








生物技术通报 ›› 2023, Vol. 39 ›› Issue (8): 126-136.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1530
收稿日期:2022-12-12
出版日期:2023-08-26
发布日期:2023-09-05
通讯作者:
杨玉菊,女,博士,教授,研究方向:微生物学与免疫学;E-mail: sh_yangyj@hrbu.edu.cn作者简介:沙珊珊,女,博士,实验师,研究方向:微生态学;E-mail: 18045187642@163.com
基金资助:
SHA Shan-shan( ), DONG Shi-rong, YANG Yu-ju(
), DONG Shi-rong, YANG Yu-ju( )
)
Received:2022-12-12
Published:2023-08-26
Online:2023-09-05
摘要:
肠道菌群是一个稳定且复杂的微生态系统,其在长期进化过程中与宿主建立了稳定的共生关系,且微生物的活动直接影响着宿主的健康。它们不仅在宿主营养物质的消化代谢和机体发育等方面发挥重要作用,而且与宿主的免疫和疾病密切相关。肠道菌群和机体免疫系统之间的相互作用机制十分复杂,受多种环境因素的影响,至今尚未完全阐明。微生物代谢物及微生物-机体共代谢物对调控免疫功能具有重要作用,逐渐引起研究者们的重视。因此,本文在介绍肠道及其在宿主防御中的作用的基础上,就肠道微生物及肠道内代谢产物如何促进宿主免疫系统发育、调节宿主免疫反应等进行了综述。旨在为进一步研究肠道微生物及代谢物与机体免疫系统互作提供参考,同时为改善畜禽肠道健康的营养措施提供理论依据。
沙珊珊, 董世荣, 杨玉菊. 肠道菌群及代谢物调控宿主肠道免疫的研究进展[J]. 生物技术通报, 2023, 39(8): 126-136.
SHA Shan-shan, DONG Shi-rong, YANG Yu-ju. Research Progress in Gut Microbiota and Metabolites Regulating Host Intestinal Immunity[J]. Biotechnology Bulletin, 2023, 39(8): 126-136.
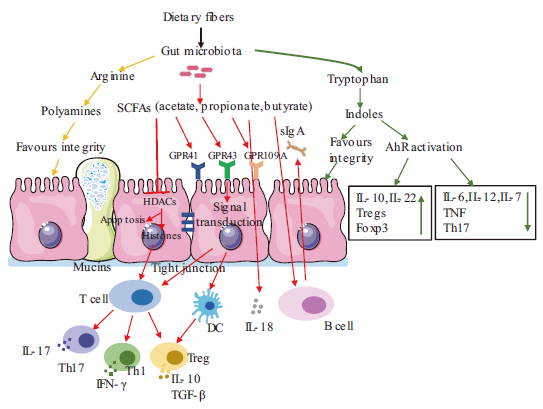
图1 肠道菌群及代谢产物对肠道免疫系统的调控作用 箭头表示促进,平头箭头表示抑制。SCFAs:短链脂肪酸;HDACs:组蛋白去乙酰化酶;IL:白细胞介素;Th:辅助性T细胞;Treg:调节性T细胞;TGF-β:转化生长因子-β;GPR:G蛋白偶联受体;sIgA:分泌型免疫球蛋白A;AhR activation:芳香烃受体激活;Foxp3:叉状头转录因子p3;TNF:肿瘤坏死因子;DC:树突状细胞;IFN-γ:γ干扰素
Fig. 1 Regulation of gut microbiota and metabolites on intestinal immune system The narrow arrows indicate promotions, flat-head arrows indicate inhibitions. SCFAs: Short chain fatty acid. HDACs: Histone deacetylase. IL: Interleukin. Th: Helper T cells. Treg: Regulatory T cell. TGF-β: Transforming growth factor beta. GPR: G-protein-coupled receptors. sIgA: Secretory immunoglobulin A. AhR activation: Aromatic hydrocarbon receptor activation. Foxp3: Fork-headed transcription factor p3. TNF: Tumor necrosis factor. DC: Dendritic cell. IFN-γ: Gamma interferon
| [1] | 李星, 曹振辉, 林秋叶, 等. 肠道微生物及其代谢产物对动物免疫机能的影响[J]. 动物营养学报, 2019, 31(2): 553-559. |
| Li X, Cao ZH, Lin QY, et al. Effects of gut microbiota and its metabolites on animal immune function[J]. Chin J Animal Nutr, 2019, 31(2): 553-559. | |
| [2] | Cummings JH, Antoine JM, Azpiroz F, et al. PASSCLAIM1? Gut health and immunity[J]. Eur J Nutr, 2004, 43(S2): ii118-ii173. |
| [3] |
Turner JR. Intestinal mucosal barrier function in health and disease[J]. Nat Rev Immunol, 2009, 9(11): 799-809.
doi: 10.1038/nri2653 pmid: 19855405 |
| [4] | 金磊, 王立志. 肠道微生物与宿主免疫关系研究进展[J]. 现代畜牧兽医, 2018(9): 52-58. |
| Jin L, Wang LZ. Research progress on the immunological relationship between intestinal microorganism and host[J]. Mod J Animal Husb Vet Med, 2018(9): 52-58. | |
| [5] |
Burgueño JF, Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease[J]. Nat Rev Gastroenterol Hepatol, 2020, 17(5): 263-278.
doi: 10.1038/s41575-019-0261-4 pmid: 32103203 |
| [6] |
Liu YH, Ajami NJ, El-Serag HB, et al. Dietary quality and the colonic mucosa-associated gut microbiome in humans[J]. Am J Clin Nutr, 2019, 110(3): 701-712.
doi: 10.1093/ajcn/nqz139 pmid: 31291462 |
| [7] |
Suzuki T. Regulation of the intestinal barrier by nutrients: The role of tight junctions[J]. Anim Sci J, 2020, 91(1): e13357.
doi: 10.1111/asj.v91.1 URL |
| [8] |
Odenwald MA, Turner JR. The intestinal epithelial barrier: a therapeutic target?[J]. Nat Rev Gastroenterol Hepatol, 2017, 14(1): 9-21.
doi: 10.1038/nrgastro.2016.169 pmid: 27848962 |
| [9] |
McLoughlin RF, Berthon BS, Jensen ME, et al. Short-chain fatty acids, prebiotics, synbiotics, and systemic inflammation: a systematic review and meta-analysis[J]. Am J Clin Nutr, 2017, 106(3): 930-945.
doi: 10.3945/ajcn.117.156265 pmid: 28793992 |
| [10] |
Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by Lamina propria dendritic cells expressing Toll-like receptor 5[J]. Nat Immunol, 2008, 9(7): 769-776.
doi: 10.1038/ni.1622 |
| [11] |
Tong TJ, Qi YJ, Bussiere LD, et al. Transport of artificial virus-like nanocarriers through intestinal monolayers via microfold cells[J]. Nanoscale, 2020, 12(30): 16339-16347.
doi: 10.1039/D0NR03680C URL |
| [12] |
Caricilli AM, Castoldi A, Câmara NOS. Intestinal barrier: a gentlemen's agreement between microbiota and immunity[J]. World J Gastrointest Pathophysiol, 2014, 5(1): 18-32.
doi: 10.4291/wjgp.v5.i1.18 pmid: 24891972 |
| [13] |
Kamada N, Seo SU, Chen GY, et al. Role of the gut microbiota in immunity and inflammatory disease[J]. Nat Rev Immunol, 2013, 13(5): 321-335.
doi: 10.1038/nri3430 pmid: 23618829 |
| [14] | Milani C, Duranti S, Bottacini F, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota[J]. Microbiol Mol Biol Rev, 2017, 81(4): e00036-e00017. |
| [15] | Zou SM, Fang LK, Lee MH. Dysbiosis of gut microbiota in promoting the development of colorectal cancer[J]. Gastroenterol Rep(Oxf), 2018, 6(1): 1-12. |
| [16] |
Mao JD. Lactobacillus rhamnosus GG attenuates lipopolysaccharide-induced inflammation and barrier dysfunction by regulating MAPK/NF-ĸB signaling and modulating metabolome in the piglet intestine[J]. J Nutr, 2020, 150(5): 1313-1323.
doi: 10.1093/jn/nxaa009 URL |
| [17] |
Brink LR, Mercer KE, Piccolo BD, et al. Neonatal diet alters fecal microbiota and metabolome profiles at different ages in infants fed breast milk or formula[J]. Am J Clin Nutr, 2020, 111(6): 1190-1202.
doi: 10.1093/ajcn/nqaa076 pmid: 32330237 |
| [18] |
Sommer F, Bäckhed F. The gut microbiota—Masters of host development and physiology[J]. Nat Rev Microbiol, 2013, 11(4): 227-238.
doi: 10.1038/nrmicro2974 pmid: 23435359 |
| [19] | 郭宏伟, 张妮妮, 张伟, 等. 抗生素诱导的菌群紊乱对幼鼠结肠黏膜屏障及免疫反应的影响[J]. 中华实用儿科临床杂志, 2019, 34(7): 505-509. |
| Guo HW, Zhang NN, Zhang W, et al. Effect of antibiotic-induced microbiota dysbiosis on colonic mucosal barrier and immune response in juvenile mice[J]. Chin J Appl Clin Pediatr, 2019, 34(7): 505-509. | |
| [20] |
Ge XL, Ding C, Zhao W, et al. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility[J]. J Transl Med, 2017, 15(1): 13.
doi: 10.1186/s12967-016-1105-4 pmid: 28086815 |
| [21] |
Franzosa EA, Hsu T, Sirota-Madi A, et al. Sequencing and beyond: integrating molecular ‘omics’ for microbial community profiling[J]. Nat Rev Microbiol, 2015, 13(6): 360-372.
doi: 10.1038/nrmicro3451 pmid: 25915636 |
| [22] |
Zuñiga C, Zaramela L, Zengler K. Elucidation of complexity and prediction of interactions in microbial communities[J]. Microb Biotechnol, 2017, 10(6): 1500-1522.
doi: 10.1111/1751-7915.12855 pmid: 28925555 |
| [23] | 侯璐文, 吴长新, 秦雪梅, 等. 肠道微生物功能宏基因组学与代谢组学关联分析方法研究进展[J]. 微生物学报, 2019, 59(9): 1813-1822. |
| Hou LW, Wu CX, Qin XM, et al. Research progress on correlation analysis methods of functional metagenomics and metabonomics of intestinal microorganisms[J]. Acta Microbiol Sin, 2019, 59(9): 1813-1822. | |
| [24] |
Zheng YP, Ran Y, Zhang HX, et al. The microbiome in autoimmune liver diseases: metagenomic and metabolomic changes[J]. Front Physiol, 2021, 12: 715852.
doi: 10.3389/fphys.2021.715852 URL |
| [25] |
Wang YL, Yin YS, Chen X, et al. Induction of intestinal Th17 cells by flagellins from segmented filamentous bacteria[J]. Front Immunol, 2019, 10: 2750.
doi: 10.3389/fimmu.2019.02750 pmid: 31824516 |
| [26] |
Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species[J]. Science, 2011, 331(6015): 337-341.
doi: 10.1126/science.1198469 pmid: 21205640 |
| [27] |
Wong SH. Zhao LY, Zhang X, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice[J]. Gastroenterology, 2017, 153(6): 1621-1633.
doi: 10.1053/j.gastro.2017.08.022 URL |
| [28] |
Thiele Orberg E, Fan H, Tam AJ, et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis[J]. Mucosal Immunol, 2017, 10(2): 421-433.
doi: 10.1038/mi.2016.53 pmid: 27301879 |
| [29] |
Coleman OI, Lobner EM, Bierwirth S, et al. Activated ATF6 induces intestinal dysbiosis and innate immune response to promote colorectal tumorigenesis[J]. Gastroenterology, 2018, 155(5): 1539-1552.
doi: S0016-5085(18)34816-9 pmid: 30063920 |
| [30] |
Jiao YH, Wu L, Huntington ND, et al. Crosstalk between gut microbiota and innate immunity and its implication in autoimmune diseases[J]. Front Immunol, 2020, 11: 282.
doi: 10.3389/fimmu.2020.00282 pmid: 32153586 |
| [31] |
Chida S, Sakamoto M, Takino T, et al. Changes in immune system and intestinal bacteria of cows during the transition period[J]. Vet Anim Sci, 2021, 14: 100222.
doi: 10.1016/j.vas.2021.100222 URL |
| [32] |
Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens[J]. Nat Rev Immunol, 2013, 13(11): 790-801.
doi: 10.1038/nri3535 pmid: 24096337 |
| [33] |
Schwartz DJ, Langdon AE, Dantas G. Understanding the impact of antibiotic perturbation on the human microbiome[J]. Genome Med, 2020, 12(1): 82.
doi: 10.1186/s13073-020-00782-x pmid: 32988391 |
| [34] |
Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review[J]. J Infect, 2019, 79(6): 471-489.
doi: S0163-4453(19)30318-4 pmid: 31629863 |
| [35] |
Wu HG, Chen Q, Liu JN, et al. Microbiome analysis reveals gut microbiota alteration in mice with the effect of matrine[J]. Microb Pathog, 2021, 156: 104926.
doi: 10.1016/j.micpath.2021.104926 URL |
| [36] |
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity[J]. Nat Rev Immunol, 2016, 16(6): 341-352.
doi: 10.1038/nri.2016.42 pmid: 27231050 |
| [37] |
Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345.
doi: S0092-8674(16)30592-X pmid: 27259147 |
| [38] |
Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer[J]. Nat Rev Microbiol, 2014, 12(10): 661-672.
doi: 10.1038/nrmicro3344 pmid: 25198138 |
| [39] |
Reichardt N, Duncan SH, Young P, et al. Phylogenetic distribution of three pathways for propionate production within the human gut microbiota[J]. ISME J, 2014, 8(6): 1323-1335.
doi: 10.1038/ismej.2014.14 pmid: 24553467 |
| [40] |
Flint HJ, Duncan SH, Scott KP, et al. Links between diet, gut microbiota composition and gut metabolism[J]. Proc Nutr Soc, 2015, 74(1): 13-22.
doi: 10.1017/S0029665114001463 pmid: 25268552 |
| [41] |
Vinolo MAR, Rodrigues HG, Hatanaka E, et al. Short-chain fatty acids stimulate the migration of neutrophils to inflammatory sites[J]. Clin Sci(Lond), 2009, 117(9): 331-338.
doi: 10.1042/CS20080642 URL |
| [42] |
Vinolo MAR, Hatanaka E, Lambertucci RH, et al. Effects of short chain fatty acids on effector mechanisms of neutrophils[J]. Cell Biochem Funct, 2009, 27(1): 48-55.
doi: 10.1002/cbf.1533 pmid: 19107872 |
| [43] |
Vinolo MAR, Rodrigues HG, Hatanaka E, et al. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils[J]. J Nutr Biochem, 2011, 22(9): 849-855.
doi: 10.1016/j.jnutbio.2010.07.009 pmid: 21167700 |
| [44] |
Nastasi C, Candela M, Bonefeld CM, et al. The effect of short-chain fatty acids on human monocyte-derived dendritic cells[J]. Sci Rep, 2015, 5: 16148.
doi: 10.1038/srep16148 pmid: 26541096 |
| [45] |
Li YJ, Chen X, Kwan TK, et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A[J]. J Am Soc Nephrol, 2020, 31(6): 1267-1281.
doi: 10.1681/ASN.2019101029 pmid: 32358041 |
| [46] | Grabarska A, Dmoszyńska-Graniczka M, Nowosadzka E, et al. Histone deacetylase inhibitors - molecular mechanisms of actions and clinical applications[J]. Postepy Hig Med Dosw(Online), 2013, 67: 722-735. |
| [47] |
Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis[J]. Science, 2013, 341(6145): 569-573.
doi: 10.1126/science.1241165 pmid: 23828891 |
| [48] |
Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells[J]. Nat Med, 2007, 13(11):1299-1307.
doi: 10.1038/nm1652 pmid: 17922010 |
| [49] |
Chang PV, Hao LM, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition[J]. Proc Natl Acad Sci USA, 2014, 111(6): 2247-2252.
doi: 10.1073/pnas.1322269111 pmid: 24390544 |
| [50] |
Singh N, Thangaraju M, Prasad PD, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter(Slc5a8)-dependent inhibition of histone deacetylases[J]. J Biol Chem, 2010, 285(36): 27601-27608.
doi: 10.1074/jbc.M110.102947 URL |
| [51] |
Hino S, Mizushima T, Kaneko K, et al. Mucin-derived O-glycans act as endogenous fiber and sustain mucosal immune homeostasis via short-chain fatty acid production in rat cecum[J]. J Nutr, 2020, 150(10): 2656-2665.
doi: 10.1093/jn/nxaa097 pmid: 32286621 |
| [52] |
Singh N, Gurav A, Sivaprakasam S, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis[J]. Immunity, 2014, 40(1): 128-139.
doi: 10.1016/j.immuni.2013.12.007 pmid: 24412617 |
| [53] |
Yang WJ, Yu TM, Huang XS, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity[J]. Nat Commun, 2020, 11(1): 4457.
doi: 10.1038/s41467-020-18262-6 pmid: 32901017 |
| [54] |
Park J, Kim M, Kang SG, et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway[J]. Mucosal Immunol, 2015, 8(1): 80-93.
doi: 10.1038/mi.2014.44 pmid: 24917457 |
| [55] |
Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation[J]. Cell, 2011, 147(3): 629-640.
doi: 10.1016/j.cell.2011.09.025 pmid: 21999944 |
| [56] |
Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome[J]. Cell Metab, 2018, 28(5): 737-749.
doi: S1550-4131(18)30444-3 pmid: 30057068 |
| [57] |
Murray IA, Nichols RG, Zhang LM, et al. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice[J]. Sci Rep, 2016, 6: 33969.
doi: 10.1038/srep33969 pmid: 27659481 |
| [58] |
Zhang LM, Nichols RG, Correll J, et al. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation[J]. Environ Health Perspect, 2015, 123(7): 679-688.
doi: 10.1289/ehp.1409055 URL |
| [59] |
Lamas B, Natividad JM, Sokol H. Aryl hydrocarbon receptor and intestinal immunity[J]. Mucosal Immunol, 2018, 11(4): 1024-1038.
doi: 10.1038/s41385-018-0019-2 pmid: 29626198 |
| [60] |
Yang F, DeLuca JAA, Menon R, et al. Effect of diet and intestinal AhR expression on fecal microbiome and metabolomic profiles[J]. Microb Cell Fact, 2020, 19(1): 219.
doi: 10.1186/s12934-020-01463-5 pmid: 33256731 |
| [61] |
Hubbard TD, Murray IA, Bisson WH, et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles[J]. Sci Rep, 2015, 5: 12689.
doi: 10.1038/srep12689 pmid: 26235394 |
| [62] |
Sun M, Ma N, He T, et al. Tryptophan(Trp)modulates gut homeostasis via aryl hydrocarbon receptor(AhR)[J]. Crit Rev Food Sci Nutr, 2020, 60(10): 1760-1768.
doi: 10.1080/10408398.2019.1598334 URL |
| [63] |
Qiu JX, Guo Z, Chen L, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora[J]. Immunity, 2013, 39(2): 386-399.
doi: 10.1016/j.immuni.2013.08.002 pmid: 23954130 |
| [64] |
Ito S, Chen C, Satoh J, et al. Dietary phytochemicals regulate whole-body CYP1A1 expression through an arylhydrocarbon receptor nuclear translocator-dependent system in gut[J]. J Clin Invest, 2007, 117(7): 1940-1950.
pmid: 17607366 |
| [65] |
Schipke J, Vital M, Schnapper-Isl A, et al. Spermidine and voluntary activity exert differential effects on sucrose- compared with fat-induced systemic changes in male mice[J]. J Nutr, 2019, 149(3): 451-462.
doi: 10.1093/jn/nxy272 pmid: 30715385 |
| [66] |
Carriche GM, Almeida L, Stüve P, et al. Regulating T-cell differentiation through the polyamine spermidine[J]. J Allergy Clin Immunol, 2021, 147(1): 335-348.e11.
doi: 10.1016/j.jaci.2020.04.037 pmid: 32407834 |
| [67] |
Wang J, Tan BE, Li JJ, et al. Regulatory role of l-proline in fetal pig growth and intestinal epithelial cell proliferation[J]. Anim Nutr, 2020, 6(4): 438-446.
doi: 10.1016/j.aninu.2020.07.001 pmid: 33364460 |
| [68] |
ter Steege JC, Buurman WA, Forget PP. Spermine induces maturation of the immature intestinal immune system in neonatal mice[J]. J Pediatr Gastroenterol Nutr, 1997, 25(3): 332-340.
doi: 10.1097/00005176-199709000-00017 URL |
| [69] |
Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases[J]. Clin Nutr, 2005, 24(2): 184-197.
doi: 10.1016/j.clnu.2004.11.001 pmid: 15784477 |
| [70] |
Pérez-Cano FJ, González-Castro A, Castellote C, et al. Influence of breast milk polyamines on suckling rat immune system maturation[J]. Dev Comp Immunol, 2010, 34(2): 210-218.
doi: 10.1016/j.dci.2009.10.001 pmid: 19825390 |
| [71] |
Yoo JY, Groer M, Dutra SVO, et al. Gut microbiota and immune system interactions[J]. Microorganisms, 2020, 8(10): 1587.
doi: 10.3390/microorganisms8101587 URL |
| [1] | 周嫒婷, 彭睿琦, 王芳, 伍建榕, 马焕成. 生防菌株DZY6715在不同生长期的代谢差异分析[J]. 生物技术通报, 2023, 39(9): 225-235. |
| [2] | 谢田朋, 张佳宁, 董永骏, 张建, 景明. 早期抽薹对当归根际土壤微环境的影响[J]. 生物技术通报, 2023, 39(7): 206-218. |
| [3] | 熊淑琪. 胆汁酸生理功能及其与肠道微生物互作研究进展[J]. 生物技术通报, 2023, 39(4): 187-200. |
| [4] | 赵艳侠, 张晶莹, 孙骏飞, 王绛辉, 孙家波, 吕晓惠. ‘重瓣红’玫瑰不同花发育阶段转录和代谢差异分析[J]. 生物技术通报, 2023, 39(3): 184-195. |
| [5] | 王松, 简晓平, 潘婉舒, 张永光, 王涛, 游玲. 玉米小曲酒糟发酵饲料对育肥猪肠道菌群的影响[J]. 生物技术通报, 2022, 38(9): 248-257. |
| [6] | 陈天赐, 武少兰, 杨国辉, 江丹霞, 江玉姬, 陈炳智. 无柄灵芝醇提物对小鼠睡眠及肠道菌群的影响[J]. 生物技术通报, 2022, 38(8): 225-232. |
| [7] | 何亚伦, 曾丽荣, 刘雄, 张铃, 王琼. 高剂量单宁酸对小鼠肠道屏障和肠道菌群的影响[J]. 生物技术通报, 2022, 38(4): 278-287. |
| [8] | 钟明月, 刘春妍, 颜妍, 张晓慧, 原海升, 徐国全, 张和平, 王玉珍. 乳双歧杆菌V9对高脂饮食诱导的NAFLD大鼠的改善作用[J]. 生物技术通报, 2022, 38(3): 181-187. |
| [9] | 杨玉萍, 张霞, 王翀翀, 王晓艳. 不同年龄大鼠尿液代谢组学研究[J]. 生物技术通报, 2022, 38(2): 166-172. |
| [10] | 刘传和, 贺涵, 何秀古, 赖秋勤, 刘开, 邵雪花, 赖多, 匡石滋, 肖维强. 转录组与代谢组联合分析菠萝网纱覆盖防寒机制[J]. 生物技术通报, 2022, 38(11): 58-69. |
| [11] | 刘传和, 贺涵, 何秀古, 刘开, 邵雪花, 赖多, 匡石滋, 肖维强. 不同连作年限菠萝园土壤差异代谢物和细菌群落结构分析[J]. 生物技术通报, 2021, 37(8): 162-175. |
| [12] | 金秋霞, 王思宏, 金丽华. 果蝇肠道干细胞及肠道菌群的研究进展[J]. 生物技术通报, 2021, 37(4): 245-250. |
| [13] | 李海超, 谢飞, 张园琦, 关若冰. 不同抗、感水稻品种对褐飞虱肠道菌群的影响[J]. 生物技术通报, 2021, 37(3): 1-9. |
| [14] | 胡紫媛, 夏嫱. 昆虫肠道菌群组学研究及功能和应用进展[J]. 生物技术通报, 2021, 37(1): 102-112. |
| [15] | 张萌, 罗芳, 王敏, 武彦泽, 王俊奎, 和东迁, 陈丽尧, 陶金忠. 奶牛分娩后早期血浆代谢物变化研究[J]. 生物技术通报, 2020, 36(6): 191-199. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||