J]. J Agric Food Chem, 2005, 53(18):7165-7171.
doi: 10.1021/jf050415+ URL |
| [12] |
Chua A, Yean CY, Ravichandran M, et al. A rapid DNA biosensor for the molecular diagnosis of infectious disease[J]. Biosens Bioelectron, 2011, 26(9):3825-3831.
doi: 10.1016/j.bios.2011.02.040 pmid: 21458979 |
| [13] |
Kim YT, Jung JH, Choi YK, et al. A packaged paper fluidic-based microdevice for detecting gene expression of influenza A virus[J]. Biosens Bioelectron, 2014, 61:485-490.
doi: 10.1016/j.bios.2014.06.006 pmid: 24949821 |
| [14] |
Jung JH, Oh SJ, Kim YT, et al. Combination of multiplex reverse-transcription loop-mediated isothermal amplification with an immunochromatographic strip for subtyping influenza A virus[J]. Anal Chim Acta, 2015, 853:541-547.
doi: S0003-2670(14)01253-7 pmid: 25467501 |
| [15] |
Darmostuk M, Rimpelova S, Gbelcova H, et al. Current approaches in SELEX:an update to aptamer selection technology[J]. Biotechnol Adv, 2015, 33(<W>6 Pt 2):1141-1161.
doi: 10.1016/j.biotechadv.2015.02.008 pmid: 25708387 |
| [16] |
Kim YS, Raston NHA, Gu MB. Aptamer-based nanobiosensors[J]. Biosens Bioelectron, 2016, 76:2-19.
doi: 10.1016/j.bios.2015.06.040 pmid: 26139320 |
| [17] |
Mascini M, Palchetti I, Tombelli S. Nucleic acid and peptide aptamers:fundamentals and bioanalytical aspects[J]. Angew Chem Int Ed Engl, 2012, 51(6):1316-1332.
doi: 10.1002/anie.201006630 URL |
| [18] |
Liu LL, Shao Y, Peng J, et al. Molecular rotor-based fluorescent probe for selective recognition of hybrid G-quadruplex and as a K+ sensor[J]. Anal Chem, 2014, 86(3):1622-1631.
doi: 10.1021/ac403326m URL |
| [19] |
Mohanty J, Barooah N, Dhamodharan V, et al. Thioflavin T as an ef-ficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA[J]. J Am Chem Soc, 2013, 135(1):367-376.
doi: 10.1021/ja309588h URL |
| [20] |
Li YN, Wang JY, Zhang B, et al. A rapid fluorometric method for determination of aflatoxin B1 in plant-derived food by using a thioflavin T-based aptasensor[J]. Mikrochim Acta, 2019, 186(4):214.
doi: 10.1007/s00604-019-3325-9 URL |
| [21] |
Kudrya VY, Yashchuk VM, Dubey IY, et al. The spectral properties of the telomere fragments[J]. Ukr J Phys, 2016, 61(6):516-518.
doi: 10.15407/ujpe61.06.0516 URL |
| [22] |
Choudhury SD, Mohanty J, Pal H, et al. Cooperative metal ion binding to a cucurbit[7]uril-thioflavin T complex:demonstration of a stimulus-responsive fluorescent supramolecular capsule[J]. J Am Chem Soc, 2010, 132(4):1395-1401.
doi: 10.1021/ja908795y URL |
| [23] | 曲瑶, 张亚旗, 肖光, 等. 基于核酸碱基猝灭荧光团的核酸适配体传感器检测赭曲霉毒素A[J]. 分析化学, 2020, 48(10):1409-1415. |
| Qu Y, Zhang YQ, Xiao G, et al. Aptasensor based on nucleic acid base quenching fluorophore for detection of ochratoxin A[J]. Chin J Anal Chem, 2020, 48(10):1409-1415. | |
| [24] |
Hao LL, Wang W, Shen XQ, et al. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of ochratoxin A[J]. J Agric Food Chem, 2020, 68(1):369-375.
doi: 10.1021/acs.jafc.9b06021 URL |
| [25] |
Liu SG, Zhang D, He Y, et al. A split aptamer sensing platform for highly sensitive detection of theophylline based on dual-color fluorescence colocalization and single molecule photobleaching[J]. Biosens Bioelectron, 2020, 166:112461.
doi: 10.1016/j.bios.2020.112461 URL |
| [26] |
Khaled A, Gionfriddo E, Acquaro V Jr, et al. Development and validation of a fully automated solid phase microextraction high throughput method for quantitative analysis of multiresidue veterinary drugs in chicken tissue[J]. Anal Chim Acta, 2019, 1056:34-46.
doi: S0003-2670(18)31493-4 pmid: 30797459 |
| [27] |
de Faria HD, Rosa MA, Silveira AT, et al. Direct extraction of tetracyclines from bovine milk using restricted access carbon nanotubes in a column switching liquid chromatography system[J]. Food Chem, 2017, 225:98-106.
doi: S0308-8146(17)30004-3 pmid: 28193438 |
| [28] |
Zhao WJ, Zuo HY, Guo Y, et al. Porous covalent triazine-terphenyl polymer as hydrophilic-lipophilic balanced sorbent for solid phase extraction of tetracyclines in animal derived foods[J]. Talanta, 2019, 201:426-432.
doi: S0039-9140(19)30399-6 pmid: 31122445 |
| [29] |
Sun CY, Su RF, Bie JX, et al. Label-free fluorescent sensor based on aptamer and thiazole orange for the detection of tetracycline[J]. Dyes Pigments, 2018, 149:867-875.
doi: 10.1016/j.dyepig.2017.11.031 URL |
| [30] |
Dai YY, Zhang Y, Liao WL, et al. G-quadruplex specific thioflavin T-based label-free fluorescence aptasensor for rapid detection of tetracycline[J]. Spectrochim Acta A Mol Biomol Spectrosc, 2020, 238:118406.
doi: 10.1016/j.saa.2020.118406 URL |
| [1] | 李典典, 粟元, 李洁, 许文涛, 朱龙佼. 抗菌适配体的筛选与应用进展[J]. 生物技术通报, 2023, 39(6): 126-132. |
| [2] | 李天顺, 李宸葳, 王佳, 朱龙佼, 许文涛. 功能核酸筛选过程中次级文库的有效制备[J]. 生物技术通报, 2023, 39(3): 116-122. |
| [3] | 周子琦, 张洋子, 兰欣悦, 刘洋儿, 朱龙佼, 许文涛. 发光核酸适配体的筛选及应用[J]. 生物技术通报, 2022, 38(5): 240-247. |
| [4] | 兰欣悦, 刘宁宁, 朱龙佼, 陈旭, 褚华硕, 李相阳, 段诺, 许文涛. 四环素双价适配体非酶免标记传感器[J]. 生物技术通报, 2022, 38(3): 276-284. |
| [5] | 郑芳芳, 林俊生. 增殖诱导配体蛋白的核酸适配体筛选与特异性研究[J]. 生物技术通报, 2021, 37(10): 196-202. |
| [6] | 彭媛媛, 肖星凝, 朱龙佼, 陶晓奇, 许文涛. 小分子物质与适配体的相互作用规律[J]. 生物技术通报, 2020, 36(8): 201-209. |
| [7] | 赵颖, 王楠, 陆安祥, 冯晓元, 郭晓军, 栾云霞. 核酸适配体侧流层析分析技术在真菌毒素检测中的应用[J]. 生物技术通报, 2020, 36(8): 217-227. |
| [8] | 方顺燕, 宋丹, 刘艳萍, 徐文娟, 刘佳瑶, 韩向峙, 龙峰. 用于Escherichia coli O157∶H7直接快速检测的倏逝波荧光核酸适配体传感器研究[J]. 生物技术通报, 2020, 36(7): 228-234. |
| [9] | 余韩洁钰, 朱丽叶, 陈旭, 贺晓云, 许文涛. 细胞特异性核酸适配体的筛选及评价策略[J]. 生物技术通报, 2020, 36(7): 235-244. |
| [10] | 杨敏, 李舒婷, 杨文平, 李相阳, 许文涛. DNA/银纳米簇介导的功能核酸生物传感器研究进展[J]. 生物技术通报, 2020, 36(6): 245-254. |
| [11] | 王琦, 颜春蕾, 高洪伟, 吴薇, 杨庆利. 基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| [12] | 吴学玲, 周翔宇, 吴晓燕, 罗奎, 顾怡超, 周晗, 廖婉晴, 曾伟民. 四环素降解菌共培养体系构建及废水修复的群落分析[J]. 生物技术通报, 2020, 36(10): 116-126. |
| [13] | 吴亚, 徐智辉, 张彪, 赵冬芳, 曹文欣, 张兴平. 核酸适配体光学生物传感器在卡那霉素检测中的研究进展[J]. 生物技术通报, 2020, 36(1): 193-201. |
| [14] | 肖冰, 罗云波, 黄昆仑, 张园, 许文涛. 功能核酸荧光标记型定量统一化检测技术的研究进展[J]. 生物技术通报, 2019, 35(7): 213-221. |
| [15] | 谢银侠, 王蔚然, 程楠, 许文涛. 电信号分子在电化学功能核酸生物传感器中的研究进展[J]. 生物技术通报, 2019, 35(5): 157-169. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||