








生物技术通报 ›› 2021, Vol. 37 ›› Issue (11): 142-157.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1173
• 食用菌生物技术专题(专题主编: 黄晨阳) • 上一篇 下一篇
王豪( ), 唐禄鑫, 马鸿飞, 钱坤, 司静(
), 唐禄鑫, 马鸿飞, 钱坤, 司静( ), 崔宝凯(
), 崔宝凯( )
)
收稿日期:2021-09-13
出版日期:2021-11-26
发布日期:2021-12-03
作者简介:王豪,男,硕士研究生,研究方向:大型真菌资源利用;E-mail: 基金资助:
WANG Hao( ), TANG Lu-xin, MA Hong-fei, QIAN Kun, SI Jing(
), TANG Lu-xin, MA Hong-fei, QIAN Kun, SI Jing( ), CUI Bao-kai(
), CUI Bao-kai( )
)
Received:2021-09-13
Published:2021-11-26
Online:2021-12-03
摘要:
漆酶是一种天然的绿色催化剂,由于具有催化效率高、底物特异性广、对辅因子和特定环境条件要求少、无毒等优点,因而在制浆造纸、生物合成、改善纤维性能、食品加工、生物传感器制造、农林废弃物的生物转化和炼制,特别是环境污染物的生物降解和生物修复等领域具有巨大的应用潜力。但游离漆酶稳定性差且成本较高,固定化方法成为解决该问题的有效手段,其能够有效增强酶的热稳定性及对极端环境的耐受力、使酶易与产物分离以提高酶回收率。本研究基于前期筛选获得的一株高产漆酶白腐真菌菌株东方栓孔菌(Trametes orientalis)所分泌的漆酶(Tolacc-T),对其纯化酶蛋白以壳聚糖作为载体、戊二醛作为交联剂进行了固定化处理,命名为Tolacc-T@Chit@GA,并优化了固定化条件为戊二醛浓度0.7%(V/V)、交联时间4 h、给酶量6.0 mL、固定化时间6 h。与Tolacc-T相比,Tolacc-T@Chit@GA的pH适应性、耐热变性能力及贮存稳定性均显著增强。循环使用时的稳定性和耐久性也十分明显,循环使用7次后,Tolacc-T@Chit@GA的相对活性仍可保持在80%以上。此外,Tolacc-T@Chit@GA还可使不同类型的染料脱色,尤其对金属络合染料萘酚绿B的脱色效果最佳,气相色谱-质谱联用(gas chromatography-mass spectroscopy,GC-MS)检测确定该染料部分代谢产物为1-萘胺、2-萘酚、1-氨基-2-萘酚、1-亚硝基-2-萘酚、1-亚硝基-2-萘酚-6-磺酸。上述结果证明,东方栓孔菌固定化漆酶Tolacc-T@Chit@GA具有较好的稳定性和可重复利用性,存在广阔的应用前景。
王豪, 唐禄鑫, 马鸿飞, 钱坤, 司静, 崔宝凯. 东方栓孔菌漆酶的固定化及其对不同类型染料的脱色作用[J]. 生物技术通报, 2021, 37(11): 142-157.
WANG Hao, TANG Lu-xin, MA Hong-fei, QIAN Kun, SI Jing, CUI Bao-kai. Immobilization of Laccase from Trametes orientalis and Its Application for Decolorization of Multifarious Dyes[J]. Biotechnology Bulletin, 2021, 37(11): 142-157.
| 染料 Dye | 颜色索引编码 Color index No. | 颜色索引名称 Color index name | 化学结构 Chemical structure | 化学分类 Chemical class | 波长 Wave length /nm |
|---|---|---|---|---|---|
| 活性亮蓝X-BR Reactive brilliant blue X-BR | 61205 | 活性蓝4 Reactive blue 4 | 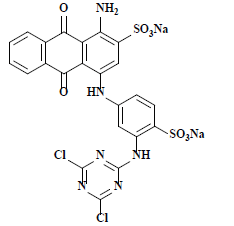 | 蒽醌类 Anthraquinone | 603 |
| 雷玛唑亮蓝R Remazol brilliant blue R | 61200 | 活性蓝19 Reactive blue 19 | 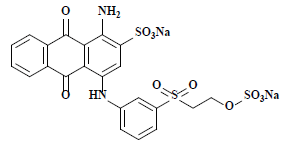 | 蒽醌类 Anthraquinone | 592 |
| 酸性黑172 Acid black 172 | 15711 | 酸性黑172 Acid black 172 | 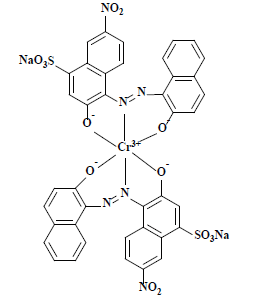 | 偶氮类(含金属) Azo(Metal-containing) | 597 |
| 刚果红 Congo red | 22120 | 直接红28 Direct red 28 | 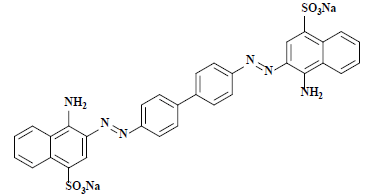 | 偶氮类 Azo | 497 |
| 亚甲基蓝 Methylene blue | 52015 | 碱性蓝9 Basic blue 9 |  | 菁类 Cyanine | 664 |
| 中性红 Neutral red | 50040 | 碱性红5 Basic red 5 | 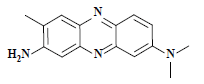 | 杂环类 Heterocycle | 553 |
| 靛蓝 Indigo blue | 73000 | 还原蓝1 Vat blue 1 |  | 靛蓝类 Indigo | 610 |
| 萘酚绿B Naphthol green B | 10020 | 酸性绿1 Acid green 1 |  | 亚硝基类 (含金属) Nitroso(Metal-containing) | 714 |
| 结晶紫 Crystal violet | 42555 | 碱性紫3 Basic violet 3 | 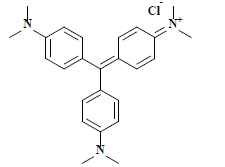 | 三苯甲烷类 Triphenylmethane | 595 |
表1 供试染料
Table 1 Dyes used in this study
| 染料 Dye | 颜色索引编码 Color index No. | 颜色索引名称 Color index name | 化学结构 Chemical structure | 化学分类 Chemical class | 波长 Wave length /nm |
|---|---|---|---|---|---|
| 活性亮蓝X-BR Reactive brilliant blue X-BR | 61205 | 活性蓝4 Reactive blue 4 |  | 蒽醌类 Anthraquinone | 603 |
| 雷玛唑亮蓝R Remazol brilliant blue R | 61200 | 活性蓝19 Reactive blue 19 |  | 蒽醌类 Anthraquinone | 592 |
| 酸性黑172 Acid black 172 | 15711 | 酸性黑172 Acid black 172 |  | 偶氮类(含金属) Azo(Metal-containing) | 597 |
| 刚果红 Congo red | 22120 | 直接红28 Direct red 28 |  | 偶氮类 Azo | 497 |
| 亚甲基蓝 Methylene blue | 52015 | 碱性蓝9 Basic blue 9 |  | 菁类 Cyanine | 664 |
| 中性红 Neutral red | 50040 | 碱性红5 Basic red 5 |  | 杂环类 Heterocycle | 553 |
| 靛蓝 Indigo blue | 73000 | 还原蓝1 Vat blue 1 |  | 靛蓝类 Indigo | 610 |
| 萘酚绿B Naphthol green B | 10020 | 酸性绿1 Acid green 1 |  | 亚硝基类 (含金属) Nitroso(Metal-containing) | 714 |
| 结晶紫 Crystal violet | 42555 | 碱性紫3 Basic violet 3 |  | 三苯甲烷类 Triphenylmethane | 595 |
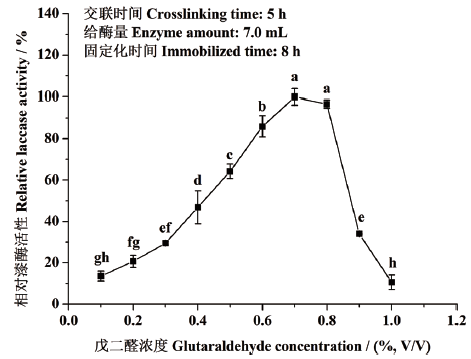
图1 戊二醛交联剂浓度对东方栓孔菌固定化漆酶Tolacc-T@Chit@GA活性的影响 不同小写字母代表差异显著(P<0.05),下同
Fig. 1 Effects of concentrations of glutaraldehyde crosslinker on the activity of the immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis Different lowercase letters refer to significant difference(P<0.05),the same below
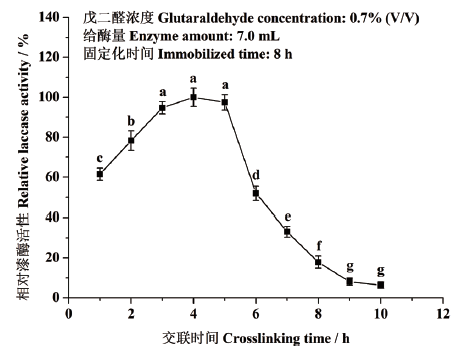
图2 交联时间对东方栓孔菌固定化漆酶Tolacc-T@Chit@GA活性的影响
Fig. 2 Effects of crosslinking time on the activity of the immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
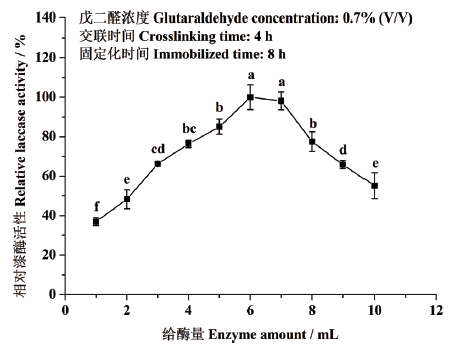
图3 给酶量对东方栓孔菌固定化漆酶Tolacc-T@Chit@GA活性的影响
Fig. 3 Effects of enzyme amounts on the activity of the immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
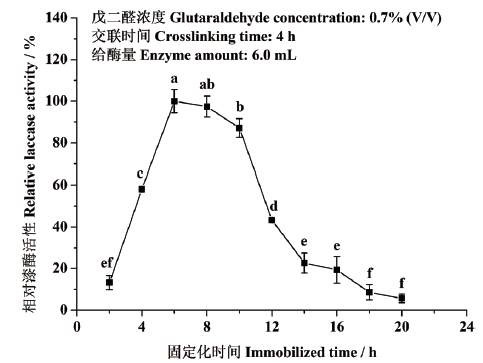
图4 固定化时间对东方栓孔菌固定化漆酶Tolacc-T@Chit@GA活性的影响
Fig. 4 Effects of immobilized time on the activity of the immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
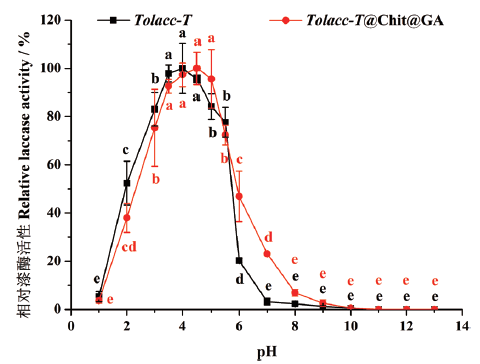
图5 pH对东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA活性的影响
Fig. 5 Effects of pH on the activity of the free laccase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus Trametes orientalis
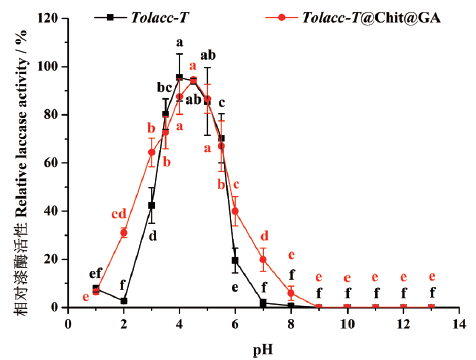
图6 pH对东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA稳定性的影响
Fig. 6 Effects of pH on the stability of the free laccase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
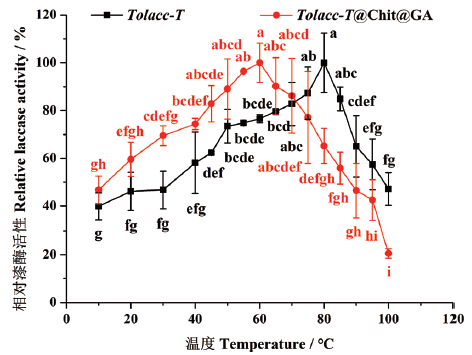
图7 温度对东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA活性的影响
Fig. 7 Effects of temperature on the activity of the free laccase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis

图8 温度对东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA稳定性的影响
Fig. 8 Effects of temperature on the stability of the free laccase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis

图9 东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA的贮存稳定性
Fig. 9 Storage stability of the free laccase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
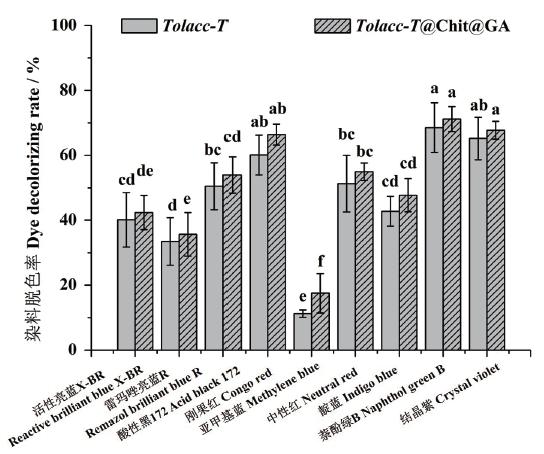
图11 东方栓孔菌游离漆酶Tolacc-T和固定化漆酶Tolacc-T@Chit@GA对不同类型染料的脱色作用
Fig. 11 Decolorization of multifarious dyes by the free lacc-ase Tolacc-T and immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
| 编号 No. | 主要片段m/z Main fragment | 化合物 Compound | 化学式 Chemical formula | CAS编码 CAS No. |
|---|---|---|---|---|
| 1 | 143 | 1-萘胺 1-naphthylamine | 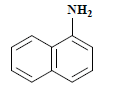 | 134-32-7 |
| 2 | 144 | 2-萘酚 2-naphthol |  | 135-19-3 |
| 3 | 159 | 1-氨基-2-萘酚 1-amino-2-naphthol |  | 2834-92-6 |
| 4 | 173 | 1-亚硝基-2-萘酚 1-nitroso-2-naphthol | 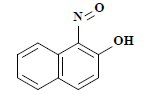 | 131-91-9 |
| 5 | 253 | 1-亚硝基-2-萘酚-6-磺酸 1-nitroso-2-naphthol-6-sulfonate | 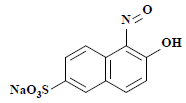 | — |
表2 萘酚绿B经东方栓孔菌固定化漆酶Tolacc-T@Chit@GA降解后的部分代谢产物
Table 2 Partially metabolic by-products of naphthol green B after degradation by the immobilized laccase Tolacc-T@Chit@GA from the white rot fungus T. orientalis
| 编号 No. | 主要片段m/z Main fragment | 化合物 Compound | 化学式 Chemical formula | CAS编码 CAS No. |
|---|---|---|---|---|
| 1 | 143 | 1-萘胺 1-naphthylamine |  | 134-32-7 |
| 2 | 144 | 2-萘酚 2-naphthol |  | 135-19-3 |
| 3 | 159 | 1-氨基-2-萘酚 1-amino-2-naphthol |  | 2834-92-6 |
| 4 | 173 | 1-亚硝基-2-萘酚 1-nitroso-2-naphthol |  | 131-91-9 |
| 5 | 253 | 1-亚硝基-2-萘酚-6-磺酸 1-nitroso-2-naphthol-6-sulfonate |  | — |
| [1] |
Shakerian F, Zhao J, Li SP. Recent development in the application of immobilized oxidative enzymes for bioremediation of hazardous micropollutants - A review[J]. Chemosphere, 2020, 239: 124716.
doi: S0045-6535(19)31946-0 pmid: 31521938 |
| [2] | 司静, 崔宝凯, 贺帅, 等. 微酸多年卧孔菌产漆酶条件优化及其在染料脱色中的应用[J]. 应用与环境生物学报, 2011, 17(5): 736-741. |
| Si J, Cui BK, He S, et al. Optimization of conditions for laccase production by Perenniporia subacida and its application in dye decolorization[J]. Chin J Appl Environ Biol, 2011, 17(5): 736-741. | |
| [3] | 司静, 李伟, 崔宝凯, 等. 真菌漆酶性质、分子生物学及其应用研究进展[J]. 生物技术通报, 2011(2): 48-55. |
| Si J, Li W, Cui BK, et al. Advances of research on characteristic, molecular biology and applications of laccase from fungi[J]. Biotechnol Bull, 2011(2): 48-55. | |
| [4] |
吴怡, 马鸿飞, 曹永佳, 等. 真菌漆酶的性质、生产、纯化及固定化研究进展[J]. 生物技术通报, 2019, 35(9): 1-10.
doi: 10.13560/j.cnki.biotech.bull.1985.2019-0614 |
| Wu Y, Ma HF, Cao YJ, et al. Advances on properties, production, purification and immobilization of fungal laccase[J]. Biotechnol Bull, 2019, 35(9): 1-10. | |
| [5] |
吴怡, 马鸿飞, 曹永佳, 等. 白腐真菌落叶松锈迷孔菌产漆酶液体培养基的优化及其对染料的脱色作用[J]. 生物技术通报, 2020, 36(1): 45-59.
doi: 10.13560/j.cnki.biotech.bull.1985.2019-0974 |
| Wu Y, Ma HF, Cao YJ, et al. Medium optimization for the laccase production by white rot fungus Porodaedalea laricis and its dye decolorizing capacity[J]. Biotechnol Bull, 2020, 36(1): 45-59. | |
| [6] |
Thurston CF. The structure and function of fungal laccases[J]. Microbiology, 1994, 140(1): 19-26.
doi: 10.1099/13500872-140-1-19 URL |
| [7] |
Baldrian P. Fungal laccases - occurrence and properties[J]. FEMS Microbiol Rev, 2006, 30(2): 215-242.
pmid: 16472305 |
| [8] |
Claus H. Laccases:structure, reactions, distribution[J]. Micron, 2004, 35(1/2): 93-96.
doi: 10.1016/j.micron.2003.10.029 URL |
| [9] |
Rivera-Hoyos CM, Morales-Álvarez ED, Poutou-Piñales RA, et al. Fungal laccases[J]. Fungal Biol Rev, 2013, 27(3/4): 67-82.
doi: 10.1016/j.fbr.2013.07.001 URL |
| [10] |
Senthivelan T, Kanagaraj J, Panda RC. Recent trends in fungal laccase for various industrial applications:an eco-friendly approach - A review[J]. Biotechnol Bioprocess Eng, 2016, 21(1): 19-38.
doi: 10.1007/s12257-015-0278-7 URL |
| [11] |
Mate DM, Alcalde M. Laccase:a multi-purpose biocatalyst at the forefront of biotechnology[J]. Microb Biotechnol, 2017, 10(6): 1457-1467.
doi: 10.1111/mbt2.2017.10.issue-6 URL |
| [12] |
Cañas AI, Camarero S. Laccases and their natural mediators:biotechnological tools for sustainable eco-friendly processes[J]. Biotechnol Adv, 2010, 28(6): 694-705.
doi: 10.1016/j.biotechadv.2010.05.002 pmid: 20471466 |
| [13] |
Si J, Peng F, Cui BK. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens[J]. Bioresour Technol, 2013, 128: 49-57.
doi: 10.1016/j.biortech.2012.10.085 URL |
| [14] |
Si J, Ma HF, Cao YJ, et al. Introducing a thermo-alkali-stable, metallic ion-tolerant laccase purified from white rot fungus Trametes hirsuta[J]. Front Microbiol, 2021, 12: 670163. DOI: 10.3389/fmicb.2021.670163.
doi: 10.3389/fmicb.2021.670163 URL |
| [15] |
Si J, Wu Y, Ma HF, et al. Selection of a pH- and temperature-stable laccase from Ganoderma australe and its application for bioremediation of textile dyes[J]. J Environ Manag, 2021, 299: 113619.
doi: 10.1016/j.jenvman.2021.113619 URL |
| [16] |
Pezzella C, Guarino L, Piscitelli A. How to enjoy laccases[J]. Cell Mol Life Sci, 2015, 72(5): 923-940.
doi: 10.1007/s00018-014-1823-9 pmid: 25577278 |
| [17] |
Bilal M, Rasheed T, Nabeel F, et al. Hazardous contaminants in the environment and their laccase-assisted degradation - A review[J]. J Environ Manage, 2019, 234: 253-264.
doi: 10.1016/j.jenvman.2019.01.001 URL |
| [18] |
Singh G, Arya SK. Utility of laccase in pulp and paper industry:a progressive step towards the green technology[J]. Int J Biol Macromol, 2019, 134: 1070-1084.
doi: 10.1016/j.ijbiomac.2019.05.168 URL |
| [19] | 曹永佳, 马鸿飞, 崔宝凯, 等. 不同固体发酵培养基下三种白腐真菌分泌的木质纤维素酶活性[J]. 菌物学报, 2021, 40(5): 1123-1139. |
| Cao YJ, Ma HF, Cui BK, et al. Lignocellulolytic enzyme activities of three white rot fungi under different solid-state fermentation media[J]. Mycosystema, 2021, 40(5): 1123-1139. | |
| [20] |
Mikolasch A, Schauer F. Fungal laccases as tools for the synjournal of new hybrid molecules and biomaterials[J]. Appl Microbiol Biotechnol, 2009, 82(4): 605-624.
doi: 10.1007/s00253-009-1869-z pmid: 19183983 |
| [21] |
Bilal M, Asgher M, Parra-Saldivar R, et al. Immobilized ligninolytic enzymes:an innovative and environmental responsive technology to tackle dye-based industrial pollutants - A review[J]. Sci Total Environ, 2017, 576: 646-659.
doi: 10.1016/j.scitotenv.2016.10.137 URL |
| [22] |
Deska M, Kończak B. Immobilized fungal laccase as “green catalyst” for the decolourization process - State of the art[J]. Process Biochem, 2019, 84: 112-123.
doi: 10.1016/j.procbio.2019.05.024 URL |
| [23] |
Cao SL, Xu P, Ma YZ, et al. Recent advances in immobilized enzymes on nanocarriers[J]. Chin J Catal, 2016, 37(11): 1814-1823.
doi: 10.1016/S1872-2067(16)62528-7 URL |
| [24] |
Ashkan Z, Hemmati R, Homaei A, et al. Immobilization of enzymes on nanoinorganic support materials:an update[J]. Int J Biol Macromol, 2021, 168: 708-721.
doi: 10.1016/j.ijbiomac.2020.11.127 pmid: 33232698 |
| [25] |
Daronch NA, Kelbert M, Pereira CS, et al. Elucidating the choice for a precise matrix for laccase immobilization:a review[J]. Chem Eng J, 2020, 397: 125506.
doi: 10.1016/j.cej.2020.125506 URL |
| [26] |
Aggarwal S, Chakravarty A, Ikram S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies[J]. Int J Biol Macromol, 2021, 167: 962-986.
doi: 10.1016/j.ijbiomac.2020.11.052 pmid: 33186644 |
| [27] |
Arica MY, Salih B, Celikbicak O, et al. Immobilization of laccase on the fibrous polymer-grafted film and study of textile dye degradation by MALDI-ToF-MS[J]. Chem Eng Res Des, 2017, 128: 107-119.
doi: 10.1016/j.cherd.2017.09.023 URL |
| [28] |
Lellis B, Fávaro-Polonio CZ, Pamphile JA, et al. Effects of textile dyes on health and the environment and bioremediation potential of living organisms[J]. Biotechnol Res Innov, 2019, 3(2): 275-290.
doi: 10.1016/j.biori.2019.09.001 URL |
| [29] |
Rovira J, Domingo JL. Human health risks due to exposure to inorganic and organic chemicals from textiles:a review[J]. Environ Res, 2019, 168: 62-69.
doi: 10.1016/j.envres.2018.09.027 URL |
| [30] | Stoll VS, Blanchard JS. Buffers:principles and practice[J]. Methods Enzymol, 1990, 182: 24-38. |
| [31] |
Lima VMG, Krieger N, Mitchell DA, et al. Activity and stability of a crude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents[J]. Biochem Eng J, 2004, 18(1): 65-71.
doi: 10.1016/S1369-703X(03)00165-7 URL |
| [32] |
Kalyani DC, Patil PS, Jadhav JP, et al. Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1[J]. Bioresour Technol, 2008, 99(11): 4635-4641.
doi: 10.1016/j.biortech.2007.06.058 URL |
| [33] | 司静, 崔宝凯, 戴玉成. 栓孔菌属漆酶高产菌株的初步筛选及其产酶条件的优化[J]. 微生物学通报, 2011, 38(3): 405-416. |
| Si J, Cui BK, Dai YC. Primary screening of effective Trametes strains with high laccase-productivity and optimization of conditions on laccase production[J]. Microbiol China, 2011, 38(3): 405-416. | |
| [34] |
Zheng F, An Q, Meng G, et al. A novel laccase from white rot fungus Trametes orientalis:Purification, characterization, and application[J]. Int J Biol Macromol, 2017, 102: 758-770.
doi: S0141-8130(16)31601-4 pmid: 28455255 |
| [35] |
Zheng F, Cui BK, Wu XJ, et al. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes[J]. Int Biodeterior Biodegrad, 2016, 110: 69-78.
doi: 10.1016/j.ibiod.2016.03.004 URL |
| [36] |
Aricov L, Leonties AR, Gîfu IC, et al. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants[J]. J Environ Manage, 2020, 276: 111326.
doi: 10.1016/j.jenvman.2020.111326 URL |
| [37] |
Yoshida H. —chemistry of lacquer(urushi). part I. communication from the chemical society of tokio[J]. J Chem Soc, Trans, 1883, 43: 472-486.
doi: 10.1039/CT8834300472 URL |
| [38] | Bertrand G. Sur la presence simultanee de la laccase et de la tyrosinase dans le suc de quelques champignons[J]. Comptes Rendus Hebdomadaires des Seances de I'Academie des Sciences, 1896, 123: 463-465. |
| [39] |
Tosa T, Mori T, Fuse N, et al. Studies on continuous enzyme reactions. I. Screening of carriers for preparation of water-insoluble aminoacylase[J]. Enzymologia, 1966, 31(4): 214-224.
pmid: 6005325 |
| [40] |
Fernández-Fernández M, Sanromán MÁ, Moldes D. Recent developments and applications of immobilized laccase[J]. Biotechnol Adv, 2013, 31(8): 1808-1825.
doi: 10.1016/j.biotechadv.2012.02.013 pmid: 22398306 |
| [41] |
Durán N, Rosa MA, D’Annibale A, et al. Applications of laccases and tyrosinases(phenoloxidases)immobilized on different supports:a review[J]. Enzyme Microb Technol, 2002, 31(7): 907-931.
doi: 10.1016/S0141-0229(02)00214-4 URL |
| [42] |
Muxika A, Etxabide A, Uranga J, et al. Chitosan as a bioactive polymer:Processing, properties and applications[J]. Int J Biol Macromol, 2017, 105(pt 2): 1358-1368.
doi: S0141-8130(17)31757-9 pmid: 28735006 |
| [43] |
Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers[J]. Appl Biochem Biotechnol, 2017, 181(4): 1314-1337.
doi: 10.1007/s12010-016-2286-2 URL |
| [44] |
Nunes YL, de Menezes FL, de Sousa IG, et al. Chemical and physical Chitosan modification for designing enzymatic industrial biocatalysts:How to choose the best strategy?[J]. Int J Biol Macromol, 2021, 181: 1124-1170.
doi: 10.1016/j.ijbiomac.2021.04.004 pmid: 33864867 |
| [45] |
Migneault I, Dartiguenave C, Bertrand MJ, et al. Glutaraldehyde:behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking[J]. Biotechniques, 2004, 37(5): 790-6, 798.
pmid: 15560135 |
| [46] |
Rodrigues RC, Berenguer-Murcia Á, Carballares D, et al. Stabilization of enzymes via immobilization:Multipoint covalent attachment and other stabilization strategies[J]. Biotechnol Adv, 2021, 52: 107821.
doi: 10.1016/j.biotechadv.2021.107821 URL |
| [47] |
Chen H, Zhang QH, Dang YC, et al. The effect of glutaraldehyde cross-linking on the enzyme activity of immobilized & beta-galactosidase on chitosan bead[J]. Adv J Food Sci Technol, 2013, 5(7): 932-935.
doi: 10.19026/ajfst.5.3185 URL |
| [48] | 付文力, 杨学山, 杨孝朴, 等. 壳聚糖固定化羊血超氧化物歧化酶工艺条件优化及酶学性质比较研究[J]. 食品科学, 2013, 34(19): 219-223. |
| Fu WL, Yang XS, Yang XP, et al. Optimization of process conditions for chitosan-immobilized SOD from sheep blood and its enzymatic characteristics[J]. Food Sci, 2013, 34(19): 219-223. | |
| [49] |
Sun J, Yang L, Jiang M, et al. Stability and activity of immobilized trypsin on carboxymethyl chitosan-functionalized magnetic nanoparticles cross-linked with carbodiimide and glutaraldehyde[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2017, 1054: 57-63.
doi: 10.1016/j.jchromb.2017.04.016 URL |
| [50] |
Gür SD, İdil N, Aksöz N. Optimization of enzyme co-immobilization with sodium alginate and glutaraldehyde-activated chitosan beads[J]. Appl Biochem Biotechnol, 2018, 184(2): 538-552.
doi: 10.1007/s12010-017-2566-5 URL |
| [51] | Kaushal J, Seema, Singh G, et al. Immobilization of catalase onto chitosan and chitosan-bentonite complex:a comparative study[J]. Biotechnol Rep:Amst, 2018, 18: e00258. |
| [52] |
Urrutia P, Bernal C, Wilson L, et al. Use of chitosan heterofunctionality for enzyme immobilization:β-galactosidase immobilization for galacto-oligosaccharide synjournal[J]. Int J Biol Macromol, 2018, 116: 182-193.
doi: 10.1016/j.ijbiomac.2018.04.112 URL |
| [53] | 周蕊, 邢炎华, 王燕. 以壳聚糖-戊二醛为载体柔性固定假丝酵母脂肪酶Candida rugosa lipase[J]. 当代化工, 2019, 48(8): 1686-1689. |
| Zhou R, Xing YH, Wang Y. Immobilization of Candida rugosa lipase by using chitosan-glutaraldehyde as support[J]. Contemp Chem Ind, 2019, 48(8): 1686-1689. | |
| [54] |
Işık M. High stability of immobilized acetylcholinesterase on chitosan beads[J]. ChemistrySelect, 2020, 5(15): 4623-4627.
doi: 10.1002/slct.v5.15 URL |
| [55] |
Mo H, Qiu J, Yang C, et al. Porous biochar/chitosan composites for high performance cellulase immobilization by glutaraldehyde[J]. Enzyme Microb Technol, 2020, 138: 109561.
doi: 10.1016/j.enzmictec.2020.109561 URL |
| [56] |
Soares AMBF, Gonçalves LMO, Ferreira RDS, et al. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications[J]. Carbohydr Polym, 2020, 243: 116498.
doi: 10.1016/j.carbpol.2020.116498 URL |
| [57] | 李黎, 马力. 磁性壳聚糖微球固定化乳糖酶及其酶学性质[J]. 中国组织工程研究, 2021, 25(4): 576-581. |
| Li L, Ma L. Immobilization of lactase on magnetic chitosan microspheres and its effect on enzymatic properties[J]. Chin J Tissue Eng Res, 2021, 25(4): 576-581. | |
| [58] |
Lopes LA, Dias LP, da Costa HPS, et al. Immobilization of a peroxidase from Moringa oleifera Lam. roots(MoPOX)on chitosan beads enhanced the decolorization of textile dyes[J]. Process Biochem, 2021, 110: 129-141.
doi: 10.1016/j.procbio.2021.07.022 URL |
| [59] |
Mohd Syukri MS, Rahman RA, Mohamad Z, et al. Optimization strategy for laccase immobilization on polyethylene terephthalate grafted with maleic anhydride electrospun nanofiber mat[J]. Int J Biol Macromol, 2021, 166: 876-883.
doi: 10.1016/j.ijbiomac.2020.10.244 URL |
| [60] |
Maryšková M, Ardao I, García-González CA, et al. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals[J]. Enzyme Microb Technol, 2016, 89: 31-38.
doi: 10.1016/j.enzmictec.2016.03.001 URL |
| [61] |
Yang J, Lin Y, Yang X, et al. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway[J]. J Hazard Mater, 2017, 322(pt b): 525-531.
doi: S0304-3894(16)30916-5 pmid: 27776862 |
| [62] |
Ma HF, Meng G, Cui BK, et al. Chitosan crosslinked with genipin as supporting matrix for biodegradation of synthetic dyes:Laccase immobilization and characterization[J]. Chem Eng Res Des, 2018, 132: 664-676.
doi: 10.1016/j.cherd.2018.02.008 URL |
| [63] |
Fortes CCS, Daniel-Da-silva AL, Xavier AMRB, et al. Optimization of enzyme immobilization on functionalized magnetic nanoparticles for laccase biocatalytic reactions[J]. Chem Eng Process:Process Intensif, 2017, 117: 1-8.
doi: 10.1016/j.cep.2017.03.009 URL |
| [64] |
Silva C, Silva CJ, Zille A, et al. Laccase immobilization on enzymatically functionalized polyamide 6, 6 fibres[J]. Enzyme Microb Technol, 2007, 41(6/7): 867-875.
doi: 10.1016/j.enzmictec.2007.07.010 URL |
| [65] |
Alver E, Metin AÜ. Chitosan based metal-chelated copolymer nanoparticles:Laccase immobilization and phenol degradation studies[J]. Int Biodeterior Biodegrad, 2017, 125: 235-242.
doi: 10.1016/j.ibiod.2017.07.012 URL |
| [66] |
Muthuvelu KS, Rajarathinam R, Selvaraj RN, et al. A novel method for improving laccase activity by immobilization onto copper ferrite nanoparticles for lignin degradation[J]. Int J Biol Macromol, 2020, 152: 1098-1107.
doi: S0141-8130(19)35419-4 pmid: 31751696 |
| [67] |
Jankowska K, Zdarta J, Grzywaczyk A, et al. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation[J]. Environ Res, 2020, 184: 109332.
doi: 10.1016/j.envres.2020.109332 URL |
| [68] |
Keshvardoostchokami M, Majidi M, Zamani A, et al. Adsorption of phenol on environmentally friendly Fe3O4/ chitosan/ zeolitic imidazolate framework-8 nanocomposite:Optimization by experimental design methodology[J]. J Mol Liq, 2021, 323: 115064.
doi: 10.1016/j.molliq.2020.115064 URL |
| [69] |
Tischer W, Kasche V. Immobilized enzymes:crystals or carriers?[J]. Trends Biotechnol, 1999, 17(8): 326-335.
pmid: 10407405 |
| [70] |
Qiu X, Qin J, Xu M, et al. Organic-inorganic nanocomposites fabricated via functional ionic liquid as the bridging agent for laccase immobilization and its application in 2, 4-dichlorophenol removal[J]. Colloids Surf B Biointerfaces, 2019, 179: 260-269.
doi: 10.1016/j.colsurfb.2019.04.002 URL |
| [71] |
Zhang K, Yang WZ, Liu Y, et al. Laccase immobilized on chitosan-coated Fe3O4 nanoparticles as reusable biocatalyst for degradation of chlorophenol[J]. J Mol Struct, 2020, 1220: 128769.
doi: 10.1016/j.molstruc.2020.128769 URL |
| [72] |
Xu R, Cui JY, Tang RZ, et al. Removal of 2, 4, 6-trichlorophenol by laccase immobilized on nano-copper incorporated electrospun fibrous membrane-high efficiency, stability and reusability[J]. Chem Eng J, 2017, 326: 647-655.
doi: 10.1016/j.cej.2017.05.083 URL |
| [73] |
Mohammadi M, As’habi MA, Salehi P, et al. Immobilization of laccase on epoxy-functionalized silica and its application in biodegradation of phenolic compounds[J]. Int J Biol Macromol, 2018, 109: 443-447.
doi: S0141-8130(17)33680-2 pmid: 29274421 |
| [74] |
Zdarta J, Jankowska K, Wyszowska M, et al. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability[J]. Mater Sci Eng C Mater Biol Appl, 2019, 103: 109789.
doi: 10.1016/j.msec.2019.109789 URL |
| [75] |
Zhang C, You S, Liu Y, et al. Construction of Luffa sponge-based magnetic carbon nanocarriers for laccase immobilization and its application in the removal of bisphenol A[J]. Bioresour Technol, 2020, 305: 123085.
doi: 10.1016/j.biortech.2020.123085 URL |
| [76] |
Jankowska K, Grzywaczyk A, Piasecki A, et al. Electrospun biosystems made of nylon 6 and laccase and its application in dyes removal[J]. Environ Technol Innov, 2021, 21: 101332.
doi: 10.1016/j.eti.2020.101332 URL |
| [77] |
Kashefi S, Borghei SM, Mahmoodi NM. Covalently immobilized laccase onto graphene oxide nanosheets:Preparation, characterization, and biodegradation of azo dyes in colored wastewater[J]. J Mol Liq, 2019, 276: 153-162.
doi: 10.1016/j.molliq.2018.11.156 |
| [78] |
Aslam S, Asgher M, Khan NA, et al. Immobilization of Pleurotus nebrodensis WC 850 laccase on glutaraldehyde cross-linked chitosan beads for enhanced biocatalytic degradation of textile dyes[J]. J Water Process Eng, 2021, 40: 101971.
doi: 10.1016/j.jwpe.2021.101971 URL |
| [79] |
Morsi R, Bilal M, Iqbal HMN, et al. Laccases and peroxidases:The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants[J]. Sci Total Environ, 2020, 714: 136572.
doi: 10.1016/j.scitotenv.2020.136572 URL |
| [1] | 陈金行, 张逸, 张军涛, 未本美, 王宏勋, 郑明明. 固定化脂肪酶的创制及其在乙酸肉桂酯无溶剂制备中的应用[J]. 生物技术通报, 2023, 39(9): 97-104. |
| [2] | 李焕敏, 高峰涛, 李伟忠, 王金庆, 封佳丽. 天然生物质材料作为固定化载体的研究应用进展[J]. 生物技术通报, 2023, 39(7): 105-112. |
| [3] | 王雨辰, 丁尊丹, 关菲菲, 田健, 刘国安, 伍宁丰. 耐热漆酶ba4基因鉴定与酶学性质分析[J]. 生物技术通报, 2022, 38(8): 252-260. |
| [4] | 贾晨波, 苏一黄, 马秀梅, 王春利, 徐春燕. 端梗霉Z45产漆酶培养基的优化及其对染料的脱色[J]. 生物技术通报, 2022, 38(6): 252-260. |
| [5] | 毛国涛, 王杰, 王凯, 王方园, 曹乐言, 张宏森, 宋安东. 水生栖热菌漆酶TaLac的性质分析及对孔雀石绿染料的脱除[J]. 生物技术通报, 2022, 38(4): 261-268. |
| [6] | 付雅丽, 彭万里, 林双君, 邓子新, 梁如冰. 香茅醇假单胞菌SJTE-3的短链脱氢酶SDR-X1的克隆及酶性质测定[J]. 生物技术通报, 2022, 38(3): 121-129. |
| [7] | 马青云, 江旭, 李情情, 宋金龙, 周义清, 阮志勇. 烟嘧磺隆降解菌Chryseobacterium sp. LAM-M5的分离、鉴定及其降解机理研究[J]. 生物技术通报, 2022, 38(2): 113-122. |
| [8] | 牛鸿宇, 舒倩, 杨海君, 颜智勇, 谭菊. 一株十二烷基硫酸钠高效降解菌的分离鉴定、降解特性及代谢途径研究[J]. 生物技术通报, 2022, 38(12): 287-299. |
| [9] | 朱永安, 王淼, 曹静, 喻鹤, 曹振, 金茂俊, 王静, 佘永新. 农药残留检测关键用酶固定化研究进展[J]. 生物技术通报, 2022, 38(1): 258-268. |
| [10] | 田嘉慧, 封佳丽, 卢俊桦, 毛林静, 胡著然, 王莹, 楚杰. 一色齿毛菌漆酶LacT-1的分离纯化与性质研究[J]. 生物技术通报, 2021, 37(8): 186-194. |
| [11] | 孙宝婷, 邱萌霞, 王子辰, 王梓源, 崔建东, 贾士儒. 半胱氨酸辅助的酶@ZIF-8固定化酶制备及其特性研究[J]. 生物技术通报, 2021, 37(8): 221-232. |
| [12] | 陈明雨, 倪烜, 司友斌, 孙凯. 固定化真菌漆酶在环境有机污染修复中的应用研究进展[J]. 生物技术通报, 2021, 37(6): 244-258. |
| [13] | 芦尚德, 刘婧婧, 冯一平, 赵鹏, 徐仰仓. 固定化小球藻产氧及光合速率的研究[J]. 生物技术通报, 2021, 37(3): 92-98. |
| [14] | 徐刘佳, 郑明明. 皮克林乳液酶反应体系的构建与应用研究进展[J]. 生物技术通报, 2021, 37(2): 187-194. |
| [15] | 肖小双, 安雪姣, 叶晗媛, 王林平, 钟斌, 张庆华. 废水中硫氰酸盐的微生物降解研究进展[J]. 生物技术通报, 2021, 37(2): 224-235. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||