








生物技术通报 ›› 2021, Vol. 37 ›› Issue (11): 158-165.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1120
• 食用菌生物技术专题(专题主编: 黄晨阳) • 上一篇 下一篇
陶治东1( ), 何艳慧1, 邓子禾2, 孙琳琳1, 武占省1(
), 何艳慧1, 邓子禾2, 孙琳琳1, 武占省1( )
)
收稿日期:2021-08-31
出版日期:2021-11-26
发布日期:2021-12-03
作者简介:陶治东,男,硕士,研究方向:固体废物资源化;E-mail: 基金资助:
TAO Zhi-dong1( ), HE Yan-hui1, DENG Zi-he2, SUN Lin-lin1, WU Zhan-sheng1(
), HE Yan-hui1, DENG Zi-he2, SUN Lin-lin1, WU Zhan-sheng1( )
)
Received:2021-08-31
Published:2021-11-26
Online:2021-12-03
摘要:
利用香菇菌渣开发生物有机肥目前是解决废弃菌渣资源化的主要趋势,为了更好地腐熟香菇菌渣,从废弃香菇菌渣中筛选出1株高效纤维素降解菌株DGW1,并优化产纤维素酶条件及评估降解菌渣能力,以期为后续利用香菇菌渣进行堆肥发酵生产生物有机肥奠定基础。采用CMC-Na平板法和刚果红染色法初步筛选,结合滤纸条崩解试验和纤维素酶活性测定进行复筛,并通过形态学观察和18S rDNA分子生物学鉴定菌株,优化菌株DGW1的产纤维素降解酶条件。结果表明:获得的高效纤维素降解菌株DGW1鉴定为里氏木霉,7 d内可将滤纸条降解为糊状。菌株DGW1最佳产酶条件为温度30℃、时间4 d,滤纸酶的酶活(FPase)为112.71 U/g,并在pH 4-7都能达到较高酶活且不低于96 U/g。菌株DGW1对香菇菌渣的降解率为31.14%(14 d)。因此,获得的菌株DGW1能够高效降解香菇菌渣,其纤维素酶活较高,对香菇菌渣发酵堆肥,制备生物有机肥具有较大应用潜力。
陶治东, 何艳慧, 邓子禾, 孙琳琳, 武占省. 香菇菌渣高效纤维素降解菌的筛选及产酶优化[J]. 生物技术通报, 2021, 37(11): 158-165.
TAO Zhi-dong, HE Yan-hui, DENG Zi-he, SUN Lin-lin, WU Zhan-sheng. Screening of High-efficiency Cellulose-degrading Microorganism from Spent Lentinula edodes Substrate and Optimization of Its Enzyme Production[J]. Biotechnology Bulletin, 2021, 37(11): 158-165.
| 菌株编号 Strain No. | 滤纸条崩解程度 Degradation degree of filter paper | 筛选温度 Screening temperature/℃ | 菌株编号 Strain No. | 滤纸条崩解程度 Degradation degree of filter paper | 筛选温度 Screening temperature/℃ | |
|---|---|---|---|---|---|---|
| DF1 | + | 28 | YD3 | +++ | 28 | |
| GF1 | ++ | 45 | YG4 | ++ | 45 | |
| GF2 | + | 45 | YD2 | ++ | 28 | |
| YG1 | + | 45 | DGW1 | ++++ | 28 | |
| DG3 | + | 28 | FYG2 | + | 45 | |
| GF3 | +++ | 45 | FYG3 | ++ | 45 | |
| YG3 | + | 45 | FFD1 | + | 28 | |
| YD4 | ++++ | 28 | FYG1 | ++ | 45 | |
| GG1 | + | 45 | FDY2 | + | 28 | |
| YG2 | +++ | 45 | FGF1 | ++ | 45 | |
| YD1 | ++ | 28 | FFD2 | + | 28 |
表1 22株分离菌株的滤纸条降解
Table 1 Filter strip degradation by 22 isolated strains
| 菌株编号 Strain No. | 滤纸条崩解程度 Degradation degree of filter paper | 筛选温度 Screening temperature/℃ | 菌株编号 Strain No. | 滤纸条崩解程度 Degradation degree of filter paper | 筛选温度 Screening temperature/℃ | |
|---|---|---|---|---|---|---|
| DF1 | + | 28 | YD3 | +++ | 28 | |
| GF1 | ++ | 45 | YG4 | ++ | 45 | |
| GF2 | + | 45 | YD2 | ++ | 28 | |
| YG1 | + | 45 | DGW1 | ++++ | 28 | |
| DG3 | + | 28 | FYG2 | + | 45 | |
| GF3 | +++ | 45 | FYG3 | ++ | 45 | |
| YG3 | + | 45 | FFD1 | + | 28 | |
| YD4 | ++++ | 28 | FYG1 | ++ | 45 | |
| GG1 | + | 45 | FDY2 | + | 28 | |
| YG2 | +++ | 45 | FGF1 | ++ | 45 | |
| YD1 | ++ | 28 | FFD2 | + | 28 |
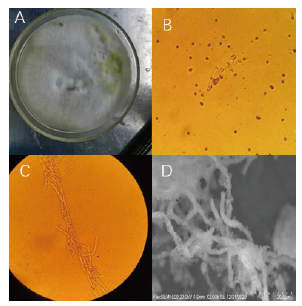
图5 菌株DGW1形态 A:菌株DGW1形态;B:菌株DGW1孢子形态;C:菌株DGW1菌丝形态;D:扫描电镜菌株DGW1菌丝形态
Fig.5 Morphology of the strain DGW1 A:Morphology of the strain DGW1;B:spore morphology of strain DGW1;C:mycelial morphology of strain DGW1;D:mycelial morphology of strain DGW1 under SCM
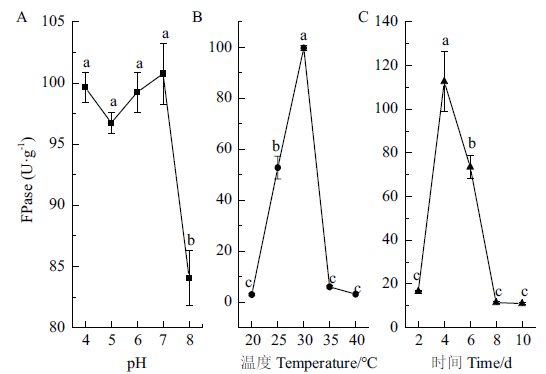
图6 不同条件下菌株DGW1的酶活 A:不同pH下菌株DGW1酶活;B:不同温度下菌株DGW1酶活;C:不同时间下菌株DGW1酶活
Fig.6 Enzyme activity of strain DGW1 under different conditions A:Enzyme activity of strain DGW1 at different pH. B:Enzyme activity of strain DGW1 at different temperature. C:Enzyme activity of strain DGW1 at different times
| [1] |
Williams BC, McMullan JT, McCahey S. An initial assessment of spent mushroom compost as a potential energy feedstock[J]. Bioresour Technol, 2001, 79(3): 227-230.
doi: 10.1016/S0960-8524(01)00073-6 URL |
| [2] |
Phan CW, Sabaratnam V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes[J]. Appl Microbiol Biotechnol, 2012, 96(4): 863-873.
doi: 10.1007/s00253-012-4446-9 URL |
| [3] |
Jordan SN, Mullen GJ, Murphy MC. Composition variability of spent mushroom compost in Ireland[J]. Bioresour Technol, 2008, 99(2): 411-418.
doi: 10.1016/j.biortech.2006.12.012 URL |
| [4] |
Lin HN, Wang YT, Zhu MJ. Evaluation of spent mushroom compost as a lignocellulosic substrate for hydrogen production by Clostridium thermocellum[J]. Int J Hydrog Energy, 2017, 42(43): 26687-26694.
doi: 10.1016/j.ijhydene.2017.09.040 URL |
| [5] | 张玲秀, 董社琴. 玉米秸秆纤维素降解处理方法研究[J]. 安徽农学通报, 2018, 24(Z1): 14-15, 20. |
| Zhang LX, Dong SQ. Study on the treatment of corn stalk cellulose degradation[J]. Anhui Agric Sci Bull, 2018, 24(Z1): 14-15, 20. | |
| [6] |
Chi CP, Chu S, Wang B, et al. Dynamic bacterial assembly driven by Streptomyces griseorubens JSD-1 inoculants correspond to composting performance in swine manure and rice straw co-composting[J]. Bioresour Technol, 2020, 313: 123692.
doi: 10.1016/j.biortech.2020.123692 URL |
| [7] |
Chen Y, Wang W, Zhou D, et al. Acetobacter orientalis XJC-C with a high lignocellulosic biomass-degrading ability improves significantly composting efficiency of banana residues by increasing metabolic activity and functional diversity of bacterial community[J]. Bioresour Technol, 2021, 324: 124661.
doi: 10.1016/j.biortech.2020.124661 URL |
| [8] |
Jiang GF, Chen PJ, Bao YZ, et al. Isolation of a novel psychrotrophic fungus for efficient low-temperature composting[J]. Bioresour Technol, 2021, 331: 125049.
doi: 10.1016/j.biortech.2021.125049 URL |
| [9] |
Sadhu S. Cellulase production by bacteria:a review[J]. Br Microbiol Res J, 2013, 3(3): 235-258.
doi: 10.9734/BMRJ URL |
| [10] | 刘晓梅, 邹亚杰, 胡清秀, 等. 菌渣纤维素降解菌的筛选与鉴定[J]. 农业环境科学学报, 2015, 34(7): 1384-1391. |
| Liu XM, Zou YJ, Hu QX, et al. Screening and identification of cellulose-degrading bacteria from spent substrate of edible mushroom[J]. J Agro Environ Sci, 2015, 34(7): 1384-1391. | |
| [11] |
Pang J, Liu ZY, Hao M, et al. An isolated cellulolytic Escherichia coli from bovine rumen produces ethanol and hydrogen from corn straw[J]. Biotechnol Biofuels, 2017, 10: 165.
doi: 10.1186/s13068-017-0852-7 URL |
| [12] | 李日强, 辛小芸, 刘继青. 天然秸秆纤维素分解菌的分离选育[J]. 上海环境科学, 2002, 21(1): 8-11. |
| Li RQ, Xin XY, Liu JQ. Isolation and Screening on Straw Cellulose-Decomposting Microorgnisms[J]. Shanghai Environment Sciences, 2002, 21(1): 8-11. | |
| [13] |
Dar MA, Pawar KD, Jadhav JP, et al. Isolation of cellulolytic bacteria from the gastro-intestinal tract of Achatina fulica(Gastropoda:Pulmonata)and their evaluation for cellulose biodegradation[J]. Int Biodeterior Biodegrad, 2015, 98: 73-80.
doi: 10.1016/j.ibiod.2014.11.016 URL |
| [14] |
Ghose TK, Bisaria VS. Measurement of hemicellulase activities:Part I Xylanases[J]. Pure Appl Chem, 1987, 59(12): 1739-1751.
doi: 10.1351/pac198759121739 URL |
| [15] |
李林超, 张超, 董庆, 等. 堆肥过程中纤维素降解菌的分离与鉴定[J]. 生物技术通报, 2019, 35(9): 165-171.
doi: 10.13560/j.cnki.biotech.bull.1985.2019-0581 |
| Li LC, Zhang C, Dong Q, et al. Isolation and identification of cellulose degrading microorganisms in composting process[J]. Biotechnol Bull, 2019, 35(9): 165-171. | |
| [16] |
Sun CY, Wei YB, Kou JN, et al. Improve spent mushroom substrate decomposition, bacterial community and mature compost quality by adding cellulase during composting[J]. J Clean Prod, 2021, 299: 126928.
doi: 10.1016/j.jclepro.2021.126928 URL |
| [17] |
Harnvoravongchai P, Singwisut R, Ounjai P, et al. Isolation and characterization of thermophilic cellulose and hemicellulose degrading bacterium, Thermoanaerobacterium sp. R63 from tropical dry deciduous forest soil[J]. PLoS One, 2020, 15(7): e0236518.
doi: 10.1371/journal.pone.0236518 URL |
| [18] | 沙沙, 刘心怡, 张玉林, 等. 一株产纤维素酶的暹罗芽孢杆菌筛选及产酶条件优化[J]. 河南科学, 2019, 37(7): 1073-1081. |
| Sha S, Liu XY, Zhang YL, et al. UV mutagenesis and fermentation conditions optimization of a cellulase-producing marine Bacillus siamensis[J]. Henan Sci, 2019, 37(7): 1073-1081. | |
| [19] |
吴婧, 聂彩娥, 朱媛媛, 等. 一株兼具产IAA能力纤维素降解菌的筛选、鉴定及条件优化[J]. 生物技术通报, 2020, 36(12): 54-63.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0454 |
| Wu Q, Nie CE, Zhu YY, et al. Isolation, identification of a cellulose-degrading bacterium with IAA-producing ability and optimization of its culture conditions[J]. Biotechnol Bull, 2020, 36(12): 54-63. | |
| [20] |
Fanuel M, Garajova S, Ropartz D, et al. The Podospora anserina lytic polysaccharide monooxygenase PaLPMO9H catalyzes oxidative cleavage of diverse plant cell wall matrix glycans[J]. Biotechnol Biofuels, 2017, 10: 63.
doi: 10.1186/s13068-017-0749-5 URL |
| [21] |
Özer CO, Kılıç B. Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid Bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef[J]. Meat Sci, 2021, 171: 108303.
doi: 10.1016/j.meatsci.2020.108303 URL |
| [22] | 梅金飞, 刚利萍, 余梅霞, 等. 烟草秸秆废弃物中纤维素降解菌的筛选、鉴定及产酶条件优化[J]. 烟草科技, 2020, 53(8): 15-23. |
| Mei JF, Gang LP, Yu MX, et al. Screening and identification of cellulose-degrading bacteria in waste tobacco stalks and optimization of enzyme production conditions[J]. Tob Sci Technol, 2020, 53(8): 15-23. | |
| [23] |
Darabzadeh N, Hamidi-Esfahani Z, Hejazi P. Optimization of cellulase production under solid-state fermentation by a new mutant strain of Trichoderma reesei[J]. Food Sci Nutr, 2019, 7(2): 572-578.
doi: 10.1002/fsn3.852 pmid: 30847136 |
| [24] |
Karthika A, Seenivasagan R, Kasimani R, et al. Cellulolytic bacteria isolation, screening and optimization of enzyme production from vermicompost of paper cup waste[J]. Waste Manag, 2020, 116: 58-65.
doi: 10.1016/j.wasman.2020.06.036 URL |
| [25] |
Dar MA, Pawar KD, Pandit RS. Prospecting the gut fluid of giant African land snail, Achatina fulica for cellulose degrading bacteria[J]. Int Biodeterior Biodegrad, 2018, 126: 103-111.
doi: 10.1016/j.ibiod.2017.10.006 URL |
| [1] | 张开平, 刘燕丽, 涂绵亮, 李继伟, 吴文标. 烟曲霉A-16产纤维素酶工艺优化及酶学特性[J]. 生物技术通报, 2022, 38(9): 215-225. |
| [2] | 袁媛, 王蕾, 石亚伟. 微生物来源碱性蛋白酶活性提高策略的研究进展[J]. 生物技术通报, 2021, 37(5): 231-236. |
| [3] | 李鹏昊, 梁严予, 王彦伟, 关洋, 逄文强, 田克恭. 非洲猪瘟病毒K196R和A240L蛋白的可溶性表达及酶活力分析[J]. 生物技术通报, 2020, 36(11): 70-75. |
| [4] | 陈建军, 刘梁涛, 曹香林. 黄孢原毛平革菌漆酶基因lac1680的克隆、表达及产酶研究[J]. 生物技术通报, 2018, 34(4): 214-220. |
| [5] | 来蒋丽, 刘姝, 胡晟源, 顾张慧, 王淑军, 房耀维. 一株产几丁质脱乙酰酶海洋细菌的筛选、鉴定及发酵优化[J]. 生物技术通报, 2017, 33(11): 153-159. |
| [6] | 李杏春, 何双辉, 戴玉成. 大伏革菌产纤维素酶条件优化及高效菌株筛选[J]. 生物技术通报, 2014, 0(4): 152-158. |
| [7] | 贾博涵,周伟,赵罗迪,杨埔,苟敏. 一株产纤维素酶细菌的分离鉴定及酶学特性研究[J]. 生物技术通报, 2014, 0(11): 187-192. |
| [8] | 王欢, 何腊平, 周换景, 张义明, 李翠芹, 陶菡. 脂肪酶活力测定方法及其在筛选产脂肪酶微生物中的应用[J]. 生物技术通报, 2013, 0(1): 203-208. |
| [9] | 田大鹏;葛娟;石峰;李鸿彬;. 棉花GhDHAR2基因克隆、功能序列分析及原核表达[J]. , 2012, 0(07): 65-69. |
| [10] | 冯慧玲;李春梅;吴振芳;陈惠;. 易错PCR技术提高黑曲霉N25植酸酶活力的研究[J]. , 2010, 0(10): 226-230. |
| [11] | 赵春雷;闫丽娟;谢振荣;高润池;李俊;唐湘华;黄遵锡;. 一株耐热脂肪酶产生菌的筛选及酶学性质研究[J]. , 2010, 0(02): 184-188. |
| [12] | 刘维英;韩亚杰;胡坤;代斌;. 合成己酸乙酯脂肪酶产生菌的筛选及发酵条件的研究[J]. , 2009, 0(03): 115-118. |
| [13] | . 酶工程[J]. , 1991, 0(10): 48-58. |
| [14] | . 酶工程[J]. , 1991, 0(09): 54-67. |
| [15] | . 酶工程[J]. , 1991, 0(08): 53-65. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||