








生物技术通报 ›› 2023, Vol. 39 ›› Issue (4): 1-9.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1305
• 酶工程专题 • 下一篇
收稿日期:2022-10-25
出版日期:2023-04-26
发布日期:2023-05-16
通讯作者:
曲戈,男,博士,副研究员,研究方向:酶理性设计与工程生物学;E-mail: qug@tib.cas.cn;基金资助:Received:2022-10-25
Published:2023-04-26
Online:2023-05-16
摘要:
作为生物催化剂,酶蛋白介导的生化反应具有条件温和、绿色环保等优点。然而相比化学催化剂,天然酶功能的局限性制约了它在生物制造领域的广泛应用。前期研究表明,酶蛋白除了催化专一性外,同时还展现出混杂性的一面,可在特定条件下催化非天然模式反应。这一特性为酶分子功能重塑提供了新思路,可用来指导人工酶设计,拓展天然酶的催化边界,实现新颖酶促反应类型,以扩大酶催化应用场景。本文从酶催化功能混杂性背后可能的进化机制入手,综述了当前诱导酶催化功能混杂性的常用策略,如定向进化、构象动力学、反应条件诱导及祖先酶重构等技术,并从催化机制、构效关系及适应性进化等多个角度,结合近年来相关研究实例,探讨了催化功能混杂性背后的分子机制,为突破天然酶促反应局限性、创制催化非天然反应的高效人工酶元件提供参考。
曲戈, 孙周通. 催化混杂性驱动的酶功能重塑[J]. 生物技术通报, 2023, 39(4): 1-9.
QU Ge, SUN Zhou-tong. Catalytic Promiscuity-driven Redesign of Enzyme Functions[J]. Biotechnology Bulletin, 2023, 39(4): 1-9.

图2 人工Kemp消除酶设计 a:Kemp消除反应方程式;b:基于不同模板获得的KE07、KE59及KE70,其中KE59以KE59 R1 7/10H突变体结构展示;c:基于木聚糖酶设计的HG3
Fig. 2 Design of artificial Kemp elimination enzymes a: Formula of Kemp elimination reaction. b: KE07, KE59 and KE70 designed from three distinct templates. KE59 is depicted using the crystallographic structure of its mutant R1 7/10H. c: HG3 designed from a xylanase
| Template | Enzyme | kcat(s-1) | Km(mM-1) | kcat/Km(M-1s-1) | Reference |
|---|---|---|---|---|---|
| 裂合酶等 | KE07 | 0.018 | 1.4 | 12.2 | [ |
| KE59 | 0.29 | 1.8 | 163 | [ | |
| KE70 | 0.16 | 2.1 | 78.3 | [ | |
| KE07.7 | 1.37 | 0.54 | 2 590 | [ | |
| KE59.13 | 9.53 | 0.16 | 60 430 | [ | |
| KE70.6 | 5.0 | 0.088 | 57 300 | [ | |
| 木聚糖酶 | HG3 | 0.68 | 1.6 | 425.0 | [ |
| HG3.17 | 700 | 3.0 | 230 000 | [ | |
| P450 BM3 | P450-BM3 | 1.5 | 6 | 240 | [ |
| A82F | 8.4 | 0.27 | 31 000 | [ |
表1 人工Kemp消除酶相关代表性突变体列表
Table 1 Representative mutants related to artificial Kemp elimination enzymes
| Template | Enzyme | kcat(s-1) | Km(mM-1) | kcat/Km(M-1s-1) | Reference |
|---|---|---|---|---|---|
| 裂合酶等 | KE07 | 0.018 | 1.4 | 12.2 | [ |
| KE59 | 0.29 | 1.8 | 163 | [ | |
| KE70 | 0.16 | 2.1 | 78.3 | [ | |
| KE07.7 | 1.37 | 0.54 | 2 590 | [ | |
| KE59.13 | 9.53 | 0.16 | 60 430 | [ | |
| KE70.6 | 5.0 | 0.088 | 57 300 | [ | |
| 木聚糖酶 | HG3 | 0.68 | 1.6 | 425.0 | [ |
| HG3.17 | 700 | 3.0 | 230 000 | [ | |
| P450 BM3 | P450-BM3 | 1.5 | 6 | 240 | [ |
| A82F | 8.4 | 0.27 | 31 000 | [ |

图3 构象动力学指导的TbSADH酶改造 a:催化反应示意图;b:底物结合口袋关键位点的运动性分析;c-d:A85及I86两位点突变后(c)与突变前(d)所在loop区域柔性分析;e:关键位点P84的空间位置;f-h:野生型(f)与两个最优突变体ΔP84/A85G(g)及P84S/I86L(h)的构象自由能路径分布
Fig. 3 Conformational dynamics-guided modification of enzyme TbSADH a: Schematic representation of catalyzed reaction. b: Dynamics analysis of the substrate binding pocket. c-d: Flexibility exploration of the loop region that accommodates residues A85 and I86 after(c)and before mutagenesis(d). e: Position of residue P84. f-h: Free energy landscapes(kcal/mol)of wild type(f), ΔP84/A85G(g)and P84S/I86L(h)
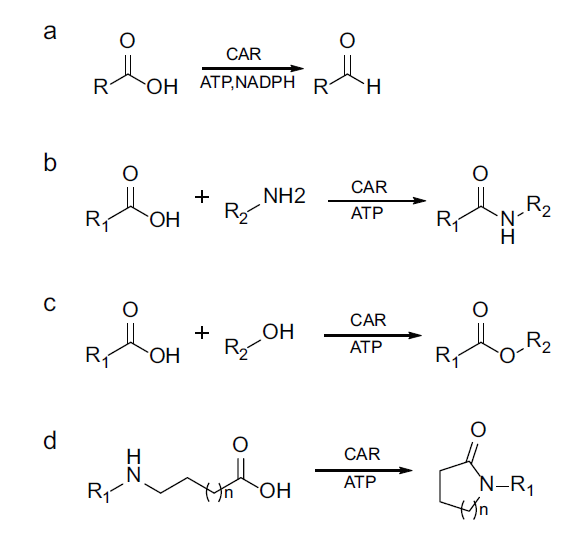
图4 羧酸还原酶介导的混杂性催化反应类型 a:氧化还原反应;b:分子间酰胺化反应;c:分子间酯化反应;d:分子内酰胺化反应
Fig. 4 Promiscuous reactions mediated by carboxylic acid reductase a: Redox reaction; b: intermolecular amidation; c: intermolecular esterification; d: intramolecular lactamization
| [1] |
Fischer E. Einfluss der configuration auf Die wirkung der enzyme[J]. Berichte Der Deutschen Chemischen Gesellschaft, 1894, 27(3): 2985-2993.
doi: 10.1002/cber.v27:3 URL |
| [2] |
Koshland DE. Application of a theory of enzyme specificity to protein synthesis[J]. Proc Natl Acad Sci USA, 1958, 44(2): 98-104.
pmid: 16590179 |
| [3] |
Ycas M. On earlier states of the biochemical system[J]. J Theor Biol, 1974, 44(1): 145-160.
pmid: 4207200 |
| [4] |
Jensen RA. Enzyme recruitment in evolution of new function[J]. Annu Rev Microbiol, 1976, 30: 409-425.
pmid: 791073 |
| [5] |
Baier F, Copp JN, Tokuriki N. Evolution of enzyme superfamilies: comprehensive exploration of sequence-function relationships[J]. Biochemistry, 2016, 55(46): 6375-6388.
pmid: 27802036 |
| [6] | Hammer SC, et al. Design and evolution of enzymes for non-natural chemistry[J]. Curr Opin Green Sustain Chem, 2017, 7: 23-30. |
| [7] |
Newton MS, Arcus VL, Gerth ML, et al. Enzyme evolution: innovation is easy, optimization is complicated[J]. Curr Opin Struct Biol, 2018, 48: 110-116.
doi: 10.1016/j.sbi.2017.11.007 URL |
| [8] | 曲戈, 赵晶, 郑平, 等. 定向进化技术的最新进展[J]. 生物工程学报, 2018, 34(1): 1-11. |
| Qu G, Zhao J, Zheng P, et al. Recent advances in directed evolution[J]. Chin J Biotechnol, 2018, 34(1): 1-11. | |
| [9] | 曲戈, 朱彤, 蒋迎迎, 等. 蛋白质工程:从定向进化到计算设计[J]. 生物工程学报, 2019, 35(10): 1843-1856. |
| Qu G, Zhu T, Jiang YY, et al. Protein engineering: from directed evolution to computational design[J]. Chin J Biotechnol, 2019, 35(10): 1843-1856. | |
| [10] |
Arnold FH. Innovation by evolution: bringing new chemistry to life(Nobel lecture)[J]. Angewandte Chemie Int Ed, 2019, 58(41): 14420-14426.
doi: 10.1002/anie.v58.41 URL |
| [11] |
Qu G, Li AT, Acevedo-Rocha CG, et al. The crucial role of methodology development in directed evolution of selective enzymes[J]. Angew Chem Int Ed Engl, 2020, 59(32): 13204-13231.
doi: 10.1002/anie.v59.32 URL |
| [12] |
Kan SBJ, Lewis RD, Chen K, et al. Directed evolution of cytochrome c for carbon-silicon bond formation: bringing silicon to life[J]. Science, 2016, 354(6315): 1048-1051.
pmid: 27885032 |
| [13] |
Kan SBJ, Huang XY, Gumulya Y, et al. Genetically programmed chiral organoborane synthesis[J]. Nature, 2017, 552(7683): 132-136.
doi: 10.1038/nature24996 URL |
| [14] |
Chen K, Huang XY, Kan SBJ, et al. Enzymatic construction of highly strained carbocycles[J]. Science, 2018, 360(6384): 71-75.
doi: 10.1126/science.aar4239 pmid: 29622650 |
| [15] |
Zhang RK, Chen K, Huang XY, et al. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization[J]. Nature, 2019, 565(7737): 67-72.
doi: 10.1038/s41586-018-0808-5 |
| [16] |
Rui JY, Zhao Q, Huls AJ, et al. Directed evolution of nonheme iron enzymes to access abiological radical-relay C(sp3)-H azidation[J]. Science, 2022, 376(6595): 869-874.
doi: 10.1126/science.abj2830 URL |
| [17] |
Röthlisberger D, Khersonsky O, Wollacott AM, et al. Kemp elimination catalysts by computational enzyme design[J]. Nature, 2008, 453(7192): 190-195.
doi: 10.1038/nature06879 |
| [18] |
Khersonsky O. Evolutionary optimization of computationally designed enzymes: kemp eliminases of the KE07 series[J]. J Mol Biol, 2010, 396(4): 1025-1042.
doi: 10.1016/j.jmb.2009.12.031 pmid: 20036254 |
| [19] |
Khersonsky O, Kiss G, Röthlisberger D, et al. Bridging the gaps in design methodologies by evolutionary optimization of the stability and proficiency of designed Kemp eliminase KE59[J]. Proc Natl Acad Sci USA, 2012, 109(26): 10358-10363.
doi: 10.1073/pnas.1121063109 pmid: 22685214 |
| [20] |
Khersonsky O, Röthlisberger D, Wollacott AM, et al. Optimization of the in-silico-designed kemp eliminase KE70 by computational design and directed evolution[J]. J Mol Biol, 2011, 407(3): 391-412.
doi: 10.1016/j.jmb.2011.01.041 pmid: 21277311 |
| [21] |
Privett HK, Kiss G, Lee TM, et al. Iterative approach to computational enzyme design[J]. Proc Natl Acad Sci USA, 2012, 109(10): 3790-3795.
doi: 10.1073/pnas.1118082108 pmid: 22357762 |
| [22] |
Blomberg R, Kries H, Pinkas DM, et al. Precision is essential for efficient catalysis in an evolved Kemp eliminase[J]. Nature, 2013, 503(7476): 418-421.
doi: 10.1038/nature12623 |
| [23] |
Li AT, Wang BJ, Ilie A, et al. A redox-mediated kemp eliminase[J]. Nat Commun, 2017, 8: 14876.
doi: 10.1038/ncomms14876 pmid: 28348375 |
| [24] |
Bar-Even A, Noor E, Savir Y, et al. The moderately efficient enzyme: evolutionary and physicochemical trends shaping enzyme parameters[J]. Biochemistry, 2011, 50(21): 4402-4410.
doi: 10.1021/bi2002289 pmid: 21506553 |
| [25] |
Otten R, Pádua RAP, Bunzel HA, et al. How directed evolution reshapes the energy landscape in an enzyme to boost catalysis[J]. Science, 2020, 370(6523): 1442-1446.
doi: 10.1126/science.abd3623 pmid: 33214289 |
| [26] |
Martí S, Tuñón I, Moliner V, et al. Are heme-dependent enzymes always using a redox mechanism? A theoretical study of the kemp elimination catalyzed by a promiscuous aldoxime dehydratase[J]. ACS Catal, 2020, 10(19): 11110-11119.
doi: 10.1021/acscatal.0c02215 URL |
| [27] |
Henzler-Wildman K, Kern D. Dynamic personalities of proteins[J]. Nature, 2007, 450(7172): 964-972.
doi: 10.1038/nature06522 |
| [28] |
Crean RM, Gardner JM, Kamerlin SCL. Harnessing conformational plasticity to generate designer enzymes[J]. J Am Chem Soc, 2020, 142(26): 11324-11342.
doi: 10.1021/jacs.0c04924 pmid: 32496764 |
| [29] |
Qu G, Lonsdale R, Yao PY, et al. Methodology development in directed evolution: exploring options when applying triple-code saturation mutagenesis[J]. Chembiochem, 2018, 19(3): 239-246.
doi: 10.1002/cbic.201700562 pmid: 29314451 |
| [30] |
Liu BB, et al. Conformational dynamics-guided loop engineering of an alcohol dehydrogenase: capture, turnover and enantioselective transformation of difficult-to-reduce ketones[J]. Adv Synth Catal, 2019, 361(13): 3182-3190.
doi: 10.1002/adsc.v361.13 URL |
| [31] |
Liu T, Bessembayeva L, Chen J, et al. Development of an economical fermentation platform for enhanced ansamitocin P-3 production in Actinosynnema pretiosum[J]. Bioresour Bioprocess, 2019, 6: 1.
doi: 10.1186/s40643-018-0235-3 |
| [32] | Qu G, Bi YX, Liu BB, et al. Unlocking the stereoselectivity and substrate acceptance of enzymes: proline-induced loop engineering test[J]. Angew Chem Int Ed Engl, 2022, 61(1): e202110793. |
| [33] |
Parra-Cruz R, Jäger CM, Lau PL, et al. Rational design of thermostable carbonic anhydrase mutants using molecular dynamics simulations[J]. J Phys Chem B, 2018, 122(36): 8526-8536.
doi: 10.1021/acs.jpcb.8b05926 URL |
| [34] |
Ouedraogo D, Souffrant M, Vasquez S, et al. Importance of loop L1 dynamics for substrate capture and catalysis in Pseudomonas aeruginosa d-arginine dehydrogenase[J]. Biochemistry, 2017, 56(19): 2477-2487.
doi: 10.1021/acs.biochem.7b00098 pmid: 28445031 |
| [35] |
Han SS, Kyeong HH, Choi JM, et al. Engineering of the conformational dynamics of an enzyme for relieving the product inhibition[J]. ACS Catal, 2016, 6(12): 8440-8445.
doi: 10.1021/acscatal.6b02793 URL |
| [36] |
Cheng ZY, Cui WJ, Liu ZM, et al. A switch in a substrate tunnel for directing regioselectivity of nitrile hydratases towards α, ω-dinitriles[J]. Catal Sci Technol, 2016, 6(5): 1292-1296.
doi: 10.1039/C5CY01997D URL |
| [37] |
Li GY, Yao PY, Gong R, et al. Simultaneous engineering of an enzyme's entrance tunnel and active site: the case of monoamine oxidase MAO-N[J]. Chem Sci, 2017, 8(5): 4093-4099.
doi: 10.1039/c6sc05381e pmid: 30155214 |
| [38] |
Jacquet P, Hiblot J, Daudé D, et al. Rational engineering of a native hyperthermostable lactonase into a broad spectrum phosphotriesterase[J]. Sci Rep, 2017, 7(1): 16745.
doi: 10.1038/s41598-017-16841-0 pmid: 29196634 |
| [39] |
Leveson-Gower RB, Mayer C, Roelfes G. The importance of catalytic promiscuity for enzyme design and evolution[J]. Nat Rev Chem, 2019, 3(12): 687-705.
doi: 10.1038/s41570-019-0143-x |
| [40] |
Qu G, Guo JG, Yang DM, et al. Biocatalysis of carboxylic acid reductases: phylogenesis, catalytic mechanism and potential applications[J]. Green Chem, 2018, 20(4): 777-792.
doi: 10.1039/C7GC03046K URL |
| [41] |
Qu G, Fu MX, Zhao LL, et al. Computational insights into the catalytic mechanism of bacterial carboxylic acid reductase[J]. J Chem Inf Model, 2019, 59(2): 832-841.
doi: 10.1021/acs.jcim.8b00763 pmid: 30688451 |
| [42] |
Cutlan R, de Rose S, Isupov MN, et al. Using enzyme cascades in biocatalysis: highlight on transaminases and carboxylic acid reductases[J]. Biochim Biophys Acta Proteins Proteom, 2020, 1868(2): 140322.
doi: 10.1016/j.bbapap.2019.140322 URL |
| [43] |
Derrington SR, Turner NJ, France SP. Carboxylic acid reductases(CARs): an industrial perspective[J]. J Biotechnol, 2019, 304: 78-88.
doi: S0168-1656(19)30827-2 pmid: 31430498 |
| [44] |
Akhtar MK, Turner NJ, Jones PR. Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities[J]. Proc Natl Acad Sci USA, 2013, 110(1): 87-92.
doi: 10.1073/pnas.1216516110 pmid: 23248280 |
| [45] |
Klumbys E, Zebec Z, Weise NJ, et al. Bio-derived production of cinnamyl alcohol via a three step biocatalytic cascade and metabolic engineering[J]. Green Chem, 2019, 20(3): 658-663.
doi: 10.1039/C7GC03325G pmid: 31168294 |
| [46] |
Bai YF, Yin H, Bi HP, et al. De novo biosynthesis of gastrodin in Escherichia coli[J]. Metab Eng, 2016, 35: 138-147.
doi: 10.1016/j.ymben.2016.01.002 URL |
| [47] |
Wood AJL, Weise NJ, Frampton JD, et al. Adenylation activity of carboxylic acid reductases enables the synthesis of amides[J]. Angew Chem Int Ed Engl, 2017, 56(46): 14498-14501.
doi: 10.1002/anie.201707918 URL |
| [48] |
Lubberink M, Schnepel C, Citoler J, et al. Biocatalytic monoacylation of symmetrical diamines and its application to the synthesis of pharmaceutically relevant amides[J]. ACS Catal, 2020, 10(17): 10005-10009.
doi: 10.1021/acscatal.0c02228 URL |
| [49] |
Pongpamorn P, Kiattisewee C, Kittipanukul N, et al. Carboxylic acid reductase can catalyze ester synthesis in aqueous environments[J]. Angew Chem Int Ed Engl, 2021, 60(11): 5749-5753.
doi: 10.1002/anie.v60.11 URL |
| [50] | Qin ZM, Zhang XH, Sang XK, et al. Carboxylic acid reductases enable intramolecular lactamization reactions[J]. Green Synth Catal, 2022, 3(3): 294-297. |
| [51] |
Yuan B, Debecker DP, Wu XF, et al. One-pot chemoenzymatic deracemisation of secondary alcohols employing variants of galactose oxidase and transfer hydrogenation[J]. ChemCatChem, 2020, 12(24): 6191-6195.
doi: 10.1002/cctc.v12.24 URL |
| [52] |
Huffman MA, Fryszkowska A, Alvizo O, et al. Design of an in vitro biocatalytic cascade for the manufacture of islatravir[J]. Science, 2019, 366(6470): 1255-1259.
doi: 10.1126/science.aay8484 pmid: 31806816 |
| [53] |
Vilím J, Knaus T, Mutti FG. Catalytic promiscuity of galactose oxidase: a mild synthesis of nitriles from alcohols, air, and ammonia[J]. Angew Chem Int Ed Engl, 2018, 57(43): 14240-14244.
doi: 10.1002/anie.201809411 URL |
| [54] |
Hyster TK. Radical biocatalysis: using non-natural single electron transfer mechanisms to access new enzymatic functions[J]. Synlett, 2020, 31(3): 248-254.
doi: 10.1055/s-0037-1611818 URL |
| [55] |
Schmermund L, Jurkaš V, Özgen FF, et al. Photo-biocatalysis: biotransformations in the presence of light[J]. ACS Catal, 2019, 9(5): 4115-4144.
doi: 10.1021/acscatal.9b00656 |
| [56] | Peng YZ, Wang ZG, Chen Y, et al. Photoinduced promiscuity of cyclohexanone monooxygenase for the enantioselective synthesis of α-fluoroketones[J]. Angew Chem Int Ed Engl, 2022, 61(50): e202211199. |
| [57] |
Emmanuel MA, Greenberg NR, Oblinsky DG, et al. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light[J]. Nature, 2016, 540(7633): 414-417.
doi: 10.1038/nature20569 |
| [58] |
Biegasiewicz KF, Cooper SJ, Gao X, et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization[J]. Science, 2019, 364(6446): 1166-1169.
doi: 10.1126/science.aaw1143 pmid: 31221855 |
| [59] | 张武元, 袁波, 曲戈, 等. 光促酶催化反应设计及生物合成应用[J]. 生物学杂志, 2021, 38(5): 1-11. |
| Zhang WY, Yuan B, Qu G, et al. Photobiocatalytic reaction design and its biosynthetic applications[J]. J Biol, 2021, 38(5): 1-11. | |
| [60] |
Sternke M, Tripp KW, Barrick D. Consensus sequence design as a general strategy to create hyperstable, biologically active proteins[J]. Proc Natl Acad Sci USA, 2019, 116(23): 11275-11284.
doi: 10.1073/pnas.1816707116 pmid: 31110018 |
| [61] |
Clifton BE, Kaczmarski JA, Carr PD, et al. Evolution of cyclohexadienyl dehydratase from an ancestral solute-binding protein[J]. Nat Chem Biol, 2018, 14(6): 542-547.
doi: 10.1038/s41589-018-0043-2 pmid: 29686357 |
| [62] |
Kaltenbach M, Burke JR, Dindo M, et al. Evolution of Chalcone isomerase from a noncatalytic ancestor[J]. Nat Chem Biol, 2018, 14(6): 548-555.
doi: 10.1038/s41589-018-0042-3 pmid: 29686356 |
| [63] |
Chen XY, Dou Z, Luo TW, et al. Directed reconstruction of a novel ancestral alcohol dehydrogenase featuring shifted pH-profile, enhanced thermostability and expanded substrate spectrum[J]. Bioresour Technol, 2022, 363: 127886.
doi: 10.1016/j.biortech.2022.127886 URL |
| [64] |
Nguyen V, Wilson C, Hoemberger M, et al. Evolutionary drivers of thermoadaptation in enzyme catalysis[J]. Science, 2017, 355(6322): 289-294.
doi: 10.1126/science.aah3717 pmid: 28008087 |
| [65] |
Zeng B, Zhou YH, Yi ZW, et al. Highly thermostable and promiscuous β-1, 3-xylanasen designed by optimized ancestral sequence reconstruction[J]. Bioresour Technol, 2021, 340: 125732.
doi: 10.1016/j.biortech.2021.125732 URL |
| [66] | Song SY, Jiang YY, Chen RD, et al. Whole-cell biotransformation of penicillin G by a three-enzyme co-expression system with engineered deacetoxycephalosporin C synthase[J]. Chembiochem, 2022, 23(11): e202200179. |
| [67] |
Schulz L, Guo Z, Zarzycki J, et al. Evolution of increased complexity and specificity at the dawn of form I Rubiscos[J]. Science, 2022, 378(6616): 155-160.
doi: 10.1126/science.abq1416 pmid: 36227987 |
| [68] |
Sandoval BA, Hyster TK. Emerging strategies for expanding the toolbox of enzymes in biocatalysis[J]. Curr Opin Chem Biol, 2020, 55: 45-51.
doi: S1367-5931(19)30150-4 pmid: 31935627 |
| [69] |
Wittwer M, Markel U, Schiffels J, et al. Engineering and emerging applications of artificial metalloenzymes with whole cells[J]. Nat Catal, 2021, 4(10): 814-827.
doi: 10.1038/s41929-021-00673-3 |
| [70] |
Bloomer BJ, Clark DS, Hartwig JF. Progress, challenges, and opportunities with artificial metalloenzymes in biosynthesis[J]. Biochemistry, 2022. DOI: 10.1021/acs.biochem.1c00829.
doi: 10.1021/acs.biochem.1c00829 |
| [71] |
Drienovská I, Mayer C, Dulson C, et al. A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue[J]. Nat Chem, 2018, 10(9): 946-952.
doi: 10.1038/s41557-018-0082-z pmid: 29967395 |
| [72] |
Burke AJ, Lovelock SL, Frese A, et al. Design and evolution of an enzyme with a non-canonical organocatalytic mechanism[J]. Nature, 2019, 570(7760): 219-223.
doi: 10.1038/s41586-019-1262-8 |
| [73] |
Yang KK, Wu Z, Arnold FH. Machine-learning-guided directed evolution for protein engineering[J]. Nat Methods, 2019, 16(8): 687-694.
doi: 10.1038/s41592-019-0496-6 pmid: 31308553 |
| [1] | 王慕镪, 陈琦, 马薇, 李春秀, 欧阳鹏飞, 许建和. 机器学习方法在酶定向进化中的应用进展[J]. 生物技术通报, 2023, 39(4): 38-48. |
| [2] | 张雪, 谭玉萌, 蒋海霞, 杨广宇. 基于单细胞超高通量筛选的α-1,2-岩藻糖基转移酶定向进化[J]. 生物技术通报, 2022, 38(1): 289-298. |
| [3] | 陈春, 宿玲恰, 夏伟, 吴敬. 定向进化提高来源于Arthrobacter ramosus 的MTHase的热稳定性[J]. 生物技术通报, 2021, 37(3): 84-91. |
| [4] | 蔡玉贞, 白巧燕, 苏敏, 唐良华. 脂肪酶底物结合口袋的分子改造策略及进展[J]. 生物技术通报, 2020, 36(11): 173-180. |
| [5] | 王叶, 贾振华, 宋水山. 宏基因组学结合合成生物学法挖掘新型生物催化剂的研究进展[J]. 生物技术通报, 2018, 34(8): 35-42. |
| [6] | 任天雷, 杨海泉, 许菲. 基于分子结构与生物信息学等多维度特征的定向进化改造甲基对硫磷水解酶[J]. 生物技术通报, 2018, 34(10): 194-200. |
| [7] | 王晓璐, 王钰,刘娇,郑平,路福平. 利用定向进化提高基因工程大肠杆菌的甲醇利用能力[J]. 生物技术通报, 2017, 33(9): 101-109. |
| [8] | 郭园, 赵仲麟. 微生物系统定向进化与合成生物学应用研究进展[J]. 生物技术通报, 2017, 33(1): 76-82. |
| [9] | 吴树丽, 刘启顺, 谭海东, 张付云, 尹恒. 5-羟甲基糠醛的生物催化氧化研究进展[J]. 生物技术通报, 2016, 32(9): 50-58. |
| [10] | 张雪玲,陈小利,李荷. 漆酶Lac1338的酶学特性测定及定向突变对其酶解染料影响[J]. 生物技术通报, 2016, 32(7): 170-177. |
| [11] | 吕永坤,堵国成,陈坚,周景文. 合成生物学技术研究进展[J]. 生物技术通报, 2015, 31(4): 134-148. |
| [12] | 王玺,段胜林,熊舒莉,郑桂兰,张贵友,王洪钟. 自诱导系统在酶促合成2’-脱氧胞苷中的应用[J]. 生物技术通报, 2014, 0(11): 225-232. |
| [13] | 刘瑜,李丕武. 黑曲霉葡萄糖氧化酶高产基因工程菌研究进展[J]. 生物技术通报, 2013, 0(7): 12-19. |
| [14] | 邵敏, 李长福, 葛正龙, 周鹤峰. 基于易错PCR技术定向进化枯草芽孢杆菌β-葡聚糖酶[J]. 生物技术通报, 2013, 0(12): 141-145. |
| [15] | 左可, 李志敏, 叶勤. 红球菌Rhodococcus. sp G14生物转化合成2-苯 基丙酸[J]. 生物技术通报, 2013, 0(12): 173-177. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
