








生物技术通报 ›› 2023, Vol. 39 ›› Issue (7): 307-315.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1429
施炜涛1,2( ), 姚春鹏3, 魏文康2, 王蕾2, 房元杰2, 仝钰洁1, 马晓姣1, 蒋文1, 张晓爱2(
), 姚春鹏3, 魏文康2, 王蕾2, 房元杰2, 仝钰洁1, 马晓姣1, 蒋文1, 张晓爱2( ), 邵伟1(
), 邵伟1( )
)
收稿日期:2022-11-18
出版日期:2023-07-26
发布日期:2023-08-17
通讯作者:
张晓爱,女,博士,副研究员,硕士生导师,研究方向:微生物;E-mail: zhangxiaoai@gdaas.cn;作者简介:施炜涛,男,硕士研究生,研究方向:基因编辑与毒素;E-mail: shiweitao19980303@163.com
基金资助:
SHI Wei-tao1,2( ), YAO Chun-peng3, WEI Wen-Kang2, WANG Lei2, FANG Yuan-jie2, TONG Yu-jie1, MA Xiao-jiao1, JIANG Wen1, ZHANG Xiao-ai2(
), YAO Chun-peng3, WEI Wen-Kang2, WANG Lei2, FANG Yuan-jie2, TONG Yu-jie1, MA Xiao-jiao1, JIANG Wen1, ZHANG Xiao-ai2( ), SHAO Wei1(
), SHAO Wei1( )
)
Received:2022-11-18
Published:2023-07-26
Online:2023-08-17
摘要:
试验旨在利用CRISPR/Cas9基因编辑技术构建稳定敲除苹果酸脱氢酶2(malate dehydrogenase 2,MDH2)基因的IPEC-J2单克隆细胞株,并研究敲除MDH2基因后抗呕吐毒素效应。针对MDH2基因序列设计sgRNA插入PX459载体中,构建PX459-sgRNA-MDH2重组质粒;将质粒通过电转导入IPEC-J2细胞中,并加入嘌呤霉素筛选阳性细胞;利用有限稀释法筛选单克隆细胞并进行基因型测序鉴定、荧光定量PCR和蛋白质印迹法验证,获得MDH2基因敲除的单克隆细胞株;最后通过CCK8试剂盒和细胞凋亡与坏死检测试剂盒检测细胞存活情况,测定MDH2敲除细胞株对呕吐毒素的抗性。测序结果显示MDH2基因敲除载体构建成功。利用荧光定量PCR及蛋白免疫印迹法验证了获得的细胞系为MDH2基因敲除的单克隆细胞株。CCK8细胞活力实验结果显示,与野生型细胞相比,敲除MDH2可使IPEC-J2细胞在不同浓度呕吐毒素(4、2、1和0.5 μg/mL)处理5 d时细胞活力分别极显著提高18.67%、19.59%、26.36%和27.01%。流式细胞仪实验结果显示,与野生型细胞相比,敲除MDH2使不同浓度呕吐毒素处理5 d的细胞死亡率分别极显著降低30.33%、15.81%、16.00%和14.70%。利用CRISPR/Cas9系统对IPEC-J2细胞中的MDH2基因进行敲除并筛选获得了MDH2-KO单克隆细胞株,并通过细胞活力和细胞死亡检测,证明该细胞株具有抗呕吐毒素毒性效应的效果,为揭示呕吐毒素诱导宿主细胞死亡的毒性机制提供借鉴,为防治呕吐毒素提供新策略。
施炜涛, 姚春鹏, 魏文康, 王蕾, 房元杰, 仝钰洁, 马晓姣, 蒋文, 张晓爱, 邵伟. 利用CRISPR/Cas9技术构建MDH2敲除细胞株及抗呕吐毒素效应研究[J]. 生物技术通报, 2023, 39(7): 307-315.
SHI Wei-tao, YAO Chun-peng, WEI Wen-Kang, WANG Lei, FANG Yuan-jie, TONG Yu-jie, MA Xiao-jiao, JIANG Wen, ZHANG Xiao-ai, SHAO Wei. Establishment of MDH2 Knockout Cell Line Using CRISPR/Cas9 Technology and Study of Anti-deoxynivalenol Effect[J]. Biotechnology Bulletin, 2023, 39(7): 307-315.
| 引物Primer | 序列 Sequence(5' -3') |
|---|---|
| sgRNA-MDH2-F | CACCGCCCGTCATTGGCGGCCACGC |
| sgRNA-MDH2-R | AAACGCGTGGCCGCCAATGACGGGC |
表1 MDH2靶向位点及sgRNA寡核苷酸序列
Table 1 MDH2 targeting sites and sgRNA oligonucleotide sequences
| 引物Primer | 序列 Sequence(5' -3') |
|---|---|
| sgRNA-MDH2-F | CACCGCCCGTCATTGGCGGCCACGC |
| sgRNA-MDH2-R | AAACGCGTGGCCGCCAATGACGGGC |
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| PCR-F | CCGTCTTCAGTCCGTCGTG |
| PCR-R | TAACCAGCGGTGCTCCAGTG |
表2 MDH2基因PCR测序引物
Table 2 Primers for PCR sequencing of MDH2 gene
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| PCR-F | CCGTCTTCAGTCCGTCGTG |
| PCR-R | TAACCAGCGGTGCTCCAGTG |
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| RT-qPCR-F | GGCCACGCCGGGAAG |
| RT-qPCR-R | GATGCCCTTTTTCCCCAGCA |
表3 MDH2基因RT-qPCR引物
Table 3 RT-qPCR primers for MDH2 gene
| 引物Primer | 序列Sequence(5'-3') |
|---|---|
| RT-qPCR-F | GGCCACGCCGGGAAG |
| RT-qPCR-R | GATGCCCTTTTTCCCCAGCA |
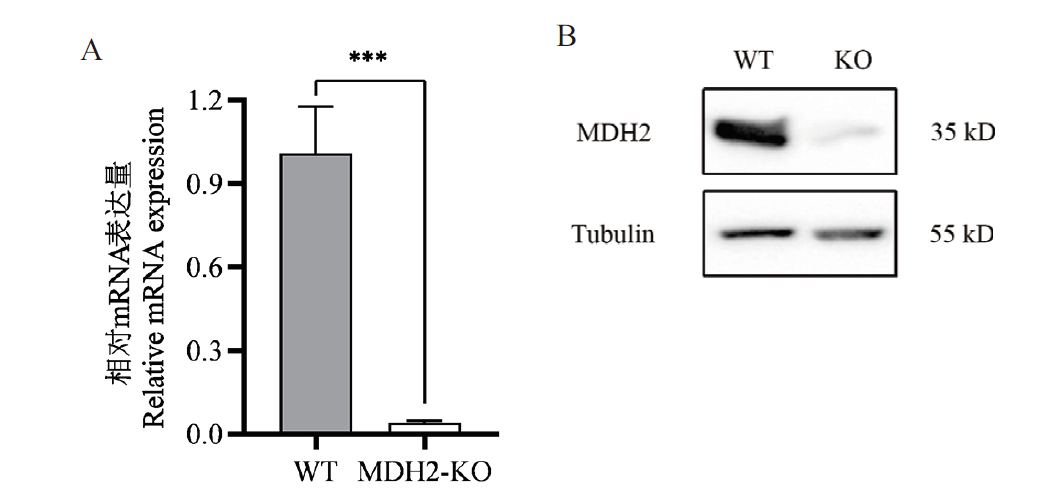
图5 MDH2-KO基因的表达检测 A: RT-qPCR检测MDH2的表达水平。B: Western blot鉴定MDH2蛋白的表达水平
Fig. 5 Expression assay of MDH2-KO gene A: RT-qPCR to detect the expression of MDH2. B: Western blot to identify the expressionof MDH2 protein
| [1] |
Zhao YJ, Guan XL, Zong Y, et al. Deoxynivalenol in wheat from the Northwestern region in China[J]. Food Addit Contam Part B Surveill, 2018, 11(4): 281-285.
doi: 10.1080/19393210.2018.1503340 URL |
| [2] |
Sobrova P, Adam V, Vasatkova A, et al. Deoxynivalenol and its toxicity[J]. Interdiscip Toxicol, 2010, 3(3): 94-99.
doi: 10.2478/v10102-010-0019-x pmid: 21217881 |
| [3] | 关蕊. 生猪养殖中霉菌毒素的危害及预防[J]. 现代畜牧科技, 2022(1): 75-76. |
| Guan R. Harm and prevention of mycotoxin in pig breeding[J]. Mod Animal Husb Sci & Technol, 2022(1): 75-76. | |
| [4] | 王庆伟, 安纲, 王金勇, 等. 2019年中国饲料与原料霉菌毒素检测报告[J]. 饲料工业, 2020, 41(24): 52-57. |
| Wang QW, An G, Wang JY, et al. China mycotoxin survey of feed and raw material in 2019[J]. Feed Ind, 2020, 41(24): 52-57. | |
| [5] | 韩业东, 李延山, 刘再胜, 等. 2020年饲料和饲料原料中霉菌毒素监测分析报告[J]. 新农业, 2021(13): 48-50. |
| Han YD, Li YS, Liu ZS, et al. Report on monitoring and analysis of mycotoxins in feed and feed raw materials in 2020[J]. Mod Agric, 2021(13): 48-50. | |
| [6] | 陈丽媛. 2018年1-6月饲料及原料霉菌毒素分析报告[J]. 国外畜牧学: 猪与禽, 2018, 38(8): 70-72. |
| Chen LY. Analysis report of mycotoxin in feed and raw materials from January to June, 2018[J]. Animal Sci Abroad Pigs Poult, 2018, 38(8): 70-72. | |
| [7] | 朱海华, 张梦雪, 胡骁飞, 等. 食品中呕吐毒素检测方法的研究进展[J]. 食品科技, 2021, 46(11): 314-320. |
| Zhu HH, Zhang MX, Hu XF, et al. Research progress on the detection method of vomitoxin in food[J]. Food Sci Technol, 2021, 46(11): 314-320. | |
| [8] | 计成, 赵丽红, 李笑樱, 等. 呕吐毒素生物降解研究进展[J]. 饲料工业, 2015, 36(10): 1-5. |
| Ji C, Zhao LH, Li XY, et al. Research advance of deoxynivalenol biodegradation[J]. Feed Ind, 2015, 36(10): 1-5. | |
| [9] |
Pestka JJ. Deoxynivalenol: Toxicity, mechanisms and animal health risks[J]. Animal Feed Sci Technol, 2007, 137(3/4): 283-298.
doi: 10.1016/j.anifeedsci.2007.06.006 URL |
| [10] |
Pierron A, Alassane-Kpembi I, Oswald IP. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health[J]. Porc Health Manag, 2016, 2(1): 21.
doi: 10.1186/s40813-016-0041-2 URL |
| [11] |
Ghareeb K, Awad WA, Böhm J, et al. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine[J]. J Appl Toxicol, 2015, 35(4): 327-337.
doi: 10.1002/jat.3083 pmid: 25352520 |
| [12] | Pedrosa K. Synergistic effect of mycotoxin contaminated feed[J]. International Pig Topics, 2010, 25: 7-9. |
| [13] |
Raymond SL, Smith TK, Swamy HV. Effects of feeding a blend of grains naturally contaminated with Fusarium mycotoxins on feed intake, serum chemistry, and hematology of horses, and the efficacy of a polymeric glucomannan mycotoxin adsorbent[J]. J Anim Sci, 2003, 81(9): 2123-2130.
pmid: 12968685 |
| [14] |
Smith TK, McMillan EG, Castillo JB. Effect of feeding blends of Fusarium mycotoxin-contaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine[J]. J Anim Sci, 1997, 75(8): 2184-2191.
pmid: 9263067 |
| [15] |
Malovrh T, Jakovac-Strajn B. Feed contaminated with Fusarium toxins alter lymphocyte proliferation and apoptosis in primiparous sows during the perinatal period[J]. Food Chem Toxicol, 2010, 48(10): 2907-2912.
doi: 10.1016/j.fct.2010.07.026 pmid: 20654678 |
| [16] | 王海飞, 渠欢, 吴圣龙, 等. 利用CRISPR/cas9敲除文库筛选呕吐毒素抗性功能基因[C]// 创新、融合、健康、未来—第九届全国畜牧兽医青年科技工作者学术研讨会论文集. 重庆: 2020: 34. |
| Wang HF Qu H, Wu SL, et al. Screening functional genes for vomitoxin resistance using CRISPR/Cas9 knockout libraries[C]. // Chinese Society of Animal Husbandry and Veterinary Medicine. Innovation, integration, health and future - Proceedings of the Ninth National Symposium for Young Veterinary Scientists and Technicians in Animal Husbandry. Chongqing: 2020: 34. | |
| [17] | 许写, 石东风, 吴嘉韵, 等. 猪METTL3基因表达水平与DON诱导IPEC-J2细胞损伤的关系[J]. 畜牧兽医学报, 2022, 53(3): 688-699. |
| Xu X, Shi DF, Wu JY, et al. Relationship between expression level of Porcine METTL3 gene and the injury of IPEC-J2 cells induced by DON[J]. Acta Vet Zootechnica Sin, 2022, 53(3): 688-699. | |
| [18] |
Xu YF, Chen XL, Yu LC, et al. SLC4A11 and MFSD3 gene expression changes in deoxynivalenol treated IPEC-J2 cells[J]. Front Genet, 2021, 12: 697883.
doi: 10.3389/fgene.2021.697883 URL |
| [19] |
Musrati RA, Kollárová M, Mernik N, et al. Malate dehydrogenase: distribution, function and properties[J]. Gen Physiol Biophys, 1998, 17(3): 193-210.
pmid: 9834842 |
| [20] |
Zhuang Y, Xiang JD, Bao W, et al. MDH2 stimulated by estrogen-GPR30 pathway down-regulated PTEN expression promoting the proliferation and invasion of cells in endometrial cancer[J]. Transl Oncol, 2017, 10(2): 203-210.
doi: S1936-5233(16)30270-4 pmid: 28189066 |
| [21] |
Xu F, Hua Q, Zhang AM, et al. LncRNA AC020978 facilitates non-small cell lung cancer progression by interacting with malate dehydrogenase 2 and activating the AKT pathway[J]. Cancer Sci, 2021, 112(11): 4501-4514.
doi: 10.1111/cas.v112.11 URL |
| [22] |
Liu Q, Harvey CT, Geng H, et al. Malate dehydrogenase 2 confers docetaxel resistance via regulations of JNK signaling and oxidative metabolism[J]. Prostate, 2013, 73(10): 1028-1037.
doi: 10.1002/pros.22650 pmid: 23389923 |
| [23] |
Tomlinson IPM, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer[J]. Nat Genet, 2002, 30(4): 406-410.
doi: 10.1038/ng849 pmid: 11865300 |
| [24] |
Wang S, Wu KT, Xue DF, et al. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: insights from mitochondrial dysfunction[J]. Food Chem Toxicol, 2021, 153: 112214.
doi: 10.1016/j.fct.2021.112214 pmid: 33930483 |
| [25] |
朱雷, 曹利, 申茂玉, 等. 脱氧雪腐镰刀菌烯醇诱导仔猪海马神经细胞凋亡的线粒体通路研究[J]. 动物营养学报, 2019, 31(10): 4675-4683.
doi: 10.3969/j.issn.1006-267x.2019.10.032 |
| Zhu L, Cao L, Shen MY, et al. Deoxynivalenol induces apoptosis in piglet hippocampal nerve cells via mitochondrial pathway[J]. Chin J Animal Nutr, 2019, 31(10): 4675-4683. | |
| [26] |
Kang RF, Li RN, Dai PY, et al. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production[J]. Environ Pollut, 2019, 251: 689-698.
doi: S0269-7491(19)30073-9 pmid: 31108302 |
| [27] |
Li DT, Ma HR, Ye YQ, et al. Deoxynivalenol induces apoptosis in mouse thymic epithelial cells through mitochondria-mediated pathway[J]. Environ Toxicol Pharmacol, 2014, 38(1): 163-171.
doi: 10.1016/j.etap.2014.05.015 URL |
| [28] |
Li DT, Ye YQ, Lin SQ, et al. Evaluation of deoxynivalenol-induced toxic effects on DF-1 cells in vitro: cell-cycle arrest, oxidative stress, and apoptosis[J]. Environ Toxicol Pharmacol, 2014, 37(1): 141-149.
doi: 10.1016/j.etap.2013.11.015 URL |
| [29] |
Yang J, Zhu C, Ye JL, et al. Protection of Porcine intestinal-epithelial cells from deoxynivalenol-induced damage by resveratrol via the Nrf2 signaling pathway[J]. J Agric Food Chem, 2019, 67(6): 1726-1735.
doi: 10.1021/acs.jafc.8b03662 URL |
| [30] |
Habrowska-Górczyńska DE, Kowalska K, Urbanek KA, et al. Deoxynivalenol modulates the viability, ROS production and apoptosis in prostate cancer cells[J]. Toxins, 2019, 11(5): 265.
doi: 10.3390/toxins11050265 URL |
| [31] |
Ait-El-Mkadem S, Dayem-Quere M, Gusic M, et al. Mutations in MDH2, encoding a Krebs cycle enzyme, cause early-onset severe encephalopathy[J]. Am J Hum Genet, 2017, 100(1): 151-159.
doi: S0002-9297(16)30492-X pmid: 27989324 |
| [32] |
Pei X, Li KY, Shen Y, et al. Palmitoylation of MDH2 by ZDHHC18 activates mitochondrial respiration and accelerates ovarian cancer growth[J]. Sci China Life Sci, 2022, 65(10): 2017-2030.
doi: 10.1007/s11427-021-2048-2 pmid: 35366151 |
| [33] |
何光明, 邓兴旺. 死亡信号传递: 叶绿体与线粒体间信号交流调控植物程序性细胞死亡[J]. 植物学报, 2018, 53(4): 441-444.
doi: 10.11983/CBB18087 |
| He GM, Deng XW. Death signal transduction: chloroplast-to-mitochondrion communication regulates programmed cell death in plants[J]. Chin Bull Bot, 2018, 53(4): 441-444. | |
| [34] |
Dubouchaud H, Walter L, Rigoulet M, et al. Mitochondrial NADH redox potential impacts the reactive oxygen species production of reverse Electron transfer through complex I[J]. J Bioenerg Biomembr, 2018, 50(5): 367-377.
doi: 10.1007/s10863-018-9767-7 pmid: 30136168 |
| [35] |
Sharma R, Agarwal A, Mohanty G, et al. Proteomic analysis of human spermatozoa proteins with oxidative stress[J]. Reprod Biol Endocrinol, 2013, 11: 48.
doi: 10.1186/1477-7827-11-48 |
| [36] |
Zhao YN, Luo LL, Xu JS, et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana[J]. Cell Res, 2018, 28(4): 448-461.
doi: 10.1038/s41422-018-0024-8 |
| [37] |
Wang L, Lam G, Thummel CS. Med24 and Mdh2are required for Drosophila larval salivary gland cell death[J]. Dev Dyn, 2010, 239(3): 954-964.
doi: 10.1002/dvdy.v239:3 URL |
| [38] |
Eleftheriadis T, Pissas G, Golfinopoulos S, et al. Inhibition of malate dehydrogenase-2 protects renal tubular epithelial cells from Anoxia-reoxygenation-induced death or senescence[J]. Biomolecules, 2022, 12(10): 1415.
doi: 10.3390/biom12101415 URL |
| [1] | 陈小玲, 廖东庆, 黄尚飞, 陈英, 芦志龙, 陈东. 利用CRISPR/Cas9系统改造酿酒酵母的研究进展[J]. 生物技术通报, 2023, 39(8): 148-158. |
| [2] | 陈宝强, 李莹莹, 马博雅, 肉扎古丽·马利克, 优丽图孜·乃比, 宋金迪, 刘君, 王希东. 西瓜食酸菌III型分泌效应物基因aop2功能分析[J]. 生物技术通报, 2023, 39(6): 286-297. |
| [3] | 苏雨, 李宗芸, 韩永华. 植物液泡加工酶研究进展[J]. 生物技术通报, 2021, 37(6): 181-191. |
| [4] | 李凯晴, 李莹, 王艺磊, 邹鹏飞. 受体相互作用蛋白的功能及在硬骨鱼类中的研究进展[J]. 生物技术通报, 2021, 37(5): 197-211. |
| [5] | 岳鹏鹏, 郭俊璠, 于鸿浩, 付灿, 王小燕, 高进涛. 基于CRISPR/cas9系统高效编辑小鼠Galt基因[J]. 生物技术通报, 2020, 36(8): 235-342. |
| [6] | 何士俊, 万毅虹, 章嘉雯, 蔡秀潮, 刘静文, 刘叔文, 姚新刚. CRISPR/Cas9慢病毒系统敲除胰岛β细胞PKA C-α的研究[J]. 生物技术通报, 2020, 36(3): 102-109. |
| [7] | 田文佳, 窦桂铭, 王莎, 孙靖雅, 马玉超. 利用CRISPR/Cas9系统建立内生链霉菌SAT1的基因簇敲除体系[J]. 生物技术通报, 2019, 35(6): 1-8. |
| [8] | 吉米拉木·加马力, 美合日班·阿不力米提, 古海尼沙·买买提, 艾尼瓦尔·吐米尔. 两种地衣共生藻对铜和锌的耐受性及吸附特性研究[J]. 生物技术通报, 2019, 35(6): 69-75. |
| [9] | 刘辉, 邓治, 杨洪, 代龙军, 李德军. 橡胶树HbMC2在酵母中的表达和抗逆性分析[J]. 生物技术通报, 2018, 34(9): 202-208. |
| [10] | 贾启鹏,申培磊,张欢,张勇. CRISPR/Cas9系统介导敲除CSN2基因奶山羊胎儿成纤维突变细胞的制备[J]. 生物技术通报, 2017, 33(9): 131-138. |
| [11] | 赵建平;姚祥坦;. 植物小孢子胚胎发生机理[J]. , 2009, 0(06): 7-11. |
| [12] | 李凤娜;陈晓安. 一氧化氮对线粒体生物合成的积极影响[J]. , 2005, 0(02): 57-57. |
| [13] | 李凤娜;陈晓安. 一氧化氮对线粒体生物合成的积极影响[J]. , 2005, 0(02): 57-57. |
| [14] | . 国外动态[J]. , 2000, 0(02): 41-46. |
| [15] | 王伟;林均民;. 细胞培养系中的编程性细胞死亡[J]. , 1997, 0(03): 4-6. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||