








生物技术通报 ›› 2025, Vol. 41 ›› Issue (9): 232-241.doi: 10.13560/j.cnki.biotech.bull.1985.2025-0064
• 研究报告 • 上一篇
于文杰1,2( ), 范斯然2, 高文丽2, 邢宇2(
), 范斯然2, 高文丽2, 邢宇2( ), 秦岭1,2(
), 秦岭1,2( )
)
收稿日期:2025-01-16
出版日期:2025-09-26
发布日期:2025-09-24
通讯作者:
邢宇,女,博士,教授,研究方向 :板栗果实发育分子生物学;E-mail: xingyu@bua.edu.cn作者简介:于文杰,男,博士研究生,研究方向 :板栗果实品质分子改良;E-mail: buayuwenjie@163.com
基金资助:
YU Wen-jie1,2( ), FAN Si-ran2, GAO Wen-li2, XING Yu2(
), FAN Si-ran2, GAO Wen-li2, XING Yu2( ), QIN Ling1,2(
), QIN Ling1,2( )
)
Received:2025-01-16
Published:2025-09-26
Online:2025-09-24
摘要:
目的 二氧四氢蝶啶合成酶(lumazine synthase, LS)和核黄素合成酶(riboflavin synthase, RS)是板栗核黄素合成的关键基因,探究其对板栗核黄素合成的影响,为板栗核黄素合成提供分子理论基础。 方法 以模式植物拟南芥核黄素合成相关基因的蛋白序列为诱饵,在板栗基因组数据库中通过BLAST方法鉴定核黄素合成相关酶基因,利用转录组数据分析相关基因在板栗果实发育3个时期的表达模式。利用超高效液相色谱仪测定8个板栗品种中核黄素含量,结合基因表达量进行组间差异分析和相关性分析,利用生物信息学方法分析关键基因的结构、蛋白理化性质、系统进化和启动子顺式作用元件。通过板栗愈伤组织瞬时转化体系初步验证关键基因功能。 结果 板栗中有2个GTP环水解酶Ⅱ(GCHⅡ),2个嘧啶脱氨酶(PYRD),1个嘧啶还原酶(PYRR),1个嘧啶磷酸酶(PYRP),2个3,4-二羟基-2-丁酮-4-磷酸合酶(DHBPs),2个二氧四氢蝶啶合成酶(LS)和1个核黄素合成酶(RS),二氧四氢蝶啶合成酶和核黄素合成酶在板栗果仁中表达量最高。板栗中核黄素含量范围在0.054‒0.104 mg/100 g,CmLS1和CmRS的基因表达量与核黄素含量分别呈显著正相关和极显著正相关。CmLS1全长693 bp,编码231个氨基酸,CmRS全长843 bp,编码281个氨基酸。CmLS1与欧榛(Corylus avellana)同源性最高,CmRS与栓皮栎(Quercus suber)、白栎(Quercus lobata)和夏栎(Corylus avellana)的同源性最高,CmLS1和CmRS启动子上主要包含激素响应顺式作用元件、光响应顺式作用元件和低温响应元件还有厌氧诱导响应元件。沉默CmLS1和CmRS后板栗愈伤组织中核黄素含量分别下降33.1%和49.1%。 结论 CmLS1和CmRS是板栗核黄素合成关键基因,正调控板栗核黄素合成。
于文杰, 范斯然, 高文丽, 邢宇, 秦岭. 板栗核黄素合成通路关键基因鉴定及功能验证[J]. 生物技术通报, 2025, 41(9): 232-241.
YU Wen-jie, FAN Si-ran, GAO Wen-li, XING Yu, QIN Ling. Identification and Functional Verification of Key Genes in Riboflavin Synthesis Pathway in Chinese Chestnut[J]. Biotechnology Bulletin, 2025, 41(9): 232-241.
参数名称 Parameter name | 参数 Parameter |
|---|---|
| 色谱柱 Chromatographic column | C18柱150 mm × 4.6 mm × 5 μm |
| 流动相 Mobile phase | 乙酸钠溶液(0.05 mol/L)-甲醇(80∶20) |
| 流速 Velocity of flow (mL/min) | 1 |
| 柱温 Column temperature (℃) | 30 |
| 检测波长 Test wavelength | 激发波长462 nm,发射波长522 nm |
| 进样体积 Injection volume (μL) | 20 |
表1 HPLC参数
Table 1 HPLC parameters
参数名称 Parameter name | 参数 Parameter |
|---|---|
| 色谱柱 Chromatographic column | C18柱150 mm × 4.6 mm × 5 μm |
| 流动相 Mobile phase | 乙酸钠溶液(0.05 mol/L)-甲醇(80∶20) |
| 流速 Velocity of flow (mL/min) | 1 |
| 柱温 Column temperature (℃) | 30 |
| 检测波长 Test wavelength | 激发波长462 nm,发射波长522 nm |
| 进样体积 Injection volume (μL) | 20 |
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| CmRS RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCTCTCACATCATTCTCCAA | 56.5 |
| CmRS RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACGCGGCTATTCTCATATCG | 57 |
| CmLS1 RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCCAGAACTCAGGGTCTTC | 60.3 |
| CmLS1 RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACCAGCCGAATTAGCAACAG | 60.4 |
表2 CmLS1和CmRS目的基因特异性片段克隆引物
Table 2 Primers for cloning specific fragments of CmLS1 and CmRS target genes
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| CmRS RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCTCTCACATCATTCTCCAA | 56.5 |
| CmRS RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACGCGGCTATTCTCATATCG | 57 |
| CmLS1 RNAi-F | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCCCAGAACTCAGGGTCTTC | 60.3 |
| CmLS1 RNAi-R | GGGGACCACTTTGTACAAGAAAGCTGGGTACCAGCCGAATTAGCAACAG | 60.4 |
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| Actin-F | TTGACTATGAGCAGGAACTT | 58.9 |
| Actin-R | TTGTAGGTGGTCTCGTGAAT | 60.8 |
| CmRS QP-F | CGGCATAGTTGAAGAAGT | 56.8 |
| CmRS QP-R | GCGGCTATTCTCATATCG | 57.0 |
| CmLS1 QP-F | GCCATCCTACACCTTAACAG | 58.4 |
| CmLS1 QP-R | CTTCCTCTCAACAGCATAGC | 59.0 |
表3 CmLS1和CmRS荧光定量PCR引物
Table 3 Primers for fluorescence quantitative PCR of CmLS1 and CmRS
引物名称 Primer name | 引物序列 Primer sequence (5'‒3') | 退火温度 Annealing temperature (℃) |
|---|---|---|
| Actin-F | TTGACTATGAGCAGGAACTT | 58.9 |
| Actin-R | TTGTAGGTGGTCTCGTGAAT | 60.8 |
| CmRS QP-F | CGGCATAGTTGAAGAAGT | 56.8 |
| CmRS QP-R | GCGGCTATTCTCATATCG | 57.0 |
| CmLS1 QP-F | GCCATCCTACACCTTAACAG | 58.4 |
| CmLS1 QP-R | CTTCCTCTCAACAGCATAGC | 59.0 |
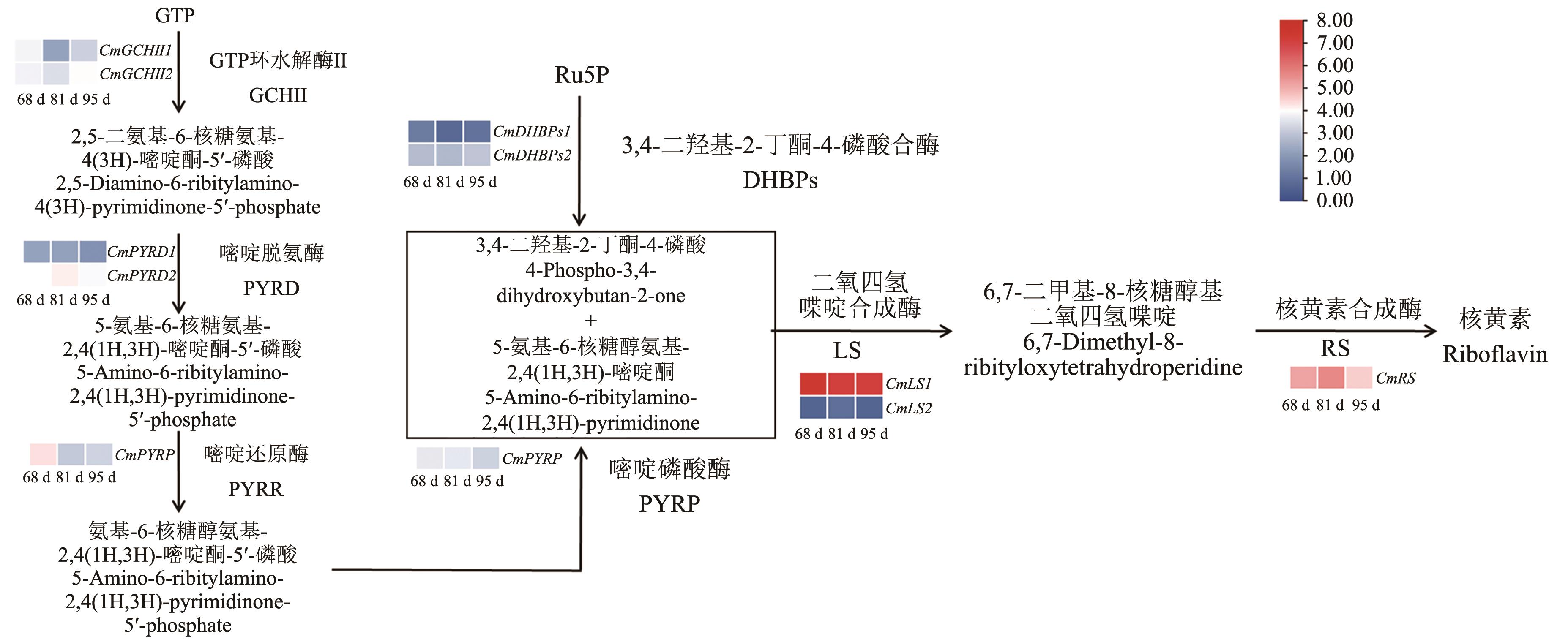
图1 板栗核黄素合成酶基因鉴定和果实不同发育时期基因表达量分析花后68、81和95 d的板栗果实基因表达量
Fig. 1 Identification of riboflavin synthetase gene and analysis of gene expression at different developmental stages of Chinese chestnutGene expression in Chinese chestnut (Castanea mollissima) at 68, 81, and 95 d after anthesis
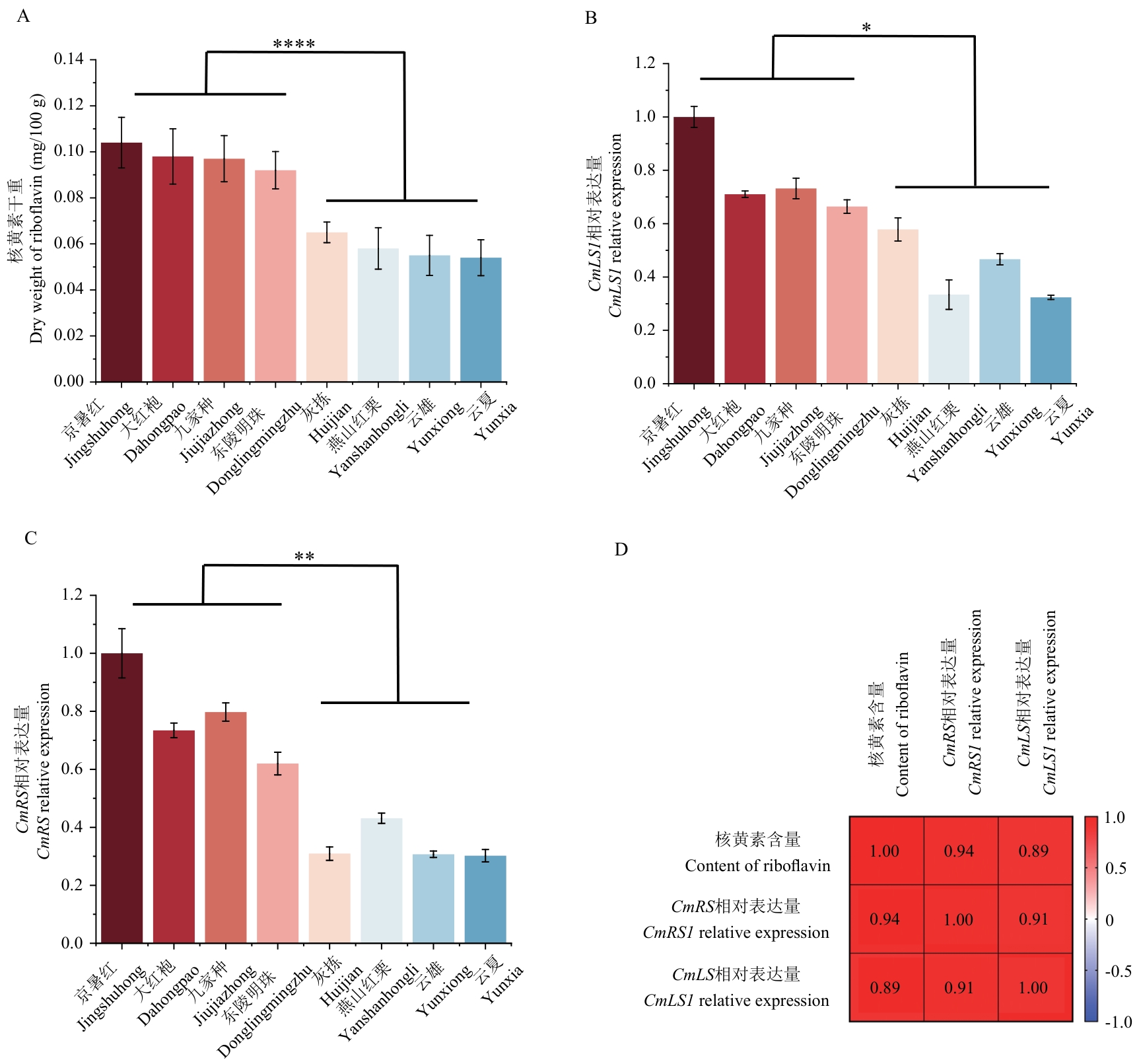
图2 板栗不同品种核黄素含量以及与CmLS1和CmRS基因表达量的相关性分析A:板栗核黄素含量和组间差异分析;B:CmLS1在板栗不同品种中表达差异和组间差异分析;C:CmRS在板栗不同品种中表达差异和组间差异分析;D:CmLS1和CmRS和核黄素含量相关性分析;**** P<0.001;** P<0.01;* P<0.05; n=3;下同
Fig. 2 Correlation analysis of riboflavin contents and expressions of CmLS1 and CmRS genes in different Chinese chestnut cultivarsA: Analysis of riboflavin content in Chinese chestnut and differences between groups. B: Differential expressions of CmLS1 in Chinese chestnut cultivars and analysis of differences between groups. C: Differential expression of CmRS in Chinese chestnut cultivars and analysis of differences between groups. D: Correlation analysis of CmLS1, CmRS and riboflavin content. **** P<0.001; ** P<0.01; * P<0.05; n=3. The same below
蛋白 Protein | 蛋白长度 Length of protein (aa) | 分子量 Molecular weight (Da) | 等电点 Isoelectric point | 不稳定系数 Coef ficient of instability | 最大亲水性 Derophilic index | 亚细胞定位 Subcel lular localization |
|---|---|---|---|---|---|---|
| CmLS1 | 230 | 24 707.02 | 8.82 | 40.54 | -0.030 | 叶绿体 |
| CmRS | 280 | 30 621.49 | 6.24 | 27.41 | 0.047 | 叶绿体 |
表4 板栗CmLS1和CmRS蛋白理化性质
Table 4 Physicochemical properties of CmLS1 and CmRS proteins in Chinese chestnut
蛋白 Protein | 蛋白长度 Length of protein (aa) | 分子量 Molecular weight (Da) | 等电点 Isoelectric point | 不稳定系数 Coef ficient of instability | 最大亲水性 Derophilic index | 亚细胞定位 Subcel lular localization |
|---|---|---|---|---|---|---|
| CmLS1 | 230 | 24 707.02 | 8.82 | 40.54 | -0.030 | 叶绿体 |
| CmRS | 280 | 30 621.49 | 6.24 | 27.41 | 0.047 | 叶绿体 |
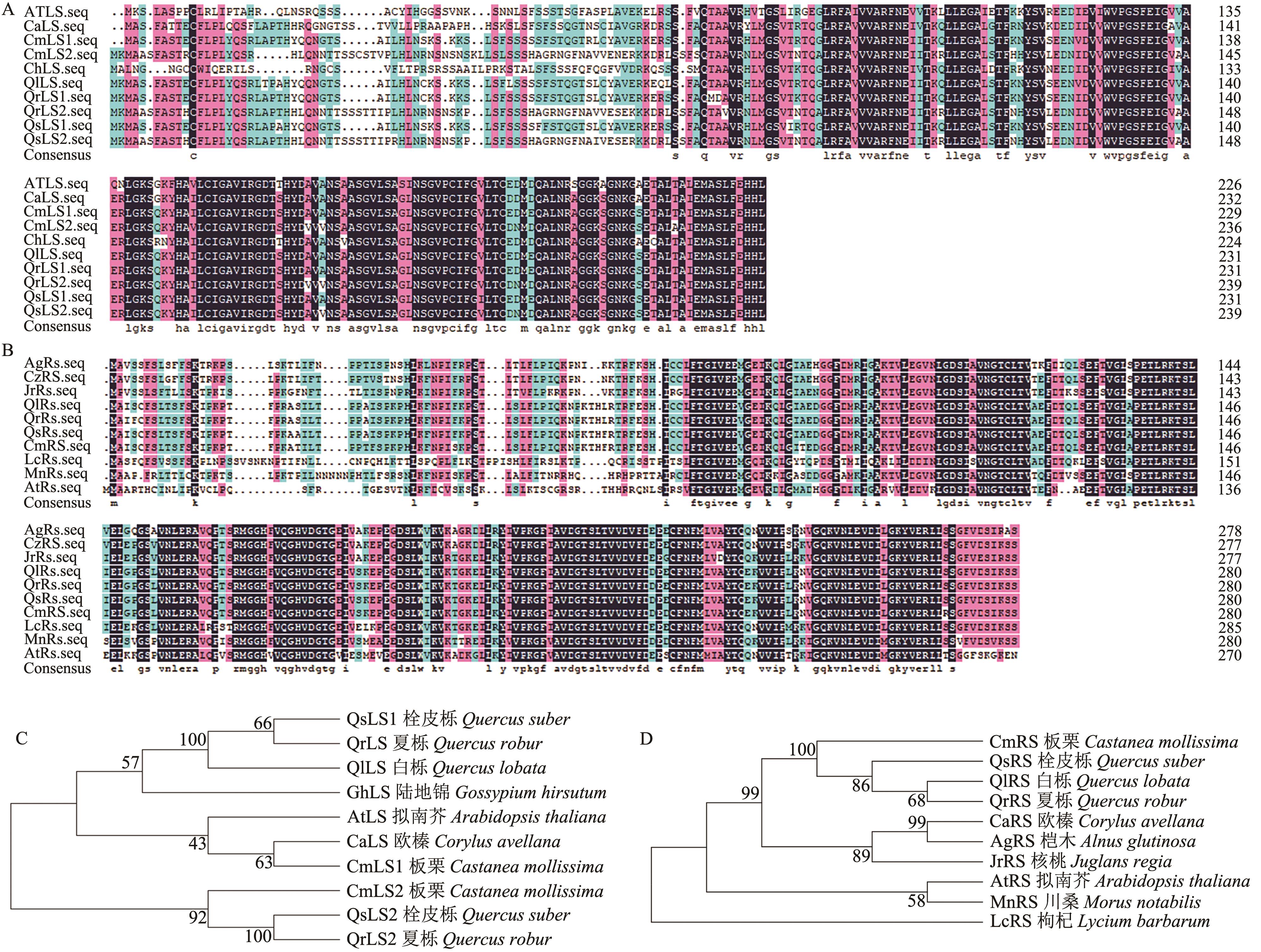
图4 二氧四氢蝶啶合成酶LS和核黄素合成酶RS蛋白序列比对和进化树分析A:板栗二氧四氢蝶啶合成酶蛋白序列比对; B:板栗核黄素合成酶蛋白序列比对;C:板栗二氧四氢蝶啶合成酶进化树分析;D:板栗核黄素合成酶进化树分析
Fig. 4 Protein sequence alignment and phylogenetic tree analysis of lumazine synthase LS and riboflavin synthetase RSA: Protein sequence alignment of lumazine synthase in Chinese chestnut. B: Protein sequence alignment of riboflavin synthetase in Chinese chestnut. C: Phylogenetic tree analysis of lumazine synthas in Chinese chestnut. D: Phylogenetic tree analysis of riboflavin synthetase in Chinese chestnut
元件名称 Name of element | 元件序列 Sequence of element | 元件类型 Type of element | CmLS1启动子元件数量 Number of CmLS1 promoter element | CmRS启动子元件数量 Number of CmRS promoter element |
|---|---|---|---|---|
| G-box | CACGTT | 光响应元件 | 2 | 1 |
| AuxRE | TGTCTCAATAAG | 生长素响应元件 | 0 | 2 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 | 2 | 1 |
| ABRE | ACGTG | 脱落酸响应元件 | 1 | 1 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 | 0 | 1 |
| ARE | AAACCA | 厌氧诱导响应元件 | 3 | 2 |
| LER | CCGAAA | 低温响应元件 | 2 | 0 |
表5 板栗二氧四氢蝶啶合成酶CmLS1和核黄素合成酶CmRS启动子顺式作用元件分析
Table 5 Analyses of cis-acting regulatory elements in the promotersoflumazine synthase CmLS1 andriboflavin synthetase CmRS
元件名称 Name of element | 元件序列 Sequence of element | 元件类型 Type of element | CmLS1启动子元件数量 Number of CmLS1 promoter element | CmRS启动子元件数量 Number of CmRS promoter element |
|---|---|---|---|---|
| G-box | CACGTT | 光响应元件 | 2 | 1 |
| AuxRE | TGTCTCAATAAG | 生长素响应元件 | 0 | 2 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应元件 | 2 | 1 |
| ABRE | ACGTG | 脱落酸响应元件 | 1 | 1 |
| TCA-element | CCATCTTTTT | 水杨酸响应元件 | 0 | 1 |
| ARE | AAACCA | 厌氧诱导响应元件 | 3 | 2 |
| LER | CCGAAA | 低温响应元件 | 2 | 0 |
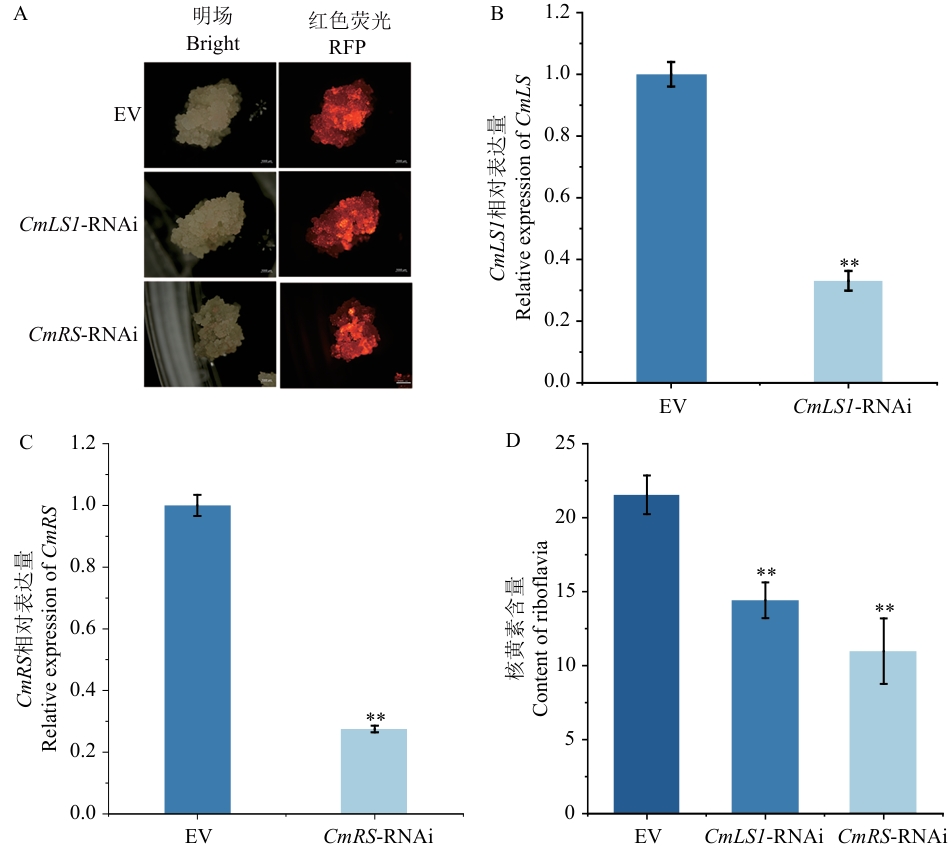
图5 CmLS1和CmRS瞬时沉默和核黄素含量测定A:愈伤组织荧光观察;B:CmLS1相对表达量;C:CmRS相对表达量;D:阳性愈伤组织核黄素含量检测;标尺=1 000 μm
Fig. 5 CmLS1 and CmRS transient silencing and determination of riboflavin contentA: Fluorescence observation of callus. B: Relative expression of CmLS1. C: Relative expression of CmRS. D: Riboflavin content detection of positive callus. Bar =1 000 μm
| [1] | Averianova LA, Balabanova LA, Son OM, et al. Production of vitamin B2 (riboflavin) by microorganisms: an overview [J]. Front Bioeng Biotechnol, 2020, 8: 570828. |
| [2] | 李俊霖, 边祥雨, 姚站馨, 等. 膳食核黄素参考摄入量国内外研究进展 [J]. 营养学报, 2022, 44(6): 530-533. |
| Li JL, Bian XY, Yao ZX, et al. Revision of dietary riboflavin reference intake: an update [J]. Acta Nutr Sin, 2022, 44(6): 530-533. | |
| [3] | 万旻. 核黄素营养状况对骨骼健康影响的研究 [D]. 北京: 军事科学院, 2023. |
| Wan M. Study of the effect of riboflavin nutritional status on bone health [D]. Beijing: Academy of Military Science, 2023. | |
| [4] | 胡海涛, 郭龙彪. 植物核黄素的生物合成及其功能研究进展 [J]. 植物学报, 2023, 58(4): 638-655. |
| Hu HT, Guo LB. Progress in the research on riboflavin biosynthesis and function in plants [J]. Chin Bull Bot, 2023, 58(4): 638-655. | |
| [5] | Ozbekova Z, Kulmyrzaev A. Study of moisture content and water activity of rice using fluorescence spectroscopy and multivariate analysis [J]. Spectrochim Acta Part A Mol Biomol Spectrosc, 2019, 223: 117357. |
| [6] | Villareal CP, Juliano BO. Variability in contents of thiamine and riboflavin in brown rice, crude oil in brown rice and bran-Polish, and silicon in hull of IR rices [J]. Plant Foods Hum Nutr, 1989, 39(3): 287-297. |
| [7] | 武妍妍, 史文石, 石新如, 等. 板栗坚果营养物质和抗氧化成分综合评价 [J]. 林业科学研究, 2022, 35(6): 12-22. |
| Wu YY, Shi WS, Shi XR, et al. Comprehensive evaluation of nutrients and antioxidant components in nuts of chestnut [J]. For Res, 2022, 35(6): 12-22. | |
| [8] | 徐志祥, 高绘菊. 板栗营养价值及其养生保健功能 [J]. 食品研究与开发, 2004, 25(5): 118-119. |
| Xu ZX, Gao HJ. Nutritional value of chestnut and its health care function [J]. Food Res Dev, 2004, 25(5): 118-119. | |
| [9] | 张瑞菊, 孙强, 张洪坤. 板栗的营养、生产现状及前景展望 [J]. 山东商业职业技术学院学报, 2014, 14(4): 106-107. |
| Zhang RJ, Sun Q, Zhang HK. Nutrition, production status and prospect of Chinese chestnut [J]. J Shandong Inst Commer Technol, 2014, 14(4): 106-107. | |
| [10] | 乔艳杰, 刘巍, 吴瑞刚, 等. 北京市板栗产业发展问题及对策研究 [J]. 中国果树, 2024(6): 125-129. |
| Qiao YJ, Liu W, Wu RG, et al. Research on the development problems and countermeasures of Chinese chestnut industry in Beijing [J]. China Fruits, 2024(6): 125-129. | |
| [11] | 韩元顺, 许林云, 周杰. 中国板栗产业与市场发展现状及趋势 [J]. 中国果树, 2021(4): 83-88. |
| Han YS, Xu LY, Zhou J. Status and trend of the development of chestnut industry in China [J]. China Fruits, 2021(4): 83-88. | |
| [12] | 蔡荣, 虢佳花, 祁春节. 板栗产业发展现状、存在问题与对策分析 [J]. 中国果菜, 2007, 27(1): 52-53. |
| Cai R, Guo JH, Qi CJ. Present situation, existing problems and countermeasures of chestnut industry development [J]. China Fruit Veg, 2007, 27(1): 52-53. | |
| [13] | Tolar JG, Li SL, Ajo-Franklin CM. The differing roles of flavins and quinones in extracellular electron transfer in lactiplantibacillus plantarum [J]. Appl Environ Microbiol, 2023, 89(1): e0131322. |
| [14] | Bacher A, Eberhardt S, Eisenreich W, et al. Biosynthesis of riboflavin [M]//Cofactor Biosynthesis. Amsterdam: Elsevier, 2001: 1-49. |
| [15] | 郭长江, 顾景范. 核黄素 [J]. 营养学报, 2013, 35(2): 119-121. |
| Guo CJ, Gu JF. Riboflavin [J]. Acta Nutr Sin, 2013, 35(2): 119-121. | |
| [16] | 王林静, 黄亿明. 核黄素与健康 [J]. 广东药学院学报, 2000, 16(3): 223-225, 228. |
| Wang LJ, Huang YM. Riboflavin and health [J]. Acad J Guangdong Coll Pharm, 2000, 16(3): 223-225, 228. | |
| [17] | Xu ZB, Lin ZQ, Wang ZW, et al. Improvement of the riboflavin production by engineering the precursor biosynthesis pathways in Escherichia coli [J]. Chin J Chem Eng, 2015, 23(11): 1834-1839. |
| [18] | Harale B, Kidwai S, Ojha D, et al. Synthesis and evaluation of antimycobacterial activity of riboflavin derivatives [J]. Bioorg Med Chem Lett, 2021, 48: 128236. |
| [19] | Tani T, Sobajima H, Okada K, et al. Identification of the OsOPR7 gene encoding 12-oxophytodienoate reductase involved in the biosynthesis of jasmonic acid in rice [J]. Planta, 2008, 227(3): 517-526. |
| [20] | Sa N, Rawat R, Thornburg C, et al. Identification and characterization of the missing phosphatase on the riboflavin biosynthesis pathway in Arabidopsis thaliana [J]. Plant J, 2016, 88(5): 705-716. |
| [21] | Xiao S, Dai LY, Liu FQ, et al. COS1: an Arabidopsis coronatine insensitive1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence [J]. Plant Cell, 2004, 16(5): 1132-1142. |
| [22] | Hu HT, Ren DY, Hu J, et al. WHITE AND LESION-MIMIC LEAF1, encoding a lumazine synthase, affects reactive oxygen species balance and chloroplast development in rice [J]. Plant J, 2021, 108(6): 1690-1703. |
| [23] | 任秀艳, 乔洁, 张江丽. 核黄素合酶的研究进展 [J]. 西北植物学报, 2011, 31(9): 1917-1926. |
| Ren XY, Qiao J, Zhang JL. Research progress of riboflavin synthase [J]. Acta Bot Boreali Occidentalia Sin, 2011, 31(9): 1917-1926. | |
| [24] | 黄伟志, 黄桂东, 钟先锋. 高效液相色谱法测定功能性饮料中维生素B1、维生素B2含量 [J]. 食品研究与开发, 2019, 40(24): 191-197. |
| Huang WZ, Huang GD, Zhong XF. Determination of vitamins B1 and vitamins B2 in energy drinks by high performance liquid chromatography [J]. Food Res Dev, 2019, 40(24): 191-197. | |
| [25] | 朱丽, 钱前. 虾青素功能米: 生物强化新思路, 优质米培育新资源 [J]. 植物学报, 2019, 54(1): 4-8. |
| Zhu L, Qian Q. Astaxanthin functional rice: new idea of biofortification, new perspectives for high-quality rice breeding [J]. Chin Bull Bot, 2019, 54(1): 4-8. | |
| [26] | Suwannasom N, Kao I, Pruß A, et al. Riboflavin: the health benefits of a forgotten natural vitamin [J]. Int J Mol Sci, 2020, 21(3): 950. |
| [27] | Tuan PA, Kim JK, Lee S, et al. Riboflavin accumulation and characterization of cDNAs encoding lumazine synthase and riboflavin synthase in bitter melon (Momordica charantia) [J]. J Agric Food Chem, 2012, 60(48): 11980-11986. |
| [28] | Zhao YY, Wang DF, Wu TQ, et al. Transgenic expression of a rice riboflavin synthase gene in tobacco enhances plant growth and resistance to Tobacco mosaic virus [J]. Can J Plant Pathol, 2014, 36(1): 100-109. |
| [29] | Namba J, Harada M, Shibata R, et al. AtDREB2G is involved in the regulation of riboflavin biosynthesis in response to low-temperature stress and abscisic acid treatment in Arabidopsis thaliana [J]. Plant Sci, 2024, 347: 112196. |
| [30] | Wang TZ, Wang J, Zhang D, et al. Protein kinase MtCIPK12 modulates iron reduction in Medicago truncatula by regulating riboflavin biosynthesis [J]. Plant Cell Environ, 2023, 46(3): 991-1003. |
| [31] | Xu XP, Zhang CY, Xu XQ, et al. Riboflavin mediates m6A modification targeted by miR408, promoting early somatic embryogenesis in Longan [J]. Plant Physiol, 2023, 192(3): 1799-1820. |
| [32] | Bosch G, Fuentes M, Erro J, et al. Hydrolysis of riboflavins in root exudates under iron deficiency and alkaline stress [J]. Plant Physiol Biochem, 2024, 210: 108573. |
| [33] | Deng BL, Dong HS. Ectopic expression of riboflavin-binding protein gene TsRfBP paradoxically enhances both plant growth and drought tolerance in transgenic Arabidopsis thaliana [J]. J Plant Growth Regul, 2013, 32(1): 170-181. |
| [34] | Wu YZ, Cheng SR, Ding XF, et al. Exogenous riboflavin application at different growth stages regulates photosynthetic accumulation and grain yield in fragrant rice [J]. Agriculture, 2024, 14(11): 1979. |
| [1] | 宋慧洋, 苏宝杰, 李京昊, 梅超, 宋倩娜, 崔福柱, 冯瑞云. 马铃薯StAS2-15基因的克隆及盐胁迫下功能分析[J]. 生物技术通报, 2025, 41(5): 119-128. |
| [2] | 杨涌, 袁国梅, 康肖肖, 刘亚明, 王东升, 张海娥. 板栗SWEET基因家族成员的鉴定及表达分析[J]. 生物技术通报, 2025, 41(2): 257-269. |
| [3] | 杨涌, 曹蕊, 康肖肖, 刘静, 王旋, 张海娥. 板栗类黄酮合成通路13个基因家族的鉴定及表达分析[J]. 生物技术通报, 2025, 41(2): 270-283. |
| [4] | 张玉, 石磊, 巩檑, 聂峰杰, 杨江伟, 刘璇, 杨文静, 张国辉, 颉瑞霞, 张丽. 马铃薯WOX基因家族的鉴定及在离体再生和非生物胁迫中的表达分析[J]. 生物技术通报, 2024, 40(3): 170-180. |
| [5] | 杨艳, 胡洋, 刘霓如, 殷璐, 杨锐, 王鹏飞, 穆霄鹏, 张帅, 程春振, 张建成. ‘红满堂’苹果MbbZIP43基因的克隆与功能研究[J]. 生物技术通报, 2024, 40(2): 146-159. |
| [6] | 赵中莹, 李青苗, 田孟良, 杨小倩, 康瑶, 邱玉洁, 张庆玲, 刘帆. 60Co-γ辐射对半夏愈伤组织成苗及植株特性的影响[J]. 生物技术通报, 2021, 37(9): 142-151. |
| [7] | 王建勇, 邹永梅, 葛言彬, 王凯, 席梦利. 植物愈伤组织诱导过程中的表观遗传修饰研究进展[J]. 生物技术通报, 2021, 37(8): 253-262. |
| [8] | 付首颖, 夏苗苗, 张祎凝, 刘川, 涂然, 张大伟. 核黄素工业菌株高通量筛选方法的建立和应用[J]. 生物技术通报, 2020, 36(4): 47-53. |
| [9] | 吴丽芳, 魏晓梅, 陆伟东. 白刺花胚性愈伤组织诱导及体细胞胚发生和萌发[J]. 生物技术通报, 2019, 35(4): 13-19. |
| [10] | 方珞, 吴小芹. 抗松针褐斑病湿地松体细胞的悬浮培养[J]. 生物技术通报, 2019, 35(3): 13-18. |
| [11] | 王俊燚, 董金金, 刘伟, 曹福亮, 汪贵斌, 王义强. 银杏愈伤组织生长、褐化与黄酮积累研究[J]. 生物技术通报, 2019, 35(2): 16-22. |
| [12] | 王玲, 李琰, 代伟娜, 严静, 张朝红. 葡萄细胞悬浮培养体系的建立和优化[J]. 生物技术通报, 2018, 34(8): 80-86. |
| [13] | 毛沛琪, 李厚华, 李嫒, 曹志秀, 韩美玲, 张延龙. 硝酸银对‘凤丹’牡丹愈伤组织褐变过程中酚类物质合成及相关基因表达的影响[J]. 生物技术通报, 2018, 34(8): 101-107. |
| [14] | 张正雪,蓝增全,吴田. 基于诺丽叶片愈伤组织的细胞悬浮系的建立[J]. 生物技术通报, 2018, 34(5): 142-147. |
| [15] | 张亚芳, 何钢, 荣广天, 刘贤桂, 倪尚格, 张世良. 鸡血藤愈伤组织培养过程中内源激素变化研究[J]. 生物技术通报, 2017, 33(3): 66-70. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||