








生物技术通报 ›› 2023, Vol. 39 ›› Issue (8): 283-290.doi: 10.13560/j.cnki.biotech.bull.1985
收稿日期:2023-01-09
出版日期:2023-08-26
发布日期:2023-09-05
通讯作者:
谢志雄,男,博士,教授,博士生导师,研究方向:微生物遗传;E-mail: zxxie@whu.edu.cn作者简介:饶紫环,女,硕士研究生,研究方向:微生物遗传;E-mail: RZHmio@whu.edu.cn
基金资助:
RAO Zi-huan( ), XIE Zhi-xiong(
), XIE Zhi-xiong( )
)
Received:2023-01-09
Published:2023-08-26
Online:2023-09-05
摘要:
为了获得常温新型纤维素降解细菌,在园林堆肥中分离纯化得到一株具有纤维素降解能力的细菌菌株18B。通过刚果红染色实验和滤纸降解实验验证其纤维素降解能力。通过16S rRNA序列比对和全基因组比对确定其属于Olivibacter属,且与Olivibacter jilunii 14-2AT最为接近,进一步生理生化特征比较分析发现,其与O. jilunii 14-2AT在生长温度、氧化酶和糖酵解等方面存在差异。菌株18B在12-48℃范围内能生长,最适生长温度在30-37℃。O. jilunii 14-2AT的生长温度在4-42℃;菌株18B的氧化酶检测为阳性,糖酵解为阴性,O. jilunii 14-2AT则不具有氧化酶活性同时糖酵解为阳性。结合基因组共线性比对结果可知,菌株18B与O. jilunii 14-2AT存在一定的进化关系。纤维素降解能力检测发现,在37℃,200 r/min培养至第5天时,菌株18B的纤维素酶活最高可达82.14+0.99-9.90 U/L,同时在以羧甲基纤维素钠为唯一碳源,仅添加无机氮源的培养条件下可维持酶活不退化。O. jilunii 14-2AT则不具有纤维素降解能力,也无法在羧甲基纤维素钠为唯一有机碳源的培养环境下存活。筛选全基因组测序结果可知菌株18B基因组上存在纤维素酶系基因。在大肠杆菌中异源表达筛选所得纤维素酶系基因,并检验表达产物的降解能力,结果表明筛选所得纤维素酶系基因确有酶活。综合以上结果,最终确定菌株18B属于具有纤维素酶活的O. jilunii新生理株。
饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290.
RAO Zi-huan, XIE Zhi-xiong. Isolation and Identification of a Cellulose-degrading Strain of Olivibacter jilunii and Analysis of Its Degradability[J]. Biotechnology Bulletin, 2023, 39(8): 283-290.
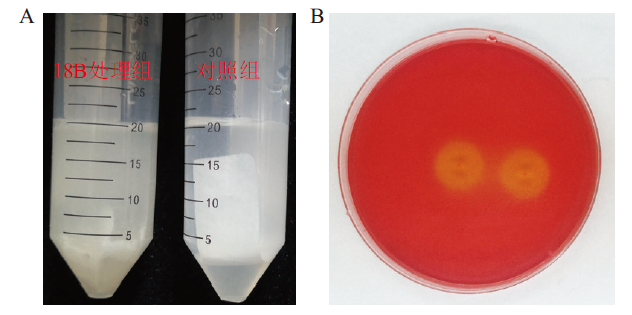
图1 菌株18B纤维素降解能力检测 A:滤纸降解;B:刚果红褪色圈检测
Fig. 1 Detection of cellulose degradation ability of strain 18B A: Degradation of filter paper. B: Congo red fading circle
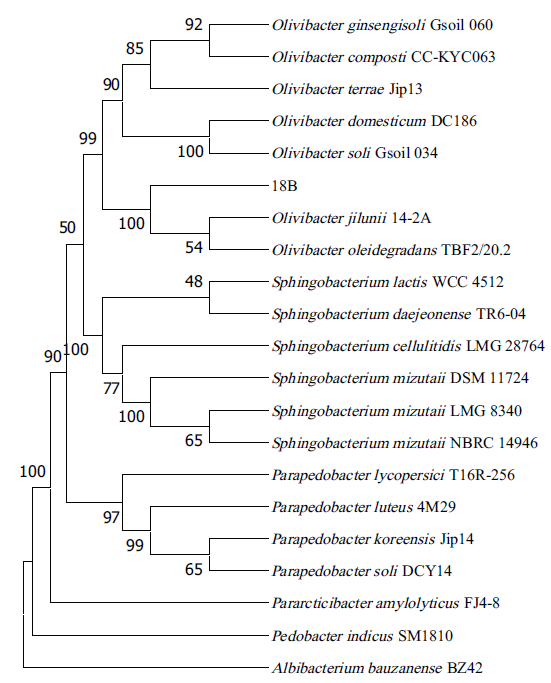
图2 基于16S rRNA序列构建的非加权组平均法进化树(以Albibacterium bauzanense BZ42为外群)
Fig. 2 UPGMA-tree based on 16S rRNA (Albibacterium bauzanense BZ42 used as the outgroup)
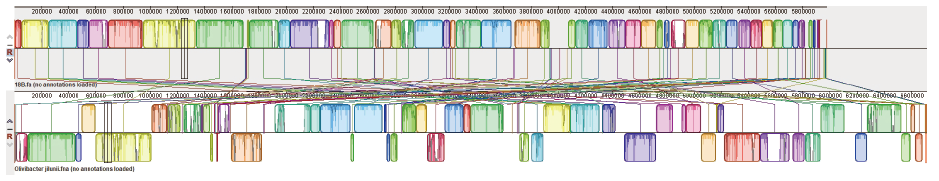
图4 菌株18B和O. jilunii 14-2AT的全基因组共线性比对 图中上方为菌株18B,下方为O. jilunii 14-2AT
Fig. 4 Genome-wide covariance alignment of strain 18B and O. jilunii 14-2AT It in the upper part of the figure it is strain 18B and in the lower part is O. jilunii 14-2AT

图5 Olivibacter属内基因组比对 从NCBI上下载目前Olivibacter属内相关菌株全基因组序列信息,与菌株18B一同进行比对。图中sitiens指菌株O. sitiensis AW-6T,domesticus指菌株O. domesticus DC186T,LS_1指菌株O. sp. LS-1,jilunii指菌株O. jilunii 14-2AT
Fig. 5 Genome alignment within the genus Olivibacter Complete genome sequence information of related strains in Olivibacter genus was downloaded from NCBI and compared with strain 18B. In the figure, sitiens indicates the strain O. sitiensis AW-6T, domesticus indicates O. domesticus DC186T, and LS_1 indicates O. sp.LS-1. jilunii refers to strain O. jilunii 14-2AT
| 项目 Item | 18B | O. jilunii 14-2AT |
|---|---|---|
| 生长温度/℃ | 12-48 | 4-42 |
| 生长pH | 6.0-8.0 | 6.0-9.0 |
| NaCl耐受/% | 0-6 | 0-5 |
| 氧化酶 | + | - |
| 硝酸盐还原 | + | + |
| 糖酵解 | - | + |
| VP | + | + |
| β-半乳糖苷酶 | + | + |
| 革兰氏染色 | - | - |
| MacConkey agar | +(Red) | +(Red) |
表1 18B和O. jilunii 14-2AT常见生理生化特征比对
Table 1 Comparison of common physiological and bioche-mical characteristics between 18B and O. jilunii 14-2AT
| 项目 Item | 18B | O. jilunii 14-2AT |
|---|---|---|
| 生长温度/℃ | 12-48 | 4-42 |
| 生长pH | 6.0-8.0 | 6.0-9.0 |
| NaCl耐受/% | 0-6 | 0-5 |
| 氧化酶 | + | - |
| 硝酸盐还原 | + | + |
| 糖酵解 | - | + |
| VP | + | + |
| β-半乳糖苷酶 | + | + |
| 革兰氏染色 | - | - |
| MacConkey agar | +(Red) | +(Red) |
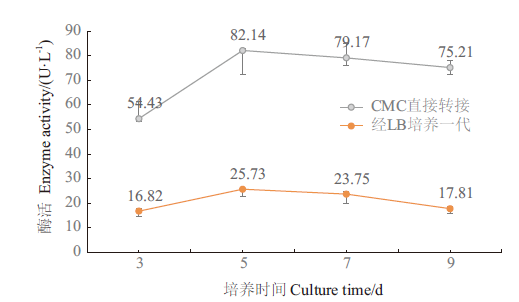
图6 18B纤维素酶活随培养时间变化 灰线为CMC-Na培养环境中转接后的菌株纤维素酶活,红线为从CMC-Na培养环境转接至LB中培养一代后的菌株纤维素酶活;O. jilunii 14-2AT的酶活未测得
Fig. 6 Variation of 18B cellulase activity with incubation time The gray line is the cellulase activity of the strain after transfer from CMC-Na culture environment, and the red line is the cellulase activity of the strain after transfer from CMC-Na culture environment to LB culture for one generation. The enzyme activity of O. jilunii 14-2AT was not detected
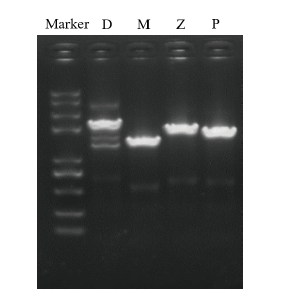
图7 诱导表达后基因检测 Marker为Trans2k plusII;D为内切葡聚糖酶D前体基因,长度为1 812 bp;M为纤维素酶M基因,长度为1 119 bp;Z为内切葡聚糖酶Z前体基因,长度为1 542 bp;P为纯内切葡聚糖酶基因,长度为1 395 bp
Fig. 7 Gene amplification after induction of expression Marker is Trans2k plusII. D is the precursor gene of endoglucanase D, the length is 1 812 bp. M is cellulase M gene with length of 1 119 bp. Z is the precursor gene of endoglucanase Z, the length of which is 1 542 bp. P is a pure endoglucanase gene with a length of 1 395 bp
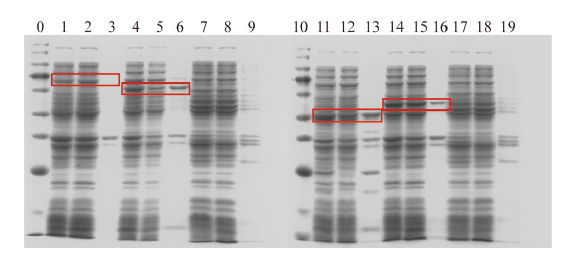
图8 纤维素酶基因诱导表达蛋白检测 0:Maker;1:D蛋白表达后破细胞全液;2:D蛋白表达后破细胞上清;3:D蛋白表达后破细胞沉淀;4:Z蛋白表达后破细胞全液;5:Z蛋白表达后破细胞上清;6:Z蛋白表达后破细胞沉淀;7:空载诱导表达后破细胞全液;8:空载诱导表达后破细胞上清;9:空载诱导表达后破细胞沉淀;10:marker;11:M蛋白表达后破细胞全液;12:M蛋白表达后破细胞上清;13:M蛋白表达后破细胞沉淀;14:P蛋白表达后破细胞全液;15:P蛋白表达后破细胞上清;16:P蛋白表达后破细胞沉淀;17:空载诱导表达后破细胞全液;18:空载诱导表达后破细胞上清;19:空载诱导表达后破细胞沉淀。红框圈出的为目的蛋白区段
Fig. 8 Protein assay of cellulase gene induced expression 0: Maker. 1: The whole solution broken after D protein expression; 2: the supernatant broken after D protein expression; 3: the precipitation broken after D protein expression; 4: the whole fluid broken after Z protein expression; 5: the supernatant broken after Z protein expression; 6: the precipitation broken after Z protein expression; 7: the whole solution of cell breaking after no-load induction expression; 8: the supernatant of cell breaking after no-load induction expression; 9: the precipitation of cell breaking after no-load induction expression; 10: marker; 11: the whole solution broken after M protein expression; 12: the supernatant broken after M protein expression; 13: the precipitation broken after M protein expression; 14: the whole fluid broken after P protein expression; 15: the supernatant broken after P protein expression; 16: the precipitation broken after P protein expression; 17: the whole solution of cell breaking after no-load induction expression; 18: the supernatant of cell breaking after no-load induction expression; 19: the precipitation of cell breaking after no-load induction expression. The red box marked the target protein region
| [1] |
Arevalo-Gallegos A, Ahmad Z, Asgher M, et al. Lignocellulose: a sustainable material to produce value-added products with a zero waste approach-a review[J]. Int J Biol Macromol, 2017, 99: 308-318.
doi: S0141-8130(16)32991-9 pmid: 28254573 |
| [2] |
Sharma A, Tewari R, Rana SS, et al. Cellulases: classification, methods of determination and industrial applications[J]. Appl Biochem Biotechnol, 2016, 179(8): 1346-1380.
doi: 10.1007/s12010-016-2070-3 pmid: 27068832 |
| [3] |
Djuric Ilic D, Dotzauer E, Trygg L, et al. Introduction of large-scale biofuel production in a district heating system-an opportunity for reduction of global greenhouse gas emissions[J]. J Clean Prod, 2014, 64: 552-561.
doi: 10.1016/j.jclepro.2013.08.029 URL |
| [4] |
Sadhu S. Cellulase production by bacteria: a review[J]. Br Microbiol Res J, 2013, 3(3): 235-258.
doi: 10.9734/BMRJ URL |
| [5] | Juturu V, Wu JC. Microbial cellulases: engineering, production and applications[J]. Renew Sustain Energy Rev, 2014, 33: 188-203. |
| [6] |
Ntougias S, Fasseas C, Zervakis GI. Olivibacter sitiensis Gen. nov., sp. nov., isolated from alkaline olive-oil mill wastes in the region of Sitia, Crete[J]. Int J Syst Evol Microbiol, 2007, 57(Pt 2): 398-404.
doi: 10.1099/ijs.0.64561-0 URL |
| [7] | Lane DJ. 16S/23S rRNA sequencing[M]//Stackebrandt E, Goodfellow M, eds. Nucleic Acid Techniques in Bacterial Systematics. Chichester, UK: J. Wiley & Sons, 1991: 115-175. |
| [8] |
Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms[J]. Mol Biol Evol, 2018, 35(6): 1547-1549.
doi: 10.1093/molbev/msy096 pmid: 29722887 |
| [9] |
Sokal RR. Numerical taxonomy[J]. Sci Am, 1966, 215(6): 106-116.
doi: 10.1038/scientificamerican1266-106 URL |
| [10] | Swofford DL. PAUP* 4.0b10: phylogenetic analysis using parsimony(and other methods)[Software]. 2003. |
| [11] |
Darling ACE, Mau B, Blattner FR, et al. Mauve: multiple alignment of conserved genomic sequence with rearrangements[J]. Genome Res, 2004, 14(7): 1394-1403.
doi: 10.1101/gr.2289704 pmid: 15231754 |
| [12] |
Alikhan NF, Petty NK, Ben Zakour NL, et al. BLAST Ring Image Generator(BRIG): simple prokaryote genome comparisons[J]. BMC Genomics, 2011, 12: 402.
doi: 10.1186/1471-2164-12-402 |
| [13] |
Chen K, Tang SK, Wang GL, et al. Olivibacter jilunii sp. nov., isolated from DDT-contaminated soil[J]. Int J Syst Evol Microbiol, 2013, 63(Pt 3): 1083-1088.
doi: 10.1099/ijs.0.042416-0 URL |
| [14] |
Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar[J]. Anal Chem, 1959, 31(3): 426-428.
doi: 10.1021/ac60147a030 URL |
| [15] |
Wang WK, Liang CM. Enhancing the compost maturation of swine manure and rice straw by applying bioaugmentation[J]. Sci Rep, 2021, 11(1): 6103.
doi: 10.1038/s41598-021-85615-6 |
| [16] |
Konovalova A, Kahne DE, Silhavy TJ. Outer membrane biogenesis[J]. Annu Rev Microbiol, 2017, 71: 539-556.
doi: 10.1146/annurev-micro-090816-093754 pmid: 28886680 |
| [17] |
Ang SK, Shaza EM, Adibah Y, et al. Production of cellulases and xylanase by Aspergillus fumigatus SK1 using untreated oil palm trunk through solid state fermentation[J]. Process Biochem, 2013, 48(9): 1293-1302.
doi: 10.1016/j.procbio.2013.06.019 URL |
| [18] |
Gilkes NR, Kilburn DG, Miller RC Jr, et al. Bacterial cellulases[J]. Bioresour Technol, 1991, 36(1): 21-35.
doi: 10.1016/0960-8524(91)90097-4 URL |
| [19] | 章沙沙, 徐健峰, 柳增善. 纤维素降解菌的研究与应用进展[J]. 工业微生物, 2021, 51(2): 46-52. |
| Zhang SS, Xu JF, Liu ZS. Progress in research and application of cellulose-degrading microorganisms[J]. Ind Microbiol, 2021, 51(2): 46-52. | |
| [20] |
Niyonzima FN. Detergent-compatible bacterial cellulases[J]. J Basic Microbiol, 2019, 59(2): 134-147.
doi: 10.1002/jobm.v59.2 URL |
| [1] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [2] | 车永梅, 郭艳苹, 刘广超, 叶青, 李雅华, 赵方贵, 刘新. 菌株C8和B4的分离鉴定及其耐盐促生效果和机制[J]. 生物技术通报, 2023, 39(5): 276-285. |
| [3] | 王凤婷, 王岩, 孙颖, 崔文婧, 乔凯彬, 潘洪玉, 刘金亮. 耐盐碱土曲霉SYAT-1的分离鉴定及抑制植物病原真菌特性研究[J]. 生物技术通报, 2023, 39(2): 203-210. |
| [4] | 祖雪, 周瑚, 朱华珺, 任佐华, 刘二明. 枯草芽孢杆菌K-268的分离鉴定及对水稻稻瘟病的防病效果[J]. 生物技术通报, 2022, 38(6): 136-146. |
| [5] | 王新光, 田磊, 王恩泽, 钟成, 田春杰. 玉米秸秆高效降解微生物复合菌系的构建及降解效果评价[J]. 生物技术通报, 2022, 38(4): 217-229. |
| [6] | 王春艳, 腊贵晓, 苏秀红, 李萌, 董诚明. 地黄不同时期内生促生细菌的筛选及其促生特性分析[J]. 生物技术通报, 2022, 38(4): 242-252. |
| [7] | 张功友, 王一涵, 郭敏, 张婷婷, 王兵, 刘红美. 重楼中一株产纤维素酶内生真菌的分离及鉴定[J]. 生物技术通报, 2022, 38(2): 95-104. |
| [8] | 牛鸿宇, 舒倩, 杨海君, 颜智勇, 谭菊. 一株十二烷基硫酸钠高效降解菌的分离鉴定、降解特性及代谢途径研究[J]. 生物技术通报, 2022, 38(12): 287-299. |
| [9] | 崔欣雨, 李荣荣, 蔡瑞, 王妍, 郑猛虎, 徐春城. 苜蓿青贮中乳酸降解菌的分离、鉴定及降解性能研究[J]. 生物技术通报, 2021, 37(9): 58-67. |
| [10] | 唐昊, 孙灿, 李沅秋, 罗朝兵. 纤维素降解菌Raoultella ornithinolytica LL1的筛选及基因组测序[J]. 生物技术通报, 2021, 37(6): 85-96. |
| [11] | 李瑾, 彭可为, 潘求一, 朱哲远, 彭迪. 解淀粉芽胞杆菌HR-2的分离鉴定及对水稻稻瘟病菌的拮抗效果[J]. 生物技术通报, 2021, 37(3): 27-34. |
| [12] | 李珍阳, 姜润, 刘琳, 李思琦, 王晓慧. 低温异养硝化菌的筛选、鉴定及降解特性研究[J]. 生物技术通报, 2021, 37(10): 45-56. |
| [13] | 卫晓博, 侯颖, 程豪杰, 秦翠丽, 牛明福, 徐建强. 一种苯酚降解菌Pseudoxanthomonas sp. BF-6的分离鉴定及其降解特性及途径研究[J]. 生物技术通报, 2021, 37(10): 72-80. |
| [14] | 林嘉铭, 葛辉, 林克冰, 杨章武, 周宸, 吴建绍, 王国栋, 张哲, 杨求华, 王艺磊. 凡纳滨对虾黑鳃病病原的分离鉴定及耐药性分析[J]. 生物技术通报, 2020, 36(8): 120-128. |
| [15] | 冯光志, 石慧, 刘博, 吴玉婷, 王月琳, 石玉. 小龙虾肠道产纤维素酶细菌的分离与鉴定[J]. 生物技术通报, 2020, 36(2): 65-70. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||