








生物技术通报 ›› 2023, Vol. 39 ›› Issue (6): 274-285.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1226
收稿日期:2022-10-08
出版日期:2023-06-26
发布日期:2023-07-07
通讯作者:
于存,博士,副教授,硕士生导师,研究方向:林业资源微生物开发与利用;E-mail: chifengyucun@163.com作者简介:徐红云,女,博士,讲师,研究方向:资源微生物、林木抗逆育种;E-mail: xhyplant@126.com
基金资助:
XU Hong-Yun1( ), LV Jun2, YU Cun2(
), LV Jun2, YU Cun2( )
)
Received:2022-10-08
Published:2023-06-26
Online:2023-07-07
摘要:
为筛选溶磷效果较好的根际细菌(phosphate-solubilizing rhizobacteria, PSB),并明确其对马尾松苗的促生效果和作用机制。利用土壤稀释平板法进行PSB菌株的分离和纯化,通过形态学和16S rDNA分子测序等方法进行PSB菌株的鉴定,最后将PSB菌株接种至马尾松苗,培养60 d后测定马尾松苗生长、生理、苗根际土壤理化性质和根际细菌群落结构和组成。结果表明:由马尾松根际土中分离获得溶磷能力较强的3个PSB菌株WJ10、WJ25和WJ41均为伯克霍尔德菌Paraburkholderia spp.;3个PSB菌株对磷酸铝的增溶能力最强,其次是磷酸三钙、磷酸氢钙和磷酸铁;盆栽试验表明,3个PSB菌株均可促进幼苗的生长,其中WJ25对苗高、根长的促进效果最明显,WJ41和WJ10次之。3个PSB菌株对苗促生的主要机制包括,PSB提高了马尾松苗的根系活力、叶绿素b、可溶性蛋白等生长指标及氮、磷和钾等养分含量;同时,提升了根际土有效磷、速效钾、活性氮、土壤养分含量、土壤酶活性;此外,3个PSB菌株的添加还影响了马尾松苗根际细菌群落的组成和多样性,促进了Bacillus、Nitrosospira、Gemmata和Cytophaga等有益菌在根际土壤中的显著富集。综上,本研究筛选获得的3个溶磷伯克霍尔德菌,它们能够通过调控植物生理及改变根际微环境从而促进马尾松苗的生长。通过本研究,为马尾松根际溶磷细菌菌肥的开发和应用提供了理论依据。
徐红云, 吕俊, 于存. 根际溶磷伯克霍尔德菌Paraburkholderia spp.对马尾松苗的促生作用[J]. 生物技术通报, 2023, 39(6): 274-285.
XU Hong-Yun, LV Jun, YU Cun. Growth Promoting of Pinus massoniana Seedlings Regulated by Rhizosphere Phosphate-solubilizing Paraburkholderia spp.[J]. Biotechnology Bulletin, 2023, 39(6): 274-285.
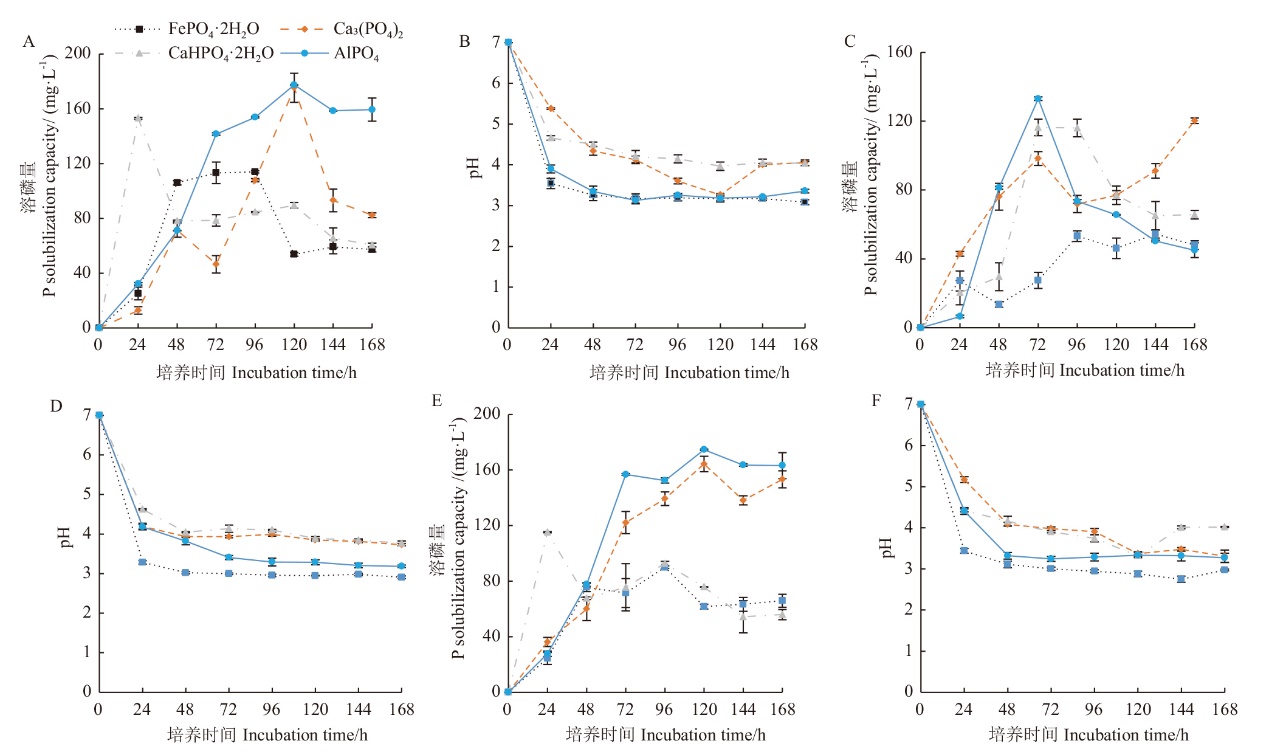
图4 三个溶磷菌株对不同磷酸盐的溶磷能力及pH变化 A和B:WJ10; C和D:WJ25; E和F:WJ41。图中的数据是均值,误差线是标准偏差,下同
Fig. 4 P solubilizing capacity of three PSB in four different phosphate medium and acidification of medium during the phosphate solubilization A and B: Strain WJ10; C and D: strain WJ25; E and F: strain WJ41. The data in the figure are means, and the error line is the SE. The same blow
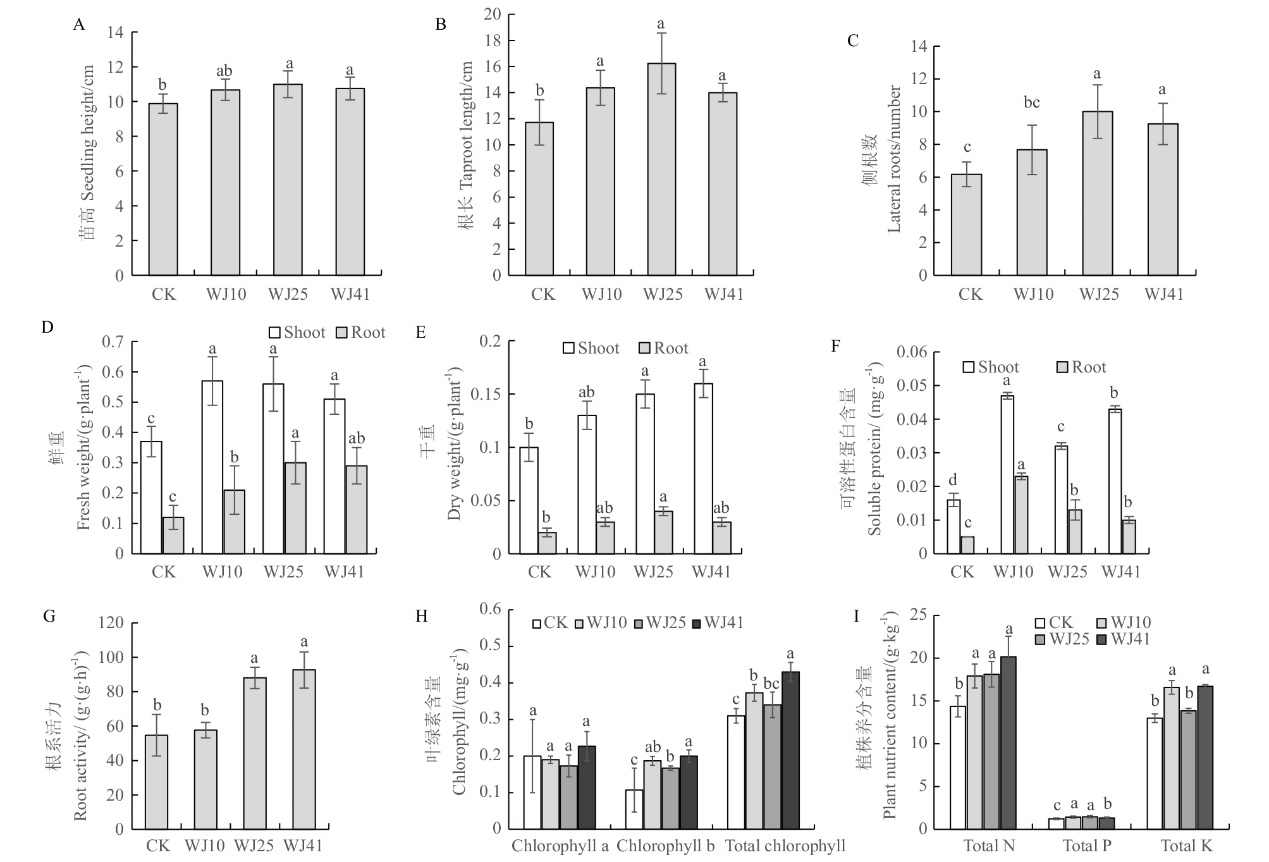
图6 接种3株溶磷菌株对马尾松苗生长、养分和生理的影响 A:苗高;B:根长;C:侧根数;D:鲜重;E:干重;F:可溶性蛋白含量;G:根系活力;H:叶绿素含量;I:植株养分。不同字母代表差异显著(P<0.05),下同
Fig. 6 Effects of PSB strain inoculations on the growth, nutrient uptake and physiological indexes of P. massoniana seedlings A: Seedling height; B: root length; C: lateral root number; D: fresh weight; E: dry weight; F: soluble protein content; G: root activity; H: chlorophyll content; I: plant nutrient. Different letters indicate siginificant difference(P<0.05). The same below
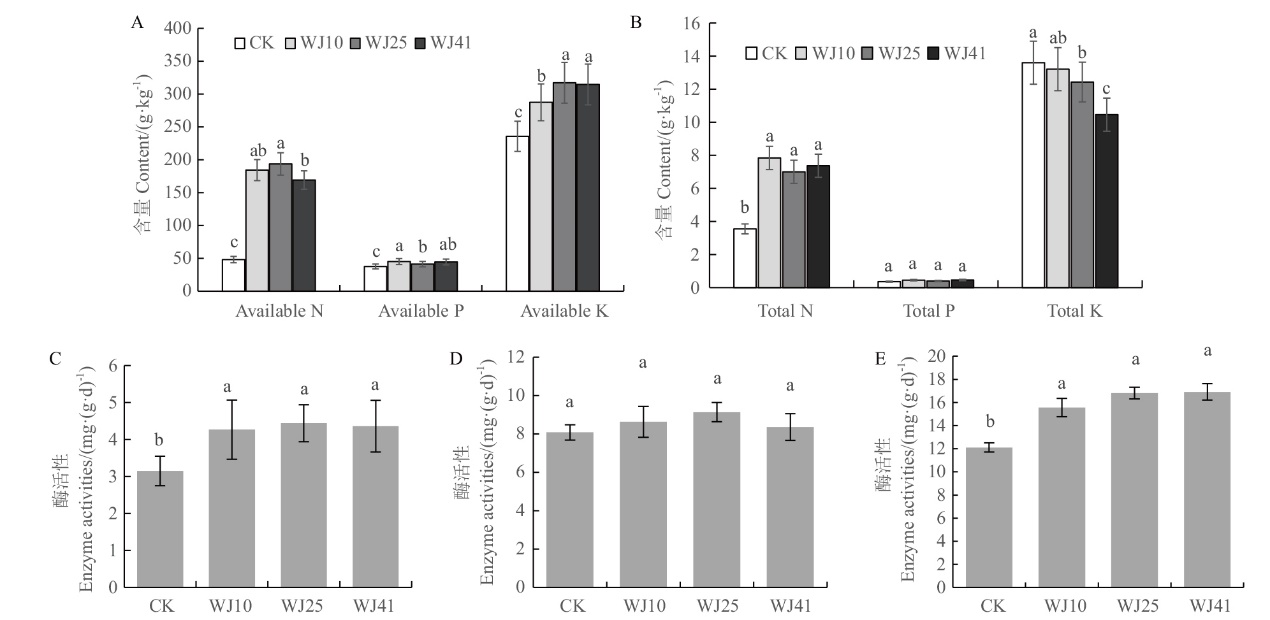
图7 接种溶磷菌株对马尾松根际土养分含量和酶活性影响 A:有效养分;B:总养分含量;C:脲酶;D:过氧化氢酶;E:磷酸酶
Fig. 7 Effects of PSB strains on soil nutrient A: Available nutrient content of soil. B: Total nutrient content of soil. C: Soil urease. D: Soil catalase. E: Soil phosphatase
| Treatment | OTUs | Shannon | ACE | Chao1 | Simpson |
|---|---|---|---|---|---|
| CK | 2181±100.00ab | 5.67±0.07b | 3126.18±107.24a | 2968.00±175.58a | 0.0134±0.002a |
| WJ10 | 1967±75.48bc | 5.82±0.14ab | 2713.49±60.82b | 2636.03±78.64b | 0.0090±0.001a |
| WJ25 | 2244±179.85a | 5.86±0.18a | 3109.78±98.45a | 2960.25±57.96a | 0.0116±0.002a |
| WJ41 | 1877±73.75c | 5.66±0.17b | 2604.44±45.00b | 2474.54±47.17b | 0.0121±0.002a |
表1 不同处理马尾松根际土壤细菌α多样性指数
Table 1 Alpha diversity index of bacteria in rhizosphere soil of P. massoniana with different treatments
| Treatment | OTUs | Shannon | ACE | Chao1 | Simpson |
|---|---|---|---|---|---|
| CK | 2181±100.00ab | 5.67±0.07b | 3126.18±107.24a | 2968.00±175.58a | 0.0134±0.002a |
| WJ10 | 1967±75.48bc | 5.82±0.14ab | 2713.49±60.82b | 2636.03±78.64b | 0.0090±0.001a |
| WJ25 | 2244±179.85a | 5.86±0.18a | 3109.78±98.45a | 2960.25±57.96a | 0.0116±0.002a |
| WJ41 | 1877±73.75c | 5.66±0.17b | 2604.44±45.00b | 2474.54±47.17b | 0.0121±0.002a |

图8 接种溶磷菌株对马尾松苗根际细菌群落门水平和属水平组成的影响 A:门水平;B:属水平
Fig. 8 Relative abundance of bacteria at phyla levels and genus levels in the rhizosphere soils under different treatments A: Phyla levels; B: genus levels
| α多样性指数 Alpha diversity index | 水解N Available N | 有效P Available P | 速效K Available K | 尿素酶 Urease | 过氧化氢酶 Catalase | 磷酸酶 Phosphatase |
|---|---|---|---|---|---|---|
| Shannon | 0.883 | 0.250 | 0.426 | 0.548 | 0.903 | 0.426 |
| ACE | -0.139 | -0.884 | -0.441 | -0.519 | -0.157 | -0.512 |
| Chao1 | -0.091 | -0.850 | -0.452 | -0.504 | -0.191 | -0.516 |
| Simpson | -0.689 | -0.773 | -0.378 | -0.605 | -0.464 | -0.449 |
表2 马尾松根际土壤有效养分、酶活性与细菌α多样性指数的相关性
Table 2 Correlation between the rhizosphere soil available nutrients, enzyme activity and bacterial alpha diversity index of P. massoniana
| α多样性指数 Alpha diversity index | 水解N Available N | 有效P Available P | 速效K Available K | 尿素酶 Urease | 过氧化氢酶 Catalase | 磷酸酶 Phosphatase |
|---|---|---|---|---|---|---|
| Shannon | 0.883 | 0.250 | 0.426 | 0.548 | 0.903 | 0.426 |
| ACE | -0.139 | -0.884 | -0.441 | -0.519 | -0.157 | -0.512 |
| Chao1 | -0.091 | -0.850 | -0.452 | -0.504 | -0.191 | -0.516 |
| Simpson | -0.689 | -0.773 | -0.378 | -0.605 | -0.464 | -0.449 |
| [1] |
Kshetri L, Pandey P, Sharma GD. Rhizosphere mediated nutrient management in Allium hookeri Thwaites by using phosphate solubilizing rhizobacteria and tricalcium phosphate amended soil[J]. J Plant Interact, 2018, 13(1): 256-269.
doi: 10.1080/17429145.2018.1472307 URL |
| [2] |
Vance CP. Symbiotic nitrogen fixation and phosphorus acquisition. Plant nutrition in a world of declining renewable resources[J]. Plant Physiol, 2001, 127(2): 390-397.
pmid: 11598215 |
| [3] |
Yu X, Liu X, Zhu TH, et al. Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization[J]. Biol Fertil Soils, 2011, 47(4): 437-446.
doi: 10.1007/s00374-011-0548-2 URL |
| [4] |
Yang PX, Ma L, Chen MH, et al. Phosphate solubilizing ability and phylogenetic diversity of bacteria from P-rich soils around Dianchi Lake drainage area of China[J]. Pedosphere, 2012, 22(5): 707-716.
doi: 10.1016/S1002-0160(12)60056-3 URL |
| [5] |
Zeng QW, Wu XQ, Wen XY. Identification and characterization of the rhizosphere phosphate-solubilizing bacterium Pseudomonas frederiksbergensis JW-SD2, and its plant growth-promoting effects on poplar seedlings[J]. Ann Microbiol, 2016, 66(4): 1343-1354.
doi: 10.1007/s13213-016-1220-8 URL |
| [6] |
Singh JS, Pandey VC, Singh DP. et al. Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development[J]. Agric Ecosyst Environ, 2011, 140(3/4): 339-353.
doi: 10.1016/j.agee.2011.01.017 URL |
| [7] |
Wang YY, Li PS, Zhang BX, et al. Identification of phosphate-solubilizing microorganisms and determination of their phosphate-solubilizing activity and growth-promoting capability[J]. BioResources, 2020, 15(2): 2560-2578.
doi: 10.15376/biores URL |
| [8] |
Li YB, Liu XM, Hao TY, et al. Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates[J]. Int J Mol Sci, 2017, 18(7): 1253.
doi: 10.3390/ijms18071253 URL |
| [9] |
Azeem M, Hassan TU, Tahir MI, et al. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity[J]. Appl Soil Ecol, 2021, 157: 103732.
doi: 10.1016/j.apsoil.2020.103732 URL |
| [10] |
Chauhan H, Bagyaraj DJ, Selvakumar G, et al. Novel plant growth promoting rhizobacteria—Prospects and potential[J]. Appl Soil Ecol, 2015, 95: 38-53.
doi: 10.1016/j.apsoil.2015.05.011 URL |
| [11] |
Liu FP, Liu HQ, Zhou HL, et al. Isolation and characterization of phosphate-solubilizing bacteria from betel nut(Areca catechu)and their effects on plant growth and phosphorus mobilization in tropical soils[J]. Biol Fertil Soils, 2014, 50(6): 927-937.
doi: 10.1007/s00374-014-0913-z URL |
| [12] |
Yu LM, Zhao MM, Wang JS, et al. Antioxidant, immunomodulatory and anti-breast cancer activities of phenolic extract from pine(Pinus massoniana Lamb)bark[J]. Innov Food Sci Emerg Technol, 2008, 9(1): 122-128.
doi: 10.1016/j.ifset.2007.06.006 URL |
| [13] |
Liu QH, Zhou ZC, Wei YC, et al. Genome-wide identification of differentially expressed genes associated with the high yielding of oleoresin in secondary xylem of Masson pine(Pinus massoniana lamb)by transcriptomic analysis[J]. PLoS One, 2015, 10(7): e0132624.
doi: 10.1371/journal.pone.0132624 URL |
| [14] | 李玲, 周运超, 刘娟, 等. 施肥对马尾松土壤磷形态的影响[J]. 亚热带水土保持, 2011, 23(4): 14-17, 57. |
| Li L, Zhou YC, Liu J, et al. Effect on soil phosphorus change of Masson pineafter fertilizer treatments[J]. Subtrop Soil Water Conserv, 2011, 23(4): 14-17, 57. | |
| [15] | 王艺, 丁贵杰. 外生菌根对马尾松幼苗生长、生理特征和养分的影响[J]. 南京林业大学学报: 自然科学版, 2013, 37(2): 97-102. |
| Wang Y, Ding GJ. Effects of ectomycorrhizal on growth, physiological characteristics and nutrition in Pinus massoniana seedlings[J]. J Nanjing For Univ Nat Sci Ed, 2013, 37(2): 97-102. | |
| [16] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册[M]. 北京: 科学出版社, 2001. |
| Dong XZ, Cai MY. Common bacterial system identification manual[M]. Beijing: Science Press, 2001. | |
| [17] | Shen P, Chen XD. Microbiology experiment[M]. Higher Education, Beijing, 2007. |
| [18] |
Heredia-Acuña C, Almaraz-Suarez JJ, Arteaga-Garibay R, et al. Isolation, characterization and effect of plant-growth-promoting rhizobacteria on pine seedlings(Pinus pseudostrobus Lindl.)[J]. J For Res, 2019, 30(5): 1727-1734.
doi: 10.1007/s11676-018-0723-5 |
| [19] |
Yin D, Deng X, Chet I, et al. Physiological responses of Pinus sylvestris var. mongolica seedlings to the interaction between Suillus luteus and Trichoderma virens[J]. Curr Microbiol, 2014, 69(3): 334-342.
doi: 10.1007/s00284-014-0589-5 pmid: 24801335 |
| [20] |
Zhang ZJ, Huang RF. Analysis of malondialdehyde, chlorophyll proline, soluble sugar, and glutathione content in Arabidopsis seedling[J]. Bio-Protocol, 2013, 3(14). DOI: 10.21769/BioProtoc.817.
doi: 10.21769/BioProtoc.817 |
| [21] |
Chu EP, Tavares AR, Kanashiro S, et al. Effects of auxins on soluble carbohydrates, starch and soluble protein content in Aechmea blanchetiana(Bromeliaceae)cultured in vitro[J]. Sci Hortic, 2010, 125(3): 451-455.
doi: 10.1016/j.scienta.2010.04.021 URL |
| [22] |
Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status[J]. Commun Soil Sci Plant Anal, 1982, 13(12): 1035-1059.
doi: 10.1080/00103628209367332 URL |
| [23] |
Javeed HMR, Qamar R, Rehman AU, et al. Improvement in soil characteristics of sandy loam soil and grain quality of spring maize by using phosphorus solublizing bacteria[J]. Sustainability, 2019, 11(24): 7049.
doi: 10.3390/su11247049 URL |
| [24] |
Lemanowicz J, Haddad SA, Bartkowiak A, et al. The role of an urban park's tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil[J]. Sci Total Environ, 2020, 741: 140446.
doi: 10.1016/j.scitotenv.2020.140446 URL |
| [25] |
Tuomisto H. A diversity of beta diversities: straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity[J]. Ecography, 2010, 33(1): 2-22.
doi: 10.1111/eco.2010.33.issue-1 URL |
| [26] |
Sarkar A, Islam T, Biswas GC, et al. Screening for phosphate solubilizing bacteria inhabiting the rhizoplane of rice grown in acidic soil in Bangladesh[J]. Acta Microbiol Immunol Hung, 2012, 59(2): 199-213.
doi: 10.1556/amicr.59.2012.2.5 URL |
| [27] |
Melo J, Carvalho L, Correia P, et al. Conventional farming disrupts cooperation among phosphate solubilising bacteria isolated from Carica papaya's rhizosphere[J]. Appl Soil Ecol, 2018, 124: 284-288.
doi: 10.1016/j.apsoil.2017.11.015 URL |
| [28] |
Panhwar QA, Naher UA, Shamshuddin J, et al. Biochemical and molecular characterization of potential phosphate-solubilizing bacteria in acid sulfate soils and their beneficial effects on rice growth[J]. PLoS One, 2014, 9(10): e97241.
doi: 10.1371/journal.pone.0097241 URL |
| [29] |
Collavino MM, Sansberro PA, Mroginski LA, et al. Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth[J]. Biol Fertil Soils, 2010, 46(7): 727-738.
doi: 10.1007/s00374-010-0480-x URL |
| [30] | Jog R, Pandya M, Nareshkumar G, et al. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth[J]. Microbiology(Reading), 2014, 160(Pt 4): 778-788. |
| [31] |
Burns RG, DeForest JL, Marxsen J, et al. Soil enzymes in a changing environment: current knowledge and future directions[J]. Soil Biol Biochem, 2013, 58: 216-234.
doi: 10.1016/j.soilbio.2012.11.009 URL |
| [32] |
Wu FY, Wan JHC, Wu SC, et al. Effects of earthworms and plant growth-promoting rhizobacteria(PGPR)on availability of nitrogen, phosphorus, and potassium in soil[J]. Z Pflanzenernähr Bodenk, 2012, 175(3): 423-433.
doi: 10.1002/jpln.v175.3 URL |
| [33] |
Heidari E, Mohammadi K, Pasari B, et al. Combining the phosphate solubilizing microorganisms with biochar types in order to improve safflower yield and soil enzyme activity[J]. Soil Sci Plant Nutr, 2020, 66(2): 255-267.
doi: 10.1080/00380768.2019.1704180 |
| [34] |
Kielak AM, Cipriano MAP, Kuramae EE. Acidobacteria strains from subdivision 1 act as plant growth-promoting bacteria[J]. Arch Microbiol, 2016, 198(10): 987-993.
pmid: 27339258 |
| [35] |
Buddrus-Schiemann K, Schmid M, Schreiner K, et al. Root colonization by Pseudomonas sp. DSMZ 13134 and impact on the indigenous rhizosphere bacterial community of barley[J]. Microb Ecol, 2010, 60(2): 381-393.
doi: 10.1007/s00248-010-9720-8 pmid: 20644925 |
| [36] |
Yang WL, Gong T, Wang JW, et al. Effects of compound microbial fertilizer on soil characteristics and yield of wheat(Triticum aestivum L.)[J]. J Soil Sci Plant Nutr, 2020, 20(4): 2740-2748.
doi: 10.1007/s42729-020-00340-9 |
| [37] |
de Feudis M, Cardelli V, Massaccesi L, et al. Altitude affects the quality of the water-extractable organic matter(WEOM)from rhizosphere and bulk soil in European beech forests[J]. Geoderma, 2017, 302: 6-13.
doi: 10.1016/j.geoderma.2017.04.015 URL |
| [38] |
Ramos B, Garcı́a JAL, Probanza A, et al. Alterations in the rhizobacterial community associated with European alder growth when inoculated with PGPR strain Bacillus licheniformis[J]. Environ Exp Bot, 2003, 49(1): 61-68.
doi: 10.1016/S0098-8472(02)00059-X URL |
| [39] |
Probanza A, Lucas Garcı́a JA, Ruiz Palomino M, et al. Pinus pinea L. seedling growth and bacterial rhizosphere structure after inoculation with PGPR Bacillus(B. licheniformis CECT 5106 and B. Pumilus CECT 5105)[J]. Appl Soil Ecol, 2002, 20(2): 75-84.
doi: 10.1016/S0929-1393(02)00007-0 URL |
| [1] | 赵光绪, 杨合同, 邵晓波, 崔志豪, 刘红光, 张杰. 一株高效溶磷产红青霉培养条件优化及其溶磷特性[J]. 生物技术通报, 2023, 39(9): 71-83. |
| [2] | 江润海, 姜冉冉, 朱城强, 侯秀丽. 微生物强化植物修复铅污染土壤的机制研究进展[J]. 生物技术通报, 2023, 39(8): 114-125. |
| [3] | 褚睿, 李昭轩, 张学青, 杨东亚, 曹行行, 张雪艳. 黄瓜枯萎病拮抗芽孢杆菌的筛选、鉴定及其生防潜力[J]. 生物技术通报, 2023, 39(8): 262-271. |
| [4] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [5] | 车永梅, 郭艳苹, 刘广超, 叶青, 李雅华, 赵方贵, 刘新. 菌株C8和B4的分离鉴定及其耐盐促生效果和机制[J]. 生物技术通报, 2023, 39(5): 276-285. |
| [6] | 罗义, 张丽娟, 黄伟, 王宁, 吾尔丽卡·买提哈斯木, 施宠, 王玮. 一株耐铀菌株的鉴定及其促生特性研究[J]. 生物技术通报, 2023, 39(5): 286-296. |
| [7] | 李善家, 雷雨昕, 孙梦格, 刘海锋, 王兴敏. 种子内生细菌多样性与植物互馈作用研究进展[J]. 生物技术通报, 2023, 39(4): 166-175. |
| [8] | 李琦, 杨晓蕾, 李晓林, 申友磊, 李建宏, 姚拓. 高寒草地燕麦根际解植酸磷促生菌鉴定及其优势菌假单胞菌属菌株功能特性[J]. 生物技术通报, 2023, 39(3): 243-253. |
| [9] | 杨东亚, 祁瑞雪, 李昭轩, 林薇, 马慧, 张雪艳. 黄瓜茄病镰刀菌拮抗芽孢杆菌的筛选、鉴定及促生效果[J]. 生物技术通报, 2023, 39(2): 211-220. |
| [10] | 车永梅, 刘广超, 郭艳苹, 叶青, 赵方贵, 刘新. 一种耐盐复合菌剂的制备和促生作用研究[J]. 生物技术通报, 2023, 39(11): 217-225. |
| [11] | 邹兰, 王茜, 李慕仪, 叶坤浩, 黄晶. 乌头内生细菌JY-3-1R的鉴定及其生防和促生能力研究[J]. 生物技术通报, 2023, 39(10): 246-255. |
| [12] | 孙卓, 王妍, 韩忠明, 王云贺, 赵淑杰, 杨利民. 防风根际真菌的分离鉴定及其生防潜力评价[J]. 生物技术通报, 2023, 39(1): 264-273. |
| [13] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| [14] | 贺丽娜, 冯源, 石慧敏, 叶建仁. 具有杀线活性马尾松内生细菌的筛选与鉴定[J]. 生物技术通报, 2022, 38(8): 159-166. |
| [15] | 江美彦, 周杨, 刘仁浪, 姚菲, 杨云舒, 侯凯, 冯冬菊, 吴卫. 白芷根际促生菌的筛选及其促生效果研究[J]. 生物技术通报, 2022, 38(8): 167-178. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||