








生物技术通报 ›› 2021, Vol. 37 ›› Issue (5): 248-258.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1206
冯翠莲1( ), 万玥2, 王俊刚1, 冯小艳1, 赵婷婷1, 王文治1, 沈林波1, 张树珍1,2(
), 万玥2, 王俊刚1, 冯小艳1, 赵婷婷1, 王文治1, 沈林波1, 张树珍1,2( )
)
收稿日期:2020-09-24
出版日期:2021-05-26
发布日期:2021-06-11
作者简介:冯翠莲,女,博士,副研究员,研究方向:甘蔗生物技术;E-mail:基金资助:
FENG Cui-lian1( ), WAN Yue2, WANG Jun-gang1, FENG Xiao-yan1, ZHAO Ting-ting1, WANG Wen-zhi1, SHEN Lin-bo1, ZHANG Shu-zhen1,2(
), WAN Yue2, WANG Jun-gang1, FENG Xiao-yan1, ZHAO Ting-ting1, WANG Wen-zhi1, SHEN Lin-bo1, ZHANG Shu-zhen1,2( )
)
Received:2020-09-24
Published:2021-05-26
Online:2021-06-11
摘要:
转基因作物外源T-DNA插入的侧翼序列是转基因事件检测的关键组分,也是转基因作物生物安全评价和监管所必需提供的重要信息。为了明确抗虫转基因甘蔗BCG-17的分子特征和建立其事件特异性检测方法,推进其生物安全性评价工作及未来的监管工作,以其T2代为研究材料,通过Southern 杂交检测外源基因在甘蔗基因组内的拷贝数;利用染色体步移技术分离外源基因在甘蔗基因组中插入位点的侧翼序列,并建立该转化体的高效灵敏的特异性PCR检测方法。结果表明:Southern杂交检测证明外源T-DNA以单拷贝方式插入BCG-17株系;经过3-4次的热不对称PCR扩增,分离到510 bp的T-DNA左侧翼序列和915 bp的右侧翼序列;以此为基础,分别设计左右侧翼检测引物,建立了BCG-17株系的转化事件特异性PCR检测方法,左侧翼的PCR检测方法特异性强、灵敏度高,检出极限为0.1%,相当于9个单倍体甘蔗基因组拷贝数。转基因株系BCG-17的分子特征及其转化事件特异性检测的完成,为该转基因甘蔗及其衍生产品的检测和身份识别提供了技术依据。
冯翠莲, 万玥, 王俊刚, 冯小艳, 赵婷婷, 王文治, 沈林波, 张树珍. 转Cry1Ac-2A-gna基因甘蔗BCG-17转化体特异性检测方法的建立[J]. 生物技术通报, 2021, 37(5): 248-258.
FENG Cui-lian, WAN Yue, WANG Jun-gang, FENG Xiao-yan, ZHAO Ting-ting, WANG Wen-zhi, SHEN Lin-bo, ZHANG Shu-zhen. Establishment of a Transformant-specific Detection Method for Cry1Ac-2A-gna Transgenic Sugarcane BCG-17[J]. Biotechnology Bulletin, 2021, 37(5): 248-258.
| 探针 Probe | 引物 Primer | 引物序列 Primer sequence(5'-3') | 退火温度Tm/℃ | 探针长度 Probe length/bp |
|---|---|---|---|---|
| BG | BG-F | CCTCGTTAGACTCAACAGCA | 58 | 857 |
| BG-R | GCTTTATCTTTCCAGCAGTAG | |||
| bar | bar2S | CATCGTCAACCACTACATCGAGACAAGC | 60 | 450 |
| bar3A | CCACTACATCGAGACAAGCACGGTCAAC | |||
| sta | sta-F | GCCGCCGTCTAAAAAGGTGATG | 60 | 682 |
| sta-R | GTCGCCCTCGGGTTCTGATTC | |||
| kan | kan-F | ATCGAAAAATACCGCTGCGTAAAAG | 56 | 637 |
| kan-R | GGACGCAGAAGGCAATGTCATAC |
表1 PCR DIG探针制备所用引物
Table 1 Primers used for PCR DIG probe preparation
| 探针 Probe | 引物 Primer | 引物序列 Primer sequence(5'-3') | 退火温度Tm/℃ | 探针长度 Probe length/bp |
|---|---|---|---|---|
| BG | BG-F | CCTCGTTAGACTCAACAGCA | 58 | 857 |
| BG-R | GCTTTATCTTTCCAGCAGTAG | |||
| bar | bar2S | CATCGTCAACCACTACATCGAGACAAGC | 60 | 450 |
| bar3A | CCACTACATCGAGACAAGCACGGTCAAC | |||
| sta | sta-F | GCCGCCGTCTAAAAAGGTGATG | 60 | 682 |
| sta-R | GTCGCCCTCGGGTTCTGATTC | |||
| kan | kan-F | ATCGAAAAATACCGCTGCGTAAAAG | 56 | 637 |
| kan-R | GGACGCAGAAGGCAATGTCATAC |
| 名称 Name | 引物序列(5'-3') Sequence(5'-3') | Tm/℃ |
|---|---|---|
| Lsp1 | CCATCGTCAACCACTACATCGAGACA | 62 |
| Lsp2 | GCCCCTGGAAGGCACGCAA | 66 |
| Lsp3 | CGTGGTCGCTGTCATCGGGCT | 65 |
| Lsp4 | CTTCAAGCACGGGAACTGGCAT | 64 |
| Rsp1 | GCCCTAAAAAGATAAAACTGTAGAGTCCTGT | 64 |
| Rsp2 | GCGCTTTATATGATTCTCTAAAACACTGATAT | 60 |
| Rsp3 | CTTCAAACAAGTGTGACAAAAAAAATATGT | 59 |
| Rsp4 | GTGACTGGGAAAACCCTGGCGTT | 62 |
表2 巢式PCR反应所需要的特异性引物
Table 2 Specific primers required for nested PCR reactions
| 名称 Name | 引物序列(5'-3') Sequence(5'-3') | Tm/℃ |
|---|---|---|
| Lsp1 | CCATCGTCAACCACTACATCGAGACA | 62 |
| Lsp2 | GCCCCTGGAAGGCACGCAA | 66 |
| Lsp3 | CGTGGTCGCTGTCATCGGGCT | 65 |
| Lsp4 | CTTCAAGCACGGGAACTGGCAT | 64 |
| Rsp1 | GCCCTAAAAAGATAAAACTGTAGAGTCCTGT | 64 |
| Rsp2 | GCGCTTTATATGATTCTCTAAAACACTGATAT | 60 |
| Rsp3 | CTTCAAACAAGTGTGACAAAAAAAATATGT | 59 |
| Rsp4 | GTGACTGGGAAAACCCTGGCGTT | 62 |
| 名称 Name | 引物序列 Primer sequence(5'-3’) | Tm/℃ | 产物长度 Fragment length/bp |
|---|---|---|---|
| LS132 LA797 | CGCTCATGTGTTGAGCATATAAGAAA | 58 | 665 |
| GCAATCCCACGGACCCACAA | |||
| LS132 LA791 | CGCTCATGTGTTGAGCATATAAGAAA | 60 | 659 |
| CCACGGACCCACAACTGTCTTTT | |||
| LS040 LA791 | GTCCTGCCCGTCACCGAGATTT | 61 | 751 |
| CCACGGACCCACAACTGTCTTTT | |||
| RS139 RA467 | GTGACTGGGAAAACCCTGGCGTT | 56 | 328 |
| CTGATGGAAGAGTGGTCAAAAGATGT | |||
| RS139 RA526 | GTGACTGGGAAAACCCTGGCGTT | 57 | 387 |
| GCTTCACACTTGTTGGACAGGATTT | |||
| RS006 RA467 | CTTCAAACAAGTGTGACAAAAAAAATATGT | 55 | 461 |
| CTGATGGAAGAGTGGTCAAAAGATGT |
表3 BCG-17事件特异性PCR检测引物
Table 3 Primers for BCG-17 event-Specific PCR detection
| 名称 Name | 引物序列 Primer sequence(5'-3’) | Tm/℃ | 产物长度 Fragment length/bp |
|---|---|---|---|
| LS132 LA797 | CGCTCATGTGTTGAGCATATAAGAAA | 58 | 665 |
| GCAATCCCACGGACCCACAA | |||
| LS132 LA791 | CGCTCATGTGTTGAGCATATAAGAAA | 60 | 659 |
| CCACGGACCCACAACTGTCTTTT | |||
| LS040 LA791 | GTCCTGCCCGTCACCGAGATTT | 61 | 751 |
| CCACGGACCCACAACTGTCTTTT | |||
| RS139 RA467 | GTGACTGGGAAAACCCTGGCGTT | 56 | 328 |
| CTGATGGAAGAGTGGTCAAAAGATGT | |||
| RS139 RA526 | GTGACTGGGAAAACCCTGGCGTT | 57 | 387 |
| GCTTCACACTTGTTGGACAGGATTT | |||
| RS006 RA467 | CTTCAAACAAGTGTGACAAAAAAAATATGT | 55 | 461 |
| CTGATGGAAGAGTGGTCAAAAGATGT |
| 探针 Probe | BCG-17 | pNUBG | ||
|---|---|---|---|---|
| 限制性内切酶 Restriction enzyme | 杂交带长度 Hybrid band length/bp | 限制性内切酶 Restriction enzyme | 杂交带长度 Hybrid band length/bp | |
| BG | BamHI | 2300 | Kpn I | 11225 |
| Nde I | ≥4650 | |||
| bar | BamHI | 1830 | Kpn I | 1823 |
| EcoRV | ≥1000 | |||
| sta | Kpn I | / | Kpn I | 11225 |
| kan | Kpn I | / | Kpn I | 11225 |
表4 BCG-17和pNUBG质粒Southern Blot杂交带大小的理论值
Table 4 Theoretical values of Southern Blot hybridization zone size in BCG-17 and pNUBG
| 探针 Probe | BCG-17 | pNUBG | ||
|---|---|---|---|---|
| 限制性内切酶 Restriction enzyme | 杂交带长度 Hybrid band length/bp | 限制性内切酶 Restriction enzyme | 杂交带长度 Hybrid band length/bp | |
| BG | BamHI | 2300 | Kpn I | 11225 |
| Nde I | ≥4650 | |||
| bar | BamHI | 1830 | Kpn I | 1823 |
| EcoRV | ≥1000 | |||
| sta | Kpn I | / | Kpn I | 11225 |
| kan | Kpn I | / | Kpn I | 11225 |
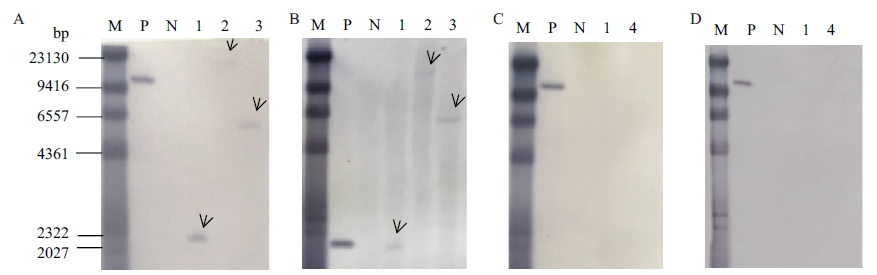
图3 转基因甘蔗BCG-17 Southern Blot分析 A:BG探针杂交;B:bar探针杂交;C:sta探针杂交;D:kan探针杂交。M:Marker;P:Kpn I酶切质粒;N:Nde I酶切非转基因甘蔗基因组;1:BamH I酶切;2:EcoRⅤ酶切;3:Nde I酶切;4:Hind Ⅲ 酶切
Fig. 3 Southern Blot analysis of transgenic sugarcane BCG-17 A: BG prob. B: Bar probe. C:sta probe. D: kan probe. M: DNA marker for southern blot, P: Kpn I enzyme digest plasmid, N: Nde I enzyme digest Non-GM sugarcane genome, 1: BamHI enzyme digestion, 2: EcoRⅤ enzyme digestion, 3: Nde I enzyme digestion, 4: Hind Ⅲ enzyme digestion
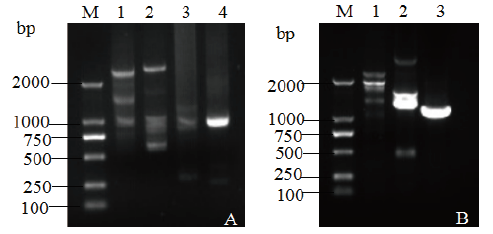
图4 BCG-17左右侧翼序列的巢式PCR扩增结果 A:左侧翼序列扩增;B:右侧翼序列扩增。M:DNA Marker;1:1st产物;2:2nd产物3:3rd产物;4:4th产物
Fig. 4 Nested PCR amplification of left and right flank sequence of BCG-17 A: Left flanking sequence. B: Right flanking sequence. M:DNA marker,1: Amplification for 1st, 2: Amplification for 2nd, 3: Amplification for 3rd,4: Amplification for 4th

图5 BCG-17左侧翼序列及拼接位置特征 A:BCG-17左侧翼序列;下划直线(大写字母):T-DNA载体序列(511~840 bp), 其余:甘蔗基因组序列。B:T-DNA拼接位置特征;pUNBG:包括T-DNA左边界在内的载体序列;箭头:T-DNA转入甘蔗基因组时的断裂位置,箭头左边为载体非T-DNA区;红色序列:T-DNA左边界核心序列;下划波浪线:T-DNA转入甘蔗基因组时缺失序列;小写字母:BCG-17甘蔗基因组序列;方框:BCG-17与pUNBG的重叠载体序列
Fig. 5 Left flanking sequence of BCG-17 and the character of junction region A: The left flanking sequence of BCG-17. The underlined sequences are T-DNA (511-840 bp),the rest are the sugarcane genome sequence. B: The character of junction region. pUNBG: vector sequences including the left border of T-DNA. The arrow, the break position when the T-DNA was transferred into the sugarcane genome, the left side of the arrow is non-T-DNA region, the red sequences,the core sequence of left T-DNA border, the wavy line, the missing sequence when the T-DNA was transferred into the sugarcane genome, lowercase letters, the left flanking sequence of BCG-17 sugarcane genome, square, overlapping vector sequence of BCG-17 and pUNBG

图6 BCG-17右侧翼序列及拼接位置特征 A:BCG-17右旁侧序列;下划直线(大写字母):T-DNA载体序列(1-318 bp), 其余(小写字母):甘蔗基因组序列。B:T-DNA拼接位置特征;pUNBG:包括T-DNA右边界在内的载体序列;箭头:T-DNA转入甘蔗基因组时的断裂位置,箭头右边为载体非T-DNA区;红色序列:T-DNA右边界核心序列;下划波浪线:T-DNA转入甘蔗基因组时缺失序列;BCG-17:右侧翼序列,小写字母为甘蔗基因组序列;方框:BCG-17与pUNBG的重叠载体序列
Fig. 6 Right flanking sequence of BCG-17 and the character of junction region A: The right flanking sequence of BCG-17. The underlined sequences are T-DNA (1-318 bp),the rest are the sugarcane genome sequence. B: The character of junction region. pUNBG: vector sequences including the right border of T-DNA. The arrow: the break position when the T-DNA was transferred into the sugarcane genome, the right side of the arrow is non-T-DNA region, the red sequences, the core sequence of right T-DNA border. The wavy line: the missing sequence when the T-DNA was transferred into the sugarcane genome. BCG-17: The obtained right flanking sequence;lowercase letters, sugarcane genome, square,overlapping vector sequence of BCG-17 and pUNBG
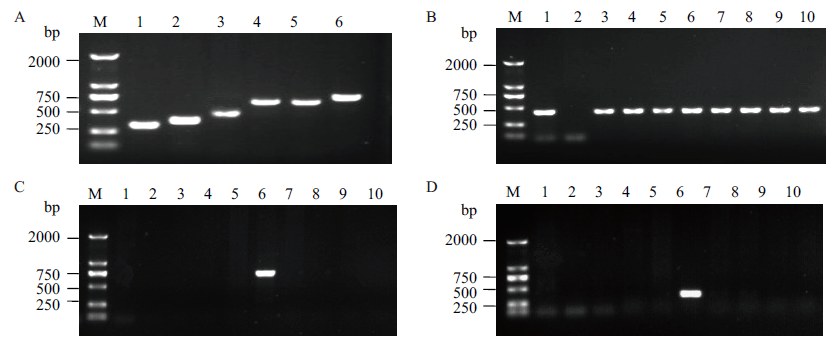
图7 甘蔗BCG-17转化事件特异性PCR检测 A: 左、右侧翼特异性引物筛选,M:DNA Marker;1-3:右侧引物RS139/RA467、RS139/RA526、RS006/RA467扩增的PCR产物;4-6:左侧引物LS132/LA797、LS132/LA791、LS040/LA791扩增的PCR产物。B:转Cry1c-2A-gna基因甘蔗bar基因PCR检测;C:转Cry1c-2A-gna基因甘蔗左侧翼事件特异性PCR检测(引物LS040/LA791);D:转Cry1c-2A-gna基因甘蔗右侧翼事件特异性PCR检测(引物RS139/RA526 ),其中M:DNA Marker;1:表达载体质粒pUNBG;2:非转基因甘蔗ROC22;3-10:分别为转Cry1c-2A-gna基因甘蔗BCG-1、BCG-2、BCG-4、BCG-17、BCG-32、BCG-35、BCG-36和BCG-41
Fig. 7 Transformation event-specific PCR detection of the BCG-17 sugarcane 170A: Screening of event-specific primers for left and right flanking. M: DNA marker. 1-3: PCR products amplified by right primers RS139/RA467, RS139/RA526, RS006/RA467. 4-6: PCR products amplified by left primersLS132/LA797, LS132/LA791, LS040/LA791. B: PCR detection of bar gene in transgenic sugarcane transformed with Cry1c-2A-gna gene. C: Event-specific PCR detection of the left flanking in transgenic sugarcane transformed with Cry1c-2A-gna gene(primer pairs: LS040/LA791 ). D: Event-specific PCR detection of the right flanking in transgenic sugarcane transformed with Cry1c-2A-gna gene(primer pairs:RS139/RA526). M: DNA marker. 1: Expression vector plasmid pUNBG. 2: Non-GM sugarcane ROC22. 3-10:transgenic sugarcane transformed with Cry1c-2A-gna gene BCG-1, BCG-2, BCG-4, BCG-17, BCG-32, BCG-35, BCG-36和BCG-41 respectively
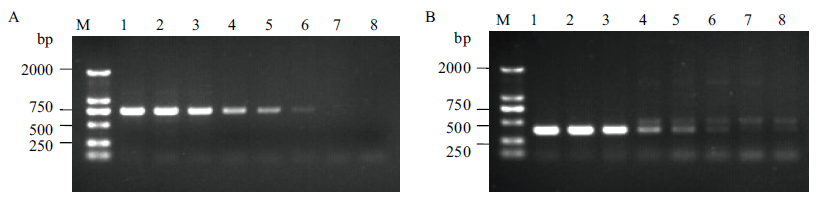
图8 左右侧翼特异性引物对PCR检测限度的测定 A:左边界特异性引物LS040/LA791对检测限度的测定;B: 右侧翼特异性引物RS139/RA526对检测限度的测定;M:DNA Marker;1-8: 转基因甘蔗BCG-17的DNA含量分别为100 %、50 %、10 %、1 %、0.5 %、0.1 %、0.05 %和0 %(非转基因甘蔗ROC22)
Fig. 8 Limitation of event-specific detection by use of specific primer pairs of left and right flanking sequences A: Limitation of event-specific detection with primer pairs of left flanking(LS040/LA791). B: Limitation of event-specific detection with primer pairs of right flanking(RS139/RA526). M: DNA marker. 1-8: The DNA content of transgenic sugarcane BCG-17 is 100 %, 50 %, 10 %, 1 %, 0.5 %, 0.1 %, 0.05 % and 0 % (non-GM sugarcane ROC22), respectively
| [1] | http://cn.agropages.com/News/NewsDetail---20390.htm. |
| [2] | 祁洋, 李燕, 王永智, 等. 扩增T-DNA插入位点侧翼序列的方法及其应用进展[J]. 安徽农业科学, 2009,37(17):7907-7908, 7927. |
| Qi Y, Li Y, Wang YZ, et al. Advances in the amplification methods and application of T-DNA insertion site flanking sequence[J]. Journal of Anhui Agricultural Sciences, 2009,37(17):7907-7908, 7927. | |
| [3] | 蒲远波, 郭翠, 平淑珍. 基因侧翼序列扩增技术的应用进展[J]. 生物技术通报, 2016,32(11):72-79. |
| Pu YB, Guo C, Ping SZ. Application progress on amplified methods to clone flanking sequence[J]. Biotechnology Bulletin, 2016,32(11):72-79. | |
| [4] | 李付鹏, 伍宝朵, 马朝芝, 等. 基于PCR的染色体步移技术研究进展[J]. 中国生物工程杂志, 2010,30(12):87-94. |
| Li FP, Wu BD, Ma CZ, et al. Progress of chromosome walking by PCR amplification techniques[J]. China Biotechnology, 2010,30(12):87-94. | |
| [5] | 康丹, 方小艳, 游腾飞, 等. 染色体步移技术克隆已知序列侧翼启动子的研究进展[J]. 农业生物技术学报, 2013,21(3):355-366. |
| Kang D, Fang XY, You TF, et al. Progress of chromosome walking the unknown promoter sequences flanked by a known segment[J]. Journal of Agricultural Biotechnology, 2013,21(3):355-366. | |
| [6] | 金永梅, 马瑞, 于志晶, 等. 转基因水稻吉生粳2号的外源基因旁侧序列分离及事件特异性PCR检测方法[J]. 东北农业科学, 2016,41(1):14-19. |
| Jin YM, Ma Rui, Yu ZJ, et al. Identification of the T-DNA flanking sequences and event-specific PCR detection of transgenic rice‘Jishengjing 2’[J]. Journal of Northeast Agricultural Sciences, 2016,41(1):14-19. | |
| [7] |
Dong Y, Jin X, Tang Q, et al. Development and event-specific detection of transgenic glyphosate-resistant rice expressing the G2-EPSPS gene[J], Front Plant Sci, 2017,8:885-895.
doi: 10.3389/fpls.2017.00885 URL |
| [8] | 崔帅, 王作平, 于江辉, 等. 转基因水稻BPL9K-2事件特异性检测方法的建立[J]. 中国生物工程杂志, 2018(11):32-41. |
| Cui S, Wang ZP, Yu JH, et al. Event-specific detection methods of genetically modified rice BPL9K-2[J]. China Biotechnology, 2018,38(11):32-41. | |
| [9] |
Rao J, Yang LT, Guo JC, et al. Development of event-specific qualitative and quantitative PCR detection methods for the transgenic maize BVLA430101[J]. Eur Food Res Technol, 2016,242(8):1277-1284.
doi: 10.1007/s00217-015-2631-7 URL |
| [10] | 王叶, 谢家建, 黄春蒙, 等. 转cry1Aa基因抗虫棉整合结构解析及转化体特异性检测方法的建立[J]. 棉花学报, 2017,29(4):307-315. |
| Wang Y, Xie JJ, Huang CM, et al. Integrated structure of the modified cry1Aa gene in cotton and its event-specific detection[J]. Cotton Science, 2017,29(4):307-315. | |
| [11] |
Zhang PQ, Xu JY, Zheng QY, et al. Flanking sequence determination and event specific detection of transgenic wheat B72-8-11b strain[J]. Appl Biochem Biotechnol, 2013,169(5):1523-1530.
doi: 10.1007/s12010-012-9989-9 URL |
| [12] |
Zhang MH, Yu YB, Gao XJ, et al. Event-specific quantitative detection of genetically modified wheat B72-8-11 based on the 3' flanking sequence[J]. Eur Food Res Technol, 2015,240(4):775-782.
doi: 10.1007/s00217-014-2383-9 URL |
| [13] | 闫建俊, 白云凤, 左静静, 等. 转基因马铃薯外源基因插入位点分析及检测方法的建立[J]. 分子植物育种, 2020,18(16):5361-5366. |
| Yan JJ, Bai YF, Zuo JJ, et al. Analysis of insertion site of transgenic potato exogenous gene and the establishment of detection method[J]. Molecular Plant Breeding, 2020,18(16):5361-5366. | |
| [14] | http://www.chinasugar.org.cn/i,35,3430,0.html |
| [15] | 黄应昆, 李文凤. 云南“双高甘蔗”病虫害综合防治[J]. 云南农业科技, 2004(4):16-18. |
| Huang YK, Li WF. Comprehensive prevention and control of diseases and pests of Yunnan “double high sugarcane”[J]. Yunnan Agricultural Science and Technology, 2004(4):16-18. | |
| [16] | Xie JJ, Yang J, Luo QW, et al. Survey of sugarcane pests in Xianggui sugarcane area and countermeasures of pest control[J]. Plant Diseases and Pests, 2020,11(1):15-18, 23. |
| [17] | 王关林, 方宏筠. 植物基因工程[M]. 第2版. 北京: 科学出版社, 2002. |
| Wang GL, Fang HJ. Plant genetic engineering[M]. 2nd ed. Beijing: Science Press, 2002. | |
| [18] | 崔学强, 张树珍, 沈林波, 等. 转基因甘蔗植株Southern杂交体系的优化[J]. 生物技术通报, 2015,31(12):105-109. |
| Cui XQ, Zhang SZ, Shen LB, et al. The optimization of southern blot for transgenic sugarcane plants[J]. Biotechnology Bulletin, 2015,31(12):105-109. | |
| [19] | 徐纪明, 胡晗, 毛文轩, 等. 利用重测序技术获取转基因植物T-DNA插入位点[J]. 遗传, 2018,40(8):676-682. |
| Xu JM, Hu H, Mao WX, et al. Identifying T-DNA insertion site(s)of transgenic plants by whole-genome resequencing[J]. Hereditas, 2018,40(8):676-682. | |
| [20] |
Zhang J, Zhang X, Tang H, et al. Allele-defined genome of the autopolyploid sugarcane Saccharum spontaneumL.[J]. Nat Genet, 2018,50:1565-1573.
doi: 10.1038/s41588-018-0237-2 URL |
| [21] | 郭超, 何行健, 邓力华, 等. 转基因水稻 BarKasalath-01事件特异性检测[J]. 分子植物育种, 2017,15(11):4466-4475. |
| Guo C, He XJ, Deng LH, et al. Event-specific detection of genetically modified rice BarKasalath-01[J]. Molecular Plant Breeding, 2017,15(11):4466-4475. | |
| [22] | 仲晓芳, 杨静, 贺红利, 等. 基于基因组重测序分析高油酸转基因大豆事件外源T-DNA旁侧序列及特异性检测[J]. 农业生物技术学报, 2018,26(12):2017-2026. |
| Zhong XF, Yang J, He HL, et al. Analysis of the T-DNA flanking sequences and event-specific PCR detection of high-content Oleic acid transgenic soybean(Glycine max)Based on Genome Re-sequencing[J]. Journal of Agricultural Biotechnology, 2018,26(12):2017-2026. | |
| [23] | Chen S, Shen AJ, Zhou XY, et al. Analysis of the flanking sequence and event-specific detection of transgenic line W-4 of Brassica napus[J]. Agr Sci Tech, 2014,15(7):1089-1094. |
| [24] |
Zhang N, Xu W, Bai W, et al. Event-specific qualitative and quantitative PCR detection of LY038 maize in mixed samples[J]. Food Control, 2011,22(8):1287-1295.
doi: 10.1016/j.foodcont.2011.01.030 URL |
| [25] |
Marmiroli N, Maestri E, Gulli M, et al. Methods for detection of GMOs in food and feed[J]. Anal Bioanal Chem, 2008,392(3):369-384.
doi: 10.1007/s00216-008-2303-6 pmid: 18726090 |
| [1] | 樊寿德,王艳. 盐穗木盐相关转录因子HcSCL13基因启动子的克隆及活性初步分析[J]. 生物技术通报, 2017, 33(5): 131-138. |
| [2] | 唐杏姣;韩科厅;胡可;孟丽;戴思兰;. 菊花CmDFR与CmANS基因启动子序列克隆与瞬时表达分析[J]. , 2012, 0(05): 81-88. |
| [3] | 谢夏青;李志勇;马继芳;刘磊;董志平;董金皋;. 谷子弯孢菌海藻糖酶基因(ClTRE)及其启动子的克隆和序列分析[J]. , 2011, 0(07): 154-159. |
| [4] | 谭颖;王凭青;何腾龙;储明星;邓腊梅;樊奇;张宝云;刘重旭;. 大足黑山羊性激素结合球蛋白基因的克隆与多态性分析[J]. , 2010, 0(10): 149-155. |
| [5] | 梁成真;张锐;郭三堆;. 染色体步移技术研究进展[J]. , 2009, 0(10): 75-82. |
| [6] | Young;N.D.;宋凤鸣;郑重;. 依据图谱克隆技术在植物病理学上的应用前景[J]. , 1992, 0(10): 1-4. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||