








生物技术通报 ›› 2023, Vol. 39 ›› Issue (8): 272-282.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0038
方澜1( ), 黎妍妍2, 江健伟1, 成胜1, 孙正祥1(
), 黎妍妍2, 江健伟1, 成胜1, 孙正祥1( ), 周燚1(
), 周燚1( )
)
收稿日期:2023-01-17
出版日期:2023-08-26
发布日期:2023-09-05
通讯作者:
孙正祥,男,博士,副教授,研究方向:植物病害生物防治;E-mail: sunzhengxiang9904@126.com;作者简介:方澜,女,硕士研究生,研究方向:生物防治;E-mail: 1605867934@qq.com
基金资助:
FANG Lan1( ), LI Yan-yan2, JIANG Jian-wei1, CHENG Sheng1, SUN Zheng-xiang1(
), LI Yan-yan2, JIANG Jian-wei1, CHENG Sheng1, SUN Zheng-xiang1( ), ZHOU Yi1(
), ZHOU Yi1( )
)
Received:2023-01-17
Published:2023-08-26
Online:2023-09-05
摘要:
真菌内生细菌是一类特殊细菌,在寄主真菌体内及生态系统中发挥重要作用。为发掘盘龙参内生真菌内具有促生功能的内生细菌资源,并探索其生物学功能,采用荧光原位杂交(fluorescence in situ hybridization, FISH)检测高粱附球菌Epicoccum sorghinum菌丝中内生细菌的存在后,利用菌丝组织研磨法分离内生细菌,通过菌落形态、生理生化特性及分子特征对其进行鉴定。运用特异性引物验证细菌的内生性,采用Salkowski比色法、CAS比色法以及钼锑抗比色法对菌株的促生特性进行测定,通过水稻种子促生长实验初步验证内生细菌的促生长能力,并对内生细菌进行全基因组测序和促生相关功能基因分析。真菌E. sorghinum菌丝内能观察到与荧光素标记的单链核酸探针杂交后的细菌,证实其菌丝内含有细菌,从菌丝内分离出一株内生栖稻根瘤菌Rhizobium oryzihabitans 7-2H,具有产吲哚乙酸(IAA)、铁载体和溶磷能力,可显著提高水稻幼苗的茎长、根长、鲜重和干重。菌株7-2H与标准菌株R. oryzihabitans M15基因组之间的平均核酸一致性(average nucleotide identity, ANI)值为96.98%,通过对基因组分析发现,菌株7-2H含有与产IAA、铁载体及溶磷能力相关的基因。分离得到一株真菌内生栖稻根瘤菌,该菌具有良好的促进植物生长特性,可作为后续研发微生物菌肥的菌种资源。
方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282.
FANG Lan, LI Yan-yan, JIANG Jian-wei, CHENG Sheng, SUN Zheng-xiang, ZHOU Yi. Isolation, Identification and Growth-promoting Characteristics of Endohyphal Bacterium 7-2H from Endophytic Fungi of Spiranthes sinensis[J]. Biotechnology Bulletin, 2023, 39(8): 272-282.
| 检测基因 Detected gene | 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 预变性 Initial denaturation | 变性Denaturation | 退火Annealing | 延伸Extension | 最终延伸 Final extension |
|---|---|---|---|---|---|---|---|
| 30 cycles | |||||||
| 16S rDNA | 27F | AGAGTTTGATCCTGGCTCAG | 94℃, 2 min | 94℃, 30 s | 55℃, 30 s | 72℃, 90 s | 72℃, 5 min |
| 1492R | TACGGCTACCTTGTTACGACTT | ||||||
| ChvE | ChvE-F101 | GCTGGTCTTCCTTGTAGTAA | 56℃, 30 s | 72℃, 60 s | |||
| ChvE-R653 | AACGCCTTCTTCTTCTATGA | ||||||
表1 PCR扩增程序
Table 1 PCR amplification procedure
| 检测基因 Detected gene | 引物名称 Primer name | 引物序列 Primer sequence(5'-3') | 预变性 Initial denaturation | 变性Denaturation | 退火Annealing | 延伸Extension | 最终延伸 Final extension |
|---|---|---|---|---|---|---|---|
| 30 cycles | |||||||
| 16S rDNA | 27F | AGAGTTTGATCCTGGCTCAG | 94℃, 2 min | 94℃, 30 s | 55℃, 30 s | 72℃, 90 s | 72℃, 5 min |
| 1492R | TACGGCTACCTTGTTACGACTT | ||||||
| ChvE | ChvE-F101 | GCTGGTCTTCCTTGTAGTAA | 56℃, 30 s | 72℃, 60 s | |||
| ChvE-R653 | AACGCCTTCTTCTTCTATGA | ||||||
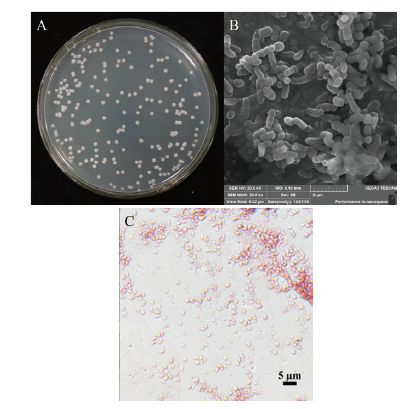
图 2 菌株7-2H的菌落形态特征 A:菌落形态图;B:扫描电镜图;C:革兰氏染色图
Fig. 2 Colony morphological characteristics of strain 7-2H A: Colony morphology. B: Scanning electron microscopic morphology. C: Gram staining
| 试验名称 Test name | 结果 Result | 试验名称 Test name | 结果 Result | |
|---|---|---|---|---|
| 淀粉水解Starch hydrolysis | - | 葡萄糖 Glucose | + | |
| 纤维素降解 Cellulose degradation | - | 乳糖 Lactose | + | |
| 蛋白降解 Protein degradation | - | 甘露醇 Mannitol | + | |
| 几丁质降解 Chitin degradation | - | 接触酶 Catalase | + | |
| 硝酸盐还原 Nitrate reduction | + | 甲基红 Methyl red | - |
表2 7-2H 的各项生理生化测定
Table 2 Physiological and biochemical determination of 7-2H
| 试验名称 Test name | 结果 Result | 试验名称 Test name | 结果 Result | |
|---|---|---|---|---|
| 淀粉水解Starch hydrolysis | - | 葡萄糖 Glucose | + | |
| 纤维素降解 Cellulose degradation | - | 乳糖 Lactose | + | |
| 蛋白降解 Protein degradation | - | 甘露醇 Mannitol | + | |
| 几丁质降解 Chitin degradation | - | 接触酶 Catalase | + | |
| 硝酸盐还原 Nitrate reduction | + | 甲基红 Methyl red | - |

图4 菌株7-2H基于特异性引物的内生验证 M:2 000 bp maker;1:真菌内生细菌7-2H;2:真菌E. sorghinum
Fig. 4 Endogenous verification of strain 7-2H based on specific primers M: 2 000 bp maker. 1: Endohyphal bacteria 7-2H. 2: E. sorghinum
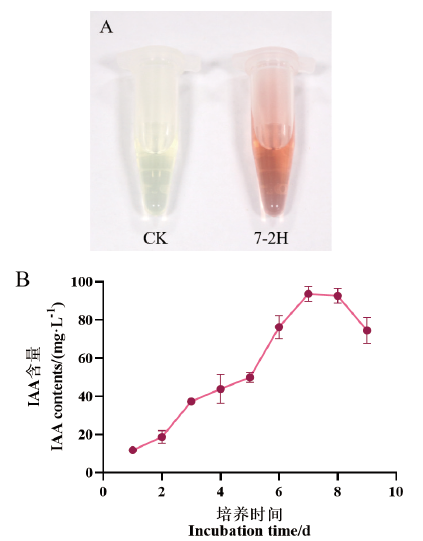
图5 菌株7-2H的产IAA能力测定 A:菌株7-2H产IAA能力的定性检测;B:菌株7-2H在不同培养时间下IAA含量
Fig. 5 Determination of IAA-producing ability of strain 7-2H A: Qualitative detection of IAA-producing ability of strain 7-2H. B: IAA contents by strain 7-2H at different culture time
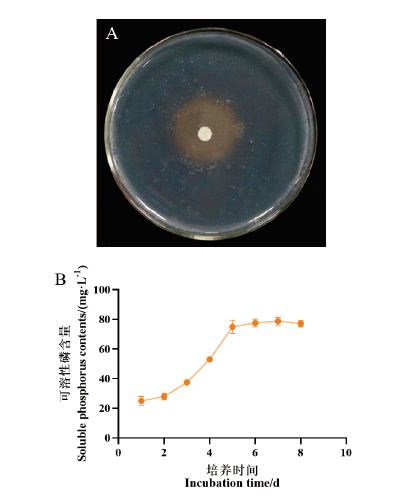
图6 菌株7-2H的产铁载体能力测定 A:菌株7-2H在CAS检测平板上生长5 d的效果;B:菌株7-2H在不同培养时间下铁载体相对含量
Fig. 6 Determination of siderophore-producing ability of strain 7-2H A: Effect of strain 7-2H growing on CAS detection plate for 5 d. B: Siderophores relative contents by strain 7-2H at different culture time
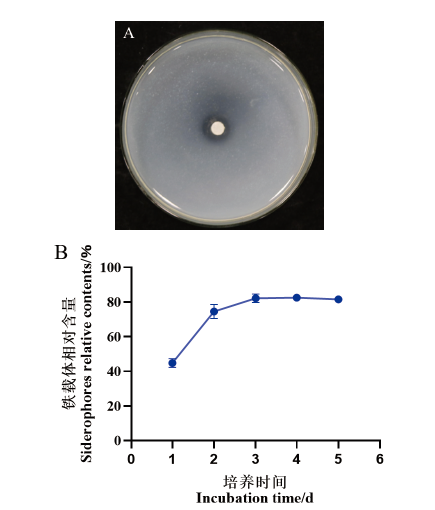
图7 菌株7-2H的溶磷能力测定 A:菌株7-2H在NBRIP无机磷平板上生长3 d的效果;B:菌株7-2H在不同培养时间下可溶性磷含量
Fig. 7 Determination of phosphate-solubilizing ability of strain 7-2H A: Effect of strain 7-2H on NBRIP inorganic phosphorus plate for 3 d. B: Soluble phosphorus contents by strain 7-2H at different culture time

图8 菌株7-2H对水稻种子幼苗生长指标的影响 A:茎长;B:根长;C:鲜重;D:干重;E:生长情况。****:极显著性差异(P<0.01);*:显著性差异(P<0.05)
Fig. 8 Effects of strain 7-2H on the growth indexes of rice seed seedlings A: Shoot length. B: Root length. C: Fresh weight. D: Dry weight. E: Growth situation. ****: Extremely significant difference(P<0.01); *: significant difference(P<0.05)
| 数据库 Database | 基因数量 Gene number | 基因注释比例 Gene annotation ratio/% |
|---|---|---|
| Uniprot | 3 081 | 58.12 |
| Pfam | 4 501 | 84.91 |
| Refseq | 5 087 | 95.96 |
| Nr | 3 014 | 56.86 |
| Tigrfam | 5 127 | 96.72 |
| GO | 2 961 | 55.86 |
| KEGG | 2 663 | 50.24 |
| COG | 2 107 | 39.75 |
表3 菌株7-2H基因功能注释数据库分布情况
Table 3 Database distribution of gene functional annotation from the strain 7-2H
| 数据库 Database | 基因数量 Gene number | 基因注释比例 Gene annotation ratio/% |
|---|---|---|
| Uniprot | 3 081 | 58.12 |
| Pfam | 4 501 | 84.91 |
| Refseq | 5 087 | 95.96 |
| Nr | 3 014 | 56.86 |
| Tigrfam | 5 127 | 96.72 |
| GO | 2 961 | 55.86 |
| KEGG | 2 663 | 50.24 |
| COG | 2 107 | 39.75 |
| [1] | 国家环境保护总局. 中国珍稀濒危保护植物名录[M]. 北京: 科学出版社, 1987: 23-28. |
| State Environmental Protection Administration. List of rare and endangered plants in China[M]. Beijing: Science Press, 1987: 23-28. | |
| [2] | 高越, 郭顺星, 邢晓科. 兰科植物种子共生萌发真菌多样性及共生萌发机制研究进展[J]. 菌物学报, 2019, 38(11): 1808-1825. |
| Gao Y, Guo SX, Xing XK. Fungal diversity and mechanisms of symbiotic germination of orchid seeds: a review[J]. Mycosystema, 2019, 38(11): 1808-1825. | |
| [3] | 裴东方, 吴秋秋, 莫俊鑫, 等. 盘龙参内生真菌多样性及其拮抗作用研究[J]. 江西农业大学学报, 2019, 41(2): 365-371. |
| Pei DF, Wu QQ, Mo JX, et al. Diversity of endophytic fungi from Spiranthes sinensis(pers.) Ames and their antifungal activity[J]. Acta Agric Univ Jiangxiensis, 2019, 41(2): 365-371. | |
| [4] |
Braga RM, Padilla G, Araújo WL. The biotechnological potential of Epicoccum spp.: diversity of secondary metabolites[J]. Crit Rev Microbiol, 2018, 44(6): 759-778.
doi: 10.1080/1040841X.2018.1514364 URL |
| [5] |
Koné Y, Alves E, Silveira PR, et al. Control of blast disease caused by Pyricularia oryzae with Epicoccum nigrum and microscopic studies of their interaction with rice plants under greenhouse conditions[J]. Biol Control, 2022, 167: 104840.
doi: 10.1016/j.biocontrol.2022.104840 URL |
| [6] |
Jin ZX, Li DD, Liu TY, et al. Cultural endophytic fungi associated with Dendrobium officinale: identification, diversity estimation and their antimicrobial potential[J]. Curr Sci, 2017, 112(8): 1690.
doi: 10.18520/cs/v112/i08/1690-1697 URL |
| [7] |
Ali Aslani M, Harighi B, Abdollahzadeh J. Screening of endofungal bacteria isolated from wild growing mushrooms as potential biological control agents against brown blotch and internal stipe necrosis diseases of Agaricus bisporus[J]. Biol Control, 2018, 119: 20-26.
doi: 10.1016/j.biocontrol.2018.01.006 URL |
| [8] |
Desirò A, Faccio A, Kaech A, et al. Endogone, one of the oldest plant-associated fungi, host unique Mollicutes-related endobacteria[J]. New Phytol, 2015, 205(4): 1464-1472.
doi: 10.1111/nph.13136 pmid: 25345989 |
| [9] |
Naumann M, Schüssler A, Bonfante P. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes[J]. ISME J, 2010, 4(7): 862-871.
doi: 10.1038/ismej.2010.21 pmid: 20237515 |
| [10] | Pakvaz S, Soltani J. Endohyphal bacteria from fungal endophytes of the Mediterranean cypress(Cupressus sempervirens)exhibit in vitro bioactivity[J]. For Pathol, 2016, 46(6): 569-581. |
| [11] |
Shaffer JP, Zalamea PC, Sarmiento C, et al. Context-dependent and variable effects of endohyphal bacteria on interactions between fungi and seeds[J]. Fungal Ecol, 2018, 36: 117-127.
doi: 10.1016/j.funeco.2018.08.008 URL |
| [12] | 刘泽, 孙翔, 刘晓玲, 等. 真菌内共生细菌研究进展[J]. 菌物学报, 2019, 38(10): 1581-1599. |
| Liu Z, Sun X, Liu XL, et al. Research progress on endofungal bacteria[J]. Mycosystema, 2019, 38(10): 1581-1599. | |
| [13] |
Ruiz-Herrera J, León-Ramírez C, Vera-Nuñez A, et al. A novel intracellular nitrogen-fixing symbiosis made by Ustilago maydis and Bacillus spp[J]. New Phytol, 2015, 207(3): 769-777.
doi: 10.1111/nph.13359 pmid: 25754368 |
| [14] |
Almendras K, García J, Carú M, et al. Nitrogen-fixing bacteria associated with Peltigera cyanolichens and Cladonia chloroli-chens[J]. Molecules, 2018, 23(12): 3077.
doi: 10.3390/molecules23123077 URL |
| [15] |
Shaffer JP, U'Ren JM, Gallery RE, et al. An endohyphal bacterium(Chitinophaga, bacteroidetes)alters carbon source use by Fusarium Keratoplasticum(F. Solani species complex, Nectriaceae)[J]. Front Microbiol, 2017, 8: 350.
doi: 10.3389/fmicb.2017.00350 pmid: 28382021 |
| [16] |
Lackner G, Hertweck C. Impact of endofungal bacteria on infection biology, food safety, and drug development[J]. PLoS Pathog, 2011, 7(6): e1002096.
doi: 10.1371/journal.ppat.1002096 URL |
| [17] |
Lumini E, Bianciotto V, Jargeat P, et al. Presymbiotic growth and sporal morphology are affected in the arbuscular mycorrhizal fungus Gigaspora margarita cured of its endobacteria[J]. Cell Microbiol, 2007, 9(7): 1716-1729.
pmid: 17331157 |
| [18] |
Hoffman MT, Gunatilaka MK, Wijeratne K, et al. Endohyphal bacterium enhances production of indole-3-acetic acid by a foliar fungal endophyte[J]. PLoS One, 2013, 8(9): e73132.
doi: 10.1371/journal.pone.0073132 URL |
| [19] |
Cheng S, Jiang JW, Tan LT, et al. Plant growth-promoting ability of mycorrhizal Fusarium strain KB-3 enhanced by its IAA producing endohyphal bacterium, Klebsiella aerogenes[J]. Front Microbiol, 2022, 13: 855399.
doi: 10.3389/fmicb.2022.855399 URL |
| [20] |
Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production[J]. Nature, 2005, 437(7060): 884-888.
doi: 10.1038/nature03997 |
| [21] |
Jiang JW, Zhang K, Cheng S, et al. Fusarium oxysporum KB-3 from Bletilla striata: an orchid mycorrhizal fungus[J]. Mycorrhiza, 2019, 29(5): 531-540.
doi: 10.1007/s00572-019-00904-3 |
| [22] | 裴东方. 盘龙参内生菌多样性及一株内生细菌的抑菌活性研究[D]. 荆州: 长江大学, 2020. |
| Pei DF. Diversity of endophytes from Spiranthes sinensis and the antifungal activity of one endophytic bacterium[D]. Jingzhou: Yangtze University, 2020. | |
| [23] | 王亚军, 冯炬威, 李雅倩, 等. 高产铁载体菌Burkholderia vietnamiensis YQ9促生特性研究及其对重金属胁迫条件下种子萌发的影响[J]. 环境科学学报, 2022, 42(2): 430-437. |
| Wang YJ, Feng JW, Li YQ, et al. Studies on growth-promoting properties of an efficient siderophore producing bacterium, Burkholderia vietnamiensis YQ9, and its effects on seed germination under heavy metal stress[J]. Acta Sci Circumstantiae, 2022, 42(2): 430-437. | |
| [24] |
Hoffman MT, Arnold AE. Diverse bacteria inhabit living hyphae of phylogenetically diverse fungal endophytes[J]. Appl Environ Microbiol, 2010, 76(12): 4063-4075.
doi: 10.1128/AEM.02928-09 URL |
| [25] | 谭利涛, 成胜, 苏杭, 等. 真菌内生蜡样芽孢杆菌7-1Y的分离鉴定及其功能探索[J]. 江西农业大学学报, 2022, 44(4): 900-909. |
| Tan LT, Cheng S, Su H, et al. Isolation, identification and functional exploration of endohyphal Bacillus cereus 7-1Y[J]. Acta Agric Univ Jiangxiensis, 2022, 44(4): 900-909. | |
| [26] | 谢家仪, 董光军, 刘振英. 扫描电镜的微生物样品制备方法[J]. 电子显微学报, 2005, 24(4): 440. |
| Xie JY, Dong GJ, Liu ZY. Preparation method of microbial samples by scanning electron microscope[J]. J Chin Electron Microsc Soc, 2005, 24(4): 440. | |
| [27] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册[M]. 北京: 科学出版社, 2001: 62-65. |
| Dong XZ, Cai MY. Handbook of identification of common bacterial systems[M]. Beijing: Science Press, 2001: 62-65. | |
| [28] |
He FL, Nair GR, Soto CS, et al. Molecular basis of ChvE function in sugar binding, sugar utilization, and virulence in Agrobacterium tumefaciens[J]. J Bacteriol, 2009, 191(18): 5802-5813.
doi: 10.1128/JB.00451-09 URL |
| [29] | 罗兴, 邹兰, 吴清山, 等. 乌头产吲哚乙酸内生细菌遗传多样性、抗逆性及其对水稻幼苗生长的影响[J]. 微生物学报, 2022, 62(4): 1485-1500. |
| Luo X, Zou L, Wu QS, et al. Genetic diversity, stress resistance, and effect on rice seedling growth of indoleacetic acid-producing endophytic bacteria isolated from Aconitum carmichaelii Debeaux[J]. Acta Microbiol Sin, 2022, 62(4): 1485-1500. | |
| [30] | 杨常娥, 鲁艳莉, 倪捍成, 等. 创伤弧菌产铁载体菌株的筛选及其诱导条件的响应面优化[J]. 食品工业科技, 2017, 38(3): 159-165. |
| Yang CE, Lu YL, Ni HC, et al. Screening of siderophore-producing Vibrio vulnificus and optimization of induction conditions using response surface methodology[J]. Sci Technol Food Ind, 2017, 38(3): 159-165. | |
| [31] |
钱婷, 叶建仁. 巨大芽孢杆菌ZS-3溶无机磷机制及其对樟树的促生作用[J]. 生物技术通报, 2020, 36(8): 45-52.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0114 |
| Qian T, Ye JR. The mechanism of dissolving inorganic phosphorus by Bacillus megaterium ZS-3 and its growth promotion of Cinnamomum camphora[J]. Biotechnol Bull, 2020, 36(8): 45-52. | |
| [32] | Gholamalizadeh R, Khodakaramian G, Ebadi AA. Assessment of rice associated bacterial ability to enhance rice seed germination and rice growth promotion[J]. Braz Arch Biol Technol, 2017, 60: e17160410. |
| [33] |
姚延轩, 接伟光, 杜燕, 等. 根瘤菌的分类、鉴定及应用技术研究现状[J]. 中国农学通报, 2020, 36(15): 100-105.
doi: 10.11924/j.issn.1000-6850.casb19010151 |
| Yao YX, Jie WG, Du Y, et al. Taxonomy, identification and application of Rhizobium[J]. Chin Agric Sci Bull, 2020, 36(15): 100-105. | |
| [34] | Saghafi D, Ghorbanpour M, Lajayer BA. Efficiency of Rhizobium strains as plant growth promoting rhizobacteria on morpho-physiological properties of Brassica napus L. under salinity stress[J]. J Soil Sci Plant Nutr, 2018(ahead). |
| [35] |
Zhao JJ, Zhao X, Wang JR, et al. Isolation, identification and characterization of endophytic bacterium Rhizobium oryzihabitans sp. nov., from rice root with biotechnological potential in agriculture[J]. Microorganisms, 2020, 8(4): 608.
doi: 10.3390/microorganisms8040608 URL |
| [36] |
Agnolucci M, Battini F, Cristani C, et al. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates[J]. Biol Fertil Soils, 2015, 51(3): 379-389.
doi: 10.1007/s00374-014-0989-5 URL |
| [37] | Shaffer JP, Sarmiento C, Zalamea PC, et al. Diversity, specificity, and phylogenetic relationships of endohyphal bacteria in fungi that inhabit tropical seeds and leaves[J]. Front Ecol Evol, 2016, 4: 116. |
| [38] |
Sharma M, Schmid M, Rothballer M, et al. Detection and identification of bacteria intimately associated with fungi of the order Sebacinales[J]. Cell Microbiol, 2008, 10(11): 2235-2246.
doi: 10.1111/j.1462-5822.2008.01202.x pmid: 18637023 |
| [39] | Guo HJ, Glaeser SP, Alabid I, et al. The abundance of endofungal bacterium Rhizobium radiobacter(syn. Agrobacterium tumefaciens)increases in its fungal host piriformospora indica during the tripartite sebacinalean symbiosis with higher plants[J]. Front Microbiol, 2017, 8: 629. |
| [40] | Swarnalakshmi K, Yadav V, Tyagi D, et al. Significance of plant growth promoting rhizobacteria in grain legumes: growth promotion and crop production[J]. Plants: Basel, 2020, 9(11): E1596. |
| [41] |
Saghafi D, Ghorbanpour M, Ajirloo HS, et al. Enhancement of growth and salt tolerance in Brassica napus L. seedlings by halotolerant Rhizobium strains containing ACC-deaminase activity[J]. Plant Physiol Rep, 2019, 24(2): 225-235.
doi: 10.1007/s40502-019-00444-0 |
| [42] |
Chaudhary T, Gera R, Shukla P. Deciphering the potential of Rhizobium pusense MB-17a, a plant growth-promoting root endophyte, and functional annotation of the genes involved in the metabolic pathway[J]. Front Bioeng Biotechnol, 2021, 8: 617034.
doi: 10.3389/fbioe.2020.617034 URL |
| [43] |
Ghignone S, Salvioli A, Anca I, et al. The genome of the obligate endobacterium of an AM fungus reveals an interphylum network of nutritional interactions[J]. ISME J, 2012, 6(1): 136-145.
doi: 10.1038/ismej.2011.110 pmid: 21866182 |
| [44] |
Li Q, Li XL, Chen C, et al. Analysis of bacterial diversity and communities associated with Tricholoma matsutake fruiting bodies by barcoded pyrosequencing in Sichuan Province, southwest China[J]. J Microbiol Biotechnol, 2016, 26(1): 89-98.
doi: 10.4014/jmb.1505.05008 URL |
| [1] | 王腾辉, 葛雯冬, 罗雅方, 范震宇, 王玉书. 基于极端混合池(BSA)全基因组重测序的羽衣甘蓝白色叶基因定位[J]. 生物技术通报, 2023, 39(9): 176-182. |
| [2] | 郭少华, 毛会丽, 刘征权, 付美媛, 赵平原, 马文博, 李旭东, 关建义. 一株鱼源致病性嗜水气单胞菌XDMG的全基因组测序及比较基因组分析[J]. 生物技术通报, 2023, 39(8): 291-306. |
| [3] | 张志霞, 李天培, 曾虹, 朱稀贤, 杨天雄, 马斯楠, 黄磊. 冰冷杆菌PG-2的基因组测序及生物信息学分析[J]. 生物技术通报, 2023, 39(3): 290-300. |
| [4] | 易希, 廖红东, 郑井元. 植物内生真菌防治根结线虫研究进展[J]. 生物技术通报, 2023, 39(3): 43-51. |
| [5] | 和梦颖, 刘文彬, 林震鸣, 黎尔彤, 汪洁, 金小宝. 一株抗革兰阳性菌的戈登氏菌WA4-43全基因组测序与分析[J]. 生物技术通报, 2023, 39(2): 232-242. |
| [6] | 张傲洁, 李青云, 宋文红, 颜少慧, 唐爱星, 刘幽燕. 基于苯酚降解的粪产碱杆菌Alcaligenes faecalis JF101的全基因组分析[J]. 生物技术通报, 2023, 39(10): 292-303. |
| [7] | 王帅, 吕鸿睿, 张昊, 吴占文, 肖翠红, 孙冬梅. 解磷菌PSB-R全基因组测序鉴定及其解磷特性分析[J]. 生物技术通报, 2023, 39(1): 274-283. |
| [8] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| [9] | 高小宁, 刘睿, 吴自林, 吴嘉云. 宿根矮化病抗感甘蔗品种茎部内生真菌和细菌群落特征分析[J]. 生物技术通报, 2022, 38(6): 166-173. |
| [10] | 张泽颖, 范清锋, 邓云峰, 韦廷舟, 周正富, 周建, 王劲, 江世杰. 一株高产脂肪酶菌株WCO-9全基因组测序及比较基因组分析[J]. 生物技术通报, 2022, 38(10): 216-225. |
| [11] | 唐嘉城, 梁毅珉, 马葭思, 彭桂香, 谭志远. 百香果内生细菌多样性及促生长特性[J]. 生物技术通报, 2022, 38(1): 86-97. |
| [12] | 薛清, 杜虹锐, 薛会英, 王译浩, 王暄, 李红梅. 苜蓿滑刃线虫线粒体基因组及其系统发育研究[J]. 生物技术通报, 2021, 37(7): 98-106. |
| [13] | 张秫华, 方千, 贾红梅, 韩桂琪, 严铸云, 何冬梅. 川芎非根际、根际及根茎内生真菌群落差异分析[J]. 生物技术通报, 2021, 37(4): 56-69. |
| [14] | 陈体强, 徐晓兰, 石林春, 钟礼义. 紫芝栽培品种‘武芝2号’(‘紫芝S2’)全基因组测序及分析[J]. 生物技术通报, 2021, 37(11): 42-56. |
| [15] | 李娥, 黄勇, 孟园园, 李璇, 杜光辉, 刘飞虎. 盐胁迫条件下‘巴麻火麻’内生真菌的分离鉴定与多样性分析[J]. 生物技术通报, 2021, 37(10): 26-33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||