








生物技术通报 ›› 2022, Vol. 38 ›› Issue (12): 287-299.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0347
牛鸿宇1( ), 舒倩2, 杨海君1, 颜智勇1, 谭菊2,3(
), 舒倩2, 杨海君1, 颜智勇1, 谭菊2,3( )
)
收稿日期:2022-03-22
出版日期:2022-12-26
发布日期:2022-12-29
作者简介:牛鸿宇,男,硕士研究生,研究方向:污水生物强化技术;E-mail:基金资助:
NIU Hong-yu1( ), SHU Qian2, YANG Hai-jun1, YAN Zhi-yong1, TAN Ju2,3(
), SHU Qian2, YANG Hai-jun1, YAN Zhi-yong1, TAN Ju2,3( )
)
Received:2022-03-22
Published:2022-12-26
Online:2022-12-29
摘要:
为获得更丰富的十二烷基硫酸钠(sodium dodecyl sulfate,SDS)降解菌资源,从本实验室筛选保存的复合菌系SDS1中,分离出1株以SDS为唯一碳源生长的高效降解菌D2,结合形态学观察、生理生化特征及16S rRNA序列分析,结果显示D2为帕拉伯克霍尔德菌(Paraburkholderia tropica)。实验结果证实,在温度30℃、pH 7、盐度0.1%(W/W)、附加氮源硝酸钠+氯化铵的条件下培养48 h,D2对初始浓度1 200 mg/L的SDS降解率达到98.3%。采用离子色谱法、气相色谱法、液相色谱法对D2在SDS降解过程中的主要代谢产物进行检测与鉴定,初步推测出其对SDS的降解途径可能为SDS→硫酸盐+1-十二烷醇→十二烷醛→十二烷酸→乙酸→CO2+H2O。本研究可为含SDS表面活性剂废水的高效降解处理提供微生物资源。
牛鸿宇, 舒倩, 杨海君, 颜智勇, 谭菊. 一株十二烷基硫酸钠高效降解菌的分离鉴定、降解特性及代谢途径研究[J]. 生物技术通报, 2022, 38(12): 287-299.
NIU Hong-yu, SHU Qian, YANG Hai-jun, YAN Zhi-yong, TAN Ju. Isolation, Identification, Degradation Characteristics and Metabolic Pathway of an Efficient Sodium Dodecyl Sulfate-degrading Bacterium[J]. Biotechnology Bulletin, 2022, 38(12): 287-299.
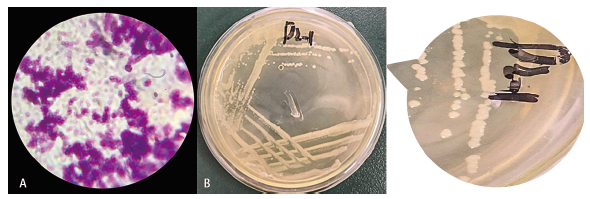
图3 菌株D2菌落特征 A:革兰氏染色形态图;B:菌株在LB固体培养基中的形态特征(平板划线)
Fig. 3 Colony characteristics of D2 strain A:Gram staining morphology. B:Morphological characteristics of strain in LB solid medium(streaked inoculation)
| 特征 Charateristics | 检测结果 Result |
|---|---|
| 利用柠檬酸盐 Use of citrate | + |
| 糖发酵 Glucose fermentation | + |
| 甲基红 Methyl red | - |
| 尿素水解 Urea hydrolysis | + |
| 产氨 Ammonia | + |
| 苯丙氨酸脱氢酶 Phenylalanine dehydrogenase | - |
| 吲哚 Indocyanine | - |
| 产硫化氢 Hydrogen sulfide | - |
表1 菌株D2的生理生化特征
Table 1 Physiological and biochemical characteristics of D2 strain
| 特征 Charateristics | 检测结果 Result |
|---|---|
| 利用柠檬酸盐 Use of citrate | + |
| 糖发酵 Glucose fermentation | + |
| 甲基红 Methyl red | - |
| 尿素水解 Urea hydrolysis | + |
| 产氨 Ammonia | + |
| 苯丙氨酸脱氢酶 Phenylalanine dehydrogenase | - |
| 吲哚 Indocyanine | - |
| 产硫化氢 Hydrogen sulfide | - |
| 物种 Species | NCBI登录号 NCBI accession no. | 相似度 Percent of similarity |
|---|---|---|
| Paraburkholderia tropica strain DSM 15359 | NR_028965.1 | 99.93% |
| Paraburkholderia bannensis strain NBRC 103871 | NR_113178.1 | 98.68% |
| Burkholderia humi Srinivasan strain JCM 18070 | NR_132708.1 | 98.53% |
表2 D2菌株16S rRNA基因序列分析鉴定
Table 2 Identification of D2 strain by 16S rDNA gene sequence analysis
| 物种 Species | NCBI登录号 NCBI accession no. | 相似度 Percent of similarity |
|---|---|---|
| Paraburkholderia tropica strain DSM 15359 | NR_028965.1 | 99.93% |
| Paraburkholderia bannensis strain NBRC 103871 | NR_113178.1 | 98.68% |
| Burkholderia humi Srinivasan strain JCM 18070 | NR_132708.1 | 98.53% |
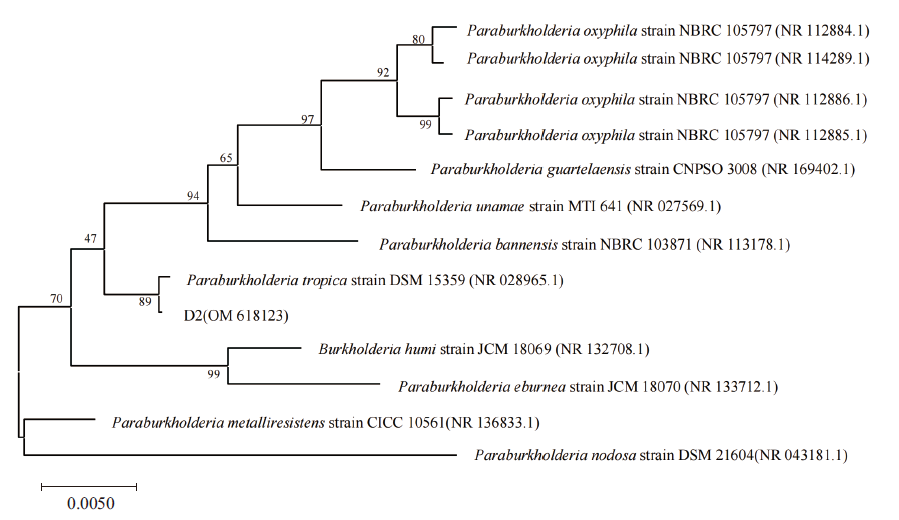
图4 菌株D2及其相关菌株系统发育树 Paraburkholderia oxyphila:嗜酸伯克霍尔德菌;Paraburkholderia guartelaensis:瓜拉伯克霍尔德菌;Paraburkholderia unamae:副伯克霍尔德菌;Paraburkholderia bannensis:假诺伯克霍尔德菌;Paraburkholderia tropica:帕拉伯克霍尔德菌;Burkholderia humi:胡氏伯克霍尔德菌;Paraburkholderia eburnea:象牙白伯克霍尔德菌;Paraburkholderia metalliresistens:抗金属伯克霍尔德菌;Paraburkholderia nodosa:根瘤伯克霍尔德菌
Fig. 4 Phylogenetic tree of strain D2 and its related strains

图5 环境因素对菌株D2降解SDS的影响 A:温度;B:pH;C:培养时间;D:NaCl浓度;E:氮源(1:硝酸钠;2:氯化铵;3:硝酸钠和氯化铵;4:脲素;5:蛋白胨)。采用ANOVA Duncan法进行分析,不同小写字母表示组间差异具有统计学意义(P<0.05)
Fig. 5 Effects of environmental factors on the degradation of SDS by strain D2 A:Temperature. B:pH. C:Incubation time. D:NaCl concentration. E:Nitrogen source(1:Sodium nitrate. 2:Ammonium chloride. 3:Sodium nitrate and ammonium chloride. 4:Urea. 5:Peptone). ANOVA Duncan method was used for analysis and different letters means significant difference at P<0.05
| 水平 Level | 因素Factors | ||||
|---|---|---|---|---|---|
| A 温度 Temperature/℃ | B pH | C 时间 Time/h | D 盐度NaCl concentration/% | ||
| 1 | 25 | 6 | 30 | 0.05 | |
| 2 | 30 | 7 | 42 | 0.1 | |
| 3 | 35 | 8 | 48 | 0.3 | |
表3 正交实验设计
Table 3 Orthogonal experimental design
| 水平 Level | 因素Factors | ||||
|---|---|---|---|---|---|
| A 温度 Temperature/℃ | B pH | C 时间 Time/h | D 盐度NaCl concentration/% | ||
| 1 | 25 | 6 | 30 | 0.05 | |
| 2 | 30 | 7 | 42 | 0.1 | |
| 3 | 35 | 8 | 48 | 0.3 | |
| 实验号 Experiment No. | 因素 Factors | SDS降解率Degrada-tion rate of SDS/% | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 2 | 1 | 2 | 2 | 81.98 |
| 2 | 2 | 2 | 3 | 1 | 80.29 |
| 3 | 2 | 3 | 1 | 3 | 57.44 |
| 4 | 3 | 3 | 2 | 1 | 83.84 |
| 5 | 1 | 1 | 1 | 1 | 77.13 |
| 6 | 3 | 2 | 1 | 2 | 91.27 |
| 7 | 1 | 3 | 3 | 2 | 49.79 |
| 8 | 1 | 2 | 2 | 3 | 57.30 |
| 9 | 3 | 1 | 3 | 3 | 67.20 |
| K1 | 198.59 | 225.70 | 189.07 | 204.80 | T=646.24 |
| K2 | 253.54 | 233.02 | 215.91 | 224.90 | |
| K3 | 194.12 | 187.52 | 241.27 | 216.55 | |
| X1 | 66.20 | 75.23 | 63.02 | 68.27 | |
| X2 | 84.51 | 77.67 | 71.97 | 74.97 | |
| X3 | 64.71 | 62.51 | 80.42 | 72.18 | |
| R | 19.81 | 15.17 | 17.40 | 6.70 | |
表4 正交实验结果
Table 4 Orthogonal experimental results
| 实验号 Experiment No. | 因素 Factors | SDS降解率Degrada-tion rate of SDS/% | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | 2 | 1 | 2 | 2 | 81.98 |
| 2 | 2 | 2 | 3 | 1 | 80.29 |
| 3 | 2 | 3 | 1 | 3 | 57.44 |
| 4 | 3 | 3 | 2 | 1 | 83.84 |
| 5 | 1 | 1 | 1 | 1 | 77.13 |
| 6 | 3 | 2 | 1 | 2 | 91.27 |
| 7 | 1 | 3 | 3 | 2 | 49.79 |
| 8 | 1 | 2 | 2 | 3 | 57.30 |
| 9 | 3 | 1 | 3 | 3 | 67.20 |
| K1 | 198.59 | 225.70 | 189.07 | 204.80 | T=646.24 |
| K2 | 253.54 | 233.02 | 215.91 | 224.90 | |
| K3 | 194.12 | 187.52 | 241.27 | 216.55 | |
| X1 | 66.20 | 75.23 | 63.02 | 68.27 | |
| X2 | 84.51 | 77.67 | 71.97 | 74.97 | |
| X3 | 64.71 | 62.51 | 80.42 | 72.18 | |
| R | 19.81 | 15.17 | 17.40 | 6.70 | |
| [1] | Morrison RT, Boyd RN. Organic Chemistry[M]. 4th ed. Massachusetts: Allyn and Bacon Press, 1983. |
| [2] |
Li SP, Su YT, Liu YD, et al. Preparation and characterization of cross-linked enzyme aggregates(CLEAs)of recombinant thermostable alkylsulfatase(SdsAP)from Pseudomonas sp. S9[J]. Process Biochem, 2016, 51(12):2084-2089.
doi: 10.1016/j.procbio.2016.09.013 URL |
| [3] |
Rebello S, Asok AK, Mundayoor S, et al. Surfactants:toxicity, remediation and green surfactants[J]. Environ Chem Lett, 2014, 12(2):275-287.
doi: 10.1007/s10311-014-0466-2 URL |
| [4] |
Lewis MA. Chronic toxicities of surfactants and detergent builders to algae:a review and risk assessment[J]. Ecotoxicol Environ Saf, 1990, 20(2):123-140.
doi: 10.1016/0147-6513(90)90052-7 URL |
| [5] |
Klebensberger J, Rui O, Fritz E, et al. Cell aggregation of Pseudomonas aeruginosa strain PAO1 as an energy-dependent stress response during growth with sodium dodecyl sulfate[J]. Arch Microbiol, 2006, 185(6):417-427.
pmid: 16775748 |
| [6] | Ambily P. Biodegradation of Anionic surfactant Sodium Dodecyl Sulphate(SDS)and analysis of its metabolic products[D]. Meghalaya: Mahatma Gandhi University, 2010 |
| [7] | Agrawal M. Performance of carbonized agricultural waste as a low-cost adsorbent for the removal of sodium dodecyl sulfate from aquatic environment[J]. Int J Sci Eng Res, 2013, 4(6):2909-2913. |
| [8] |
Rahmani Z, Samadi MT. Preparation of magnetic multi-walled carbon nanotubes to adsorb sodium dodecyl sulfate(SDS)[J]. Avicenna J Environ Health Eng, 2017, 4(1):61902.
doi: 10.5812/ajehe.61902 URL |
| [9] | 付凯, 刘志红, 耿超, 等. 改性粉煤灰吸附十二烷基硫酸钠的研究[J]. 矿产保护与利用, 2019, 39(1):90-94, 99. |
| Fu K, Liu ZH, Geng C, et al. Adsorption of sodium dodecyl sulfate by modified fly ash[J]. Conserv Util Miner Resour, 2019, 39(1):90-94, 99. | |
| [10] |
Romanelli MF, Moraes MCF, Villavicencio ALCH, et al. Evaluation of toxicity reduction of sodium dodecyl sulfate submitted to electron beam radiation[J]. Radiat Phys Chem, 2004, 71(1/2):411-413.
doi: 10.1016/j.radphyschem.2004.03.038 URL |
| [11] |
Flilissa A, Méléard P, Darchen A. Selective removal of dodecyl sulfate during electrolysis with aluminum electrodes[J]. Desalination Water Treat, 2013, 51(34/35/36):6719-6728.
doi: 10.1080/19443994.2013.769915 URL |
| [12] |
Aoudjit F, Cherifi O, Halliche D. Simultaneously efficient adsorption and photocatalytic degradation of sodium dodecyl sulfate surfactant by one-pot synthesized TiO2/layered double hydroxide materials[J]. Sep Sci Technol, 2019, 54(7):1095-1105.
doi: 10.1080/01496395.2018.1527352 |
| [13] |
Bhandari PS, Makwana BP, Gogate PR. Microwave and ultrasound assisted dual oxidant based degradation of sodium dodecyl sulfate:efficacy of irradiation approaches and oxidants[J]. J Water Process Eng, 2020, 36:101316.
doi: 10.1016/j.jwpe.2020.101316 URL |
| [14] |
Kıran I, Bektaş N, Cengiz Yatmaz H, et al. Photocatalytic Fenton oxidation of sodium dodecyl sulfate solution using iron-modified zeolite catalyst[J]. Desalination Water Treat, 2013, 51(28/29/30):5768-5775.
doi: 10.1080/19443994.2012.759517 URL |
| [15] |
Mondal B, Adak A, Datta P. Degradation of anionic surfactant in municipal wastewater by UV-H2O2:process optimization using response surface methodology[J]. J Photochem Photobiol A Chem, 2019, 375:237-243.
doi: 10.1016/j.jphotochem.2019.02.030 URL |
| [16] |
Arslan A, Topkaya E, Bingöl D, et al. Removal of anionic surfactant sodium dodecyl sulfate from aqueous solutions by O3/UV/H2O2 advanced oxidation process:process optimization with response surface methodology approach[J]. Sustain Environ Res, 2018, 28(2):65-71.
doi: 10.1016/j.serj.2017.11.002 URL |
| [17] |
Yasin M, Tauseef M, Zafar Z, et al. Plant-microbe synergism in floating treatment wetlands for the enhanced removal of sodium dodecyl sulphate from water[J]. Sustainability, 2021, 13(5):2883.
doi: 10.3390/su13052883 URL |
| [18] |
Othman AR, Yusof MT, Shukor MY. Biodegradation of sodium dodecyl sulphate(SDS)by Serratia marcescens strain DRY6[J]. Bioremed Sci Technol Res, 2019, 7(2):9-14.
doi: 10.54987/bstr.v7i2.486 URL |
| [19] |
Yalaoui-Guellal D, Fella-Temzi S, Djafri-Dib S, et al. The petroleum-degrading bacteria Alcaligenes aquatilis strain YGD 2906 as a potential source of lipopeptide biosurfactant[J]. Fuel, 2021, 285:119112.
doi: 10.1016/j.fuel.2020.119112 URL |
| [20] | Ibrahim AG, Elsalam HEA. Enhancement the biodegradation of sodium dodecyl sulfate by Pseudomonas aeruginosa and Pseudomonas otitidis isolated from waste water in Saudi Arabia[J]. Annu Res Rev Biol, 2018, 28:1-7. |
| [21] |
Zhu Q, Hu ZQ, Ruan MY. Characteristics of sulfate-reducing bacteria and organic bactericides and their potential to mitigate pollution caused by coal gangue acidification[J]. Environ Technol Innov, 2020, 20:101142.
doi: 10.1016/j.eti.2020.101142 URL |
| [22] |
Furmanczyk EM, Kaminski MA, Spolnik G, et al. Isolation and characterization of Pseudomonas spp. strains that efficiently decompose sodium dodecyl sulfate[J]. Front Microbiol, 2017, 8:1872.
doi: 10.3389/fmicb.2017.01872 pmid: 29163375 |
| [23] |
Furmanczyk EM, Kaminski MA, Lipinski L, et al. Pseudomonas laurylsulfatovorans sp. nov., sodium dodecyl sulfate degrading bacteria, isolated from the peaty soil of a wastewater treatment plant[J]. Syst Appl Microbiol, 2018, 41(4):348-354.
doi: S0723-2020(18)30141-3 pmid: 29752019 |
| [24] |
Furmanczyk EM, Lipinski L, Dziembowski A, et al. Genomic and functional characterization of environmental strains of SDS-degrading Pseudomonas spp., providing a source of new sulfatases[J]. Front Microbiol, 2018, 9:1795.
doi: 10.3389/fmicb.2018.01795 pmid: 30174655 |
| [25] |
Halmi MIE, Hussin WSW, Aqlima A, et al. Characterization of a sodium dodecyl sulphate-degrading Pseudomonas sp. strain DRY15 from Antarctic soil[J]. J Environ Biol, 2013, 34(6):1077-1082.
pmid: 24555340 |
| [26] |
Chen YW, Wang L, Dai FZ, et al. Biostimulants application for bacterial metabolic activity promotion and sodium dodecyl sulfate degradation under copper stress[J]. Chemosphere, 2019, 226:736-743.
doi: S0045-6535(19)30626-5 pmid: 30965244 |
| [27] | Panasia G, Philipp B. LaoABCR, a novel system for oxidation of long-chain alcohols derived from SDS and alkane degradation in Pseudomonas aeruginosa[J]. Appl Environ Microbiol, 2018, 84(13):e00626-e00618. |
| [28] |
Hosseini F, Malekzadeh F, Amirmozafari N, et al. Biodegradation of anionic surfactants by isolated bacteria from activated sludge[J]. Int J Environ Sci Technol, 2007, 4(1):127-132.
doi: 10.1007/BF03325970 URL |
| [29] |
Singh KL, Kumar A, Kumar A. Bacillus cereus capable of degrading SDS shows growth with a variety of detergents[J]. World J Microbiol Biotechnol, 1998, 14(5):777-779.
doi: 10.1023/A:1008883915003 URL |
| [30] |
Abboud MM, Khleifat KM, Batarseh M, et al. Different optimization conditions required for enhancing the biodegradation of linear alkylbenzosulfonate and sodium dodecyl sulfate surfactants by novel consortium of Acinetobacter calcoaceticus and Pantoea agglomerans[J]. Enzyme Microb Technol, 2007, 41(4):432-439.
doi: 10.1016/j.enzmictec.2007.03.011 URL |
| [31] | Masdor N, Shukor MSA, Khan A, et al. Isolation and characterization of a molybdenum-reducing and SDS-degrading Klebsiella oxytoca strain Aft-7 and its bioremediation application in the environment[J]. Biodiversitas, 2015, 16(2):238-246. |
| [32] | Adekanmbi AO, Usinola IM. Biodegradation of sodium dodecyl sulphate(SDS)by two bacteria isolated from wastewater generated by a detergent-manufacturing plant in Nigeria[J]. Jordan J Bio Sciences, 2017, 10(4):251-255. |
| [33] |
Chaturvedi V, Kumar A. Metabolism dependent chemotaxis of Pseudomonas aeruginosa N1 towards anionic detergent sodium dodecyl sulfate[J]. Indian J Microbiol, 2014, 54(2):134-138.
doi: 10.1007/s12088-013-0426-8 pmid: 25320412 |
| [34] |
Icgen B, Salik SB, Goksu L, et al. Higher alkyl sulfatase activity required by microbial inhabitants to remove anionic surfactants in the contaminated surface waters[J]. Water Sci Technol, 2017, 76(9/10):2357-2366.
doi: 10.2166/wst.2017.402 URL |
| [35] |
Rusnam M, Gusmanizar N. Characterization of the growth on SDS by Enterobacter sp. strain Neni-13[J]. J Biochem Microbiol Biotechnol, 2017, 5(2):28-32.
doi: 10.54987/jobimb.v5i2.374 URL |
| [36] | Rahman MF, Rusnam M, Gusmanizar N, et al. Molybdate-reducing and SDS-degrading Enterobacter sp. strain neni-13[J]. Nova Biotechnol Chimica, 2016, 15(2):166-181. |
| [37] | 颜丙花, 杨海君, 罗琳, 等. 十二烷基硫酸钠降解菌的分离、鉴定及其降解能力[J]. 化工环保, 2011, 31(2):110-113. |
| Yan BH, Yang HJ, Luo L, et al. Isolation, identification of SDS-degrading strain and its degrading capability[J]. Environ Prot Chem Ind, 2011, 31(2):110-113. | |
| [38] | 刘标, 喻孟元, 王震, 等. 耐镉邻苯二甲酸二辛酯降解菌的筛选及特性研究[J]. 农业资源与环境学报, 2021, 38(2):208-214. |
| Liu B, Yu MY, Wang Z, et al. Isolating and characteristics of a Cd-resistant microorganism used in the biodegradation of di-n-octyl phthalate[J]. J Agric Resour Environ, 2021, 38(2):208-214. | |
| [39] | 李莎, 崔鹤, 尹秀贞, 等. 离子色谱法测定富锂锰基正极材料中的硫酸根[J]. 化学研究与应用, 2021, 33(9):1844-1848. |
| Li S, Cui H, Yin XZ, et al. Determination of sulfate in lithium-riched manganese cathode material by ion chromatography[J]. Chem Res Appl, 2021, 33(9):1844-1848. | |
| [40] | 王发, 徐春燕, 王莉, 等. 毛细管气相色谱法测定聚桂醇原料中乙二醇、月桂醇、二甘醇的含量[J]. 药物分析杂志, 2013, 33(1):138-140. |
| Wang F, Xu CY, Wang L, et al. Capillary GC determination of ethylene glycol, lauryl alcohol and diethylene glycol in lauromacrogol[J]. Chin J Pharm Anal, 2013, 33(1):138-140. | |
| [41] | 王静静, 郭兵, 张庆建, 等. 十通阀双柱切换技术高灵敏检测汽油中甲缩醛和酯类[J]. 分析科学学报, 2019, 35(1):75-79. |
| Wang JJ, Guo B, Zhang QJ, et al. The technology of the ten-pass valve/double column switch for highly sensitive determination of methyl acetals and esters in gasoline[J]. J Anal Sci, 2019, 35(1):75-79. | |
| [42] | 杨丽峰. 洗涤剂中十二酸含量的高效液相色谱法测定[J]. 日用化学品科学, 2013, 36(7):21-23. |
| Yang LF. Determination of lauric acid by HPLC[J]. Deterg Cosmet, 2013, 36(7):21-23. | |
| [43] | 王丹, 吕冰, 周爽, 等. 高效液相色谱法检测淀粉及含淀粉食品中6种有机酸[J]. 中国食品添加剂, 2014(2):108-113. |
| Wang D, Lv B, Zhou S, et al. Determination of six organic acids in starchy food by high performance liquid chromatography[J]. China Food Addit, 2014(2):108-113. | |
| [44] |
Shukor MY, Husin WSW, Rahman MFA, et al. Isolation and characterization of an SDS-degrading Klebsiella oxytoca[J]. J Environ Biol, 2009, 30(1):129-134.
pmid: 20112874 |
| [45] |
Ambily PS, Jisha MS. Metabolic profile of sodium dodecyl sulphate(SDS)biodegradation by Pseudomonas aeruginosa(MTCC 10311)[J]. J Environ Biol, 2014, 35(5):827-831.
pmid: 25204054 |
| [46] | 张若木. 十二烷基硫酸钠降解菌株的筛选与特性研究[D]. 成都: 成都理工大学, 2016. |
| Zhang RM. Screening and characteristics research of SDS degrading strain[D]. Chengdu: Chengdu University of Technology, 2016. | |
| [47] |
Marchesi JR, Owen SA, White GF, et al. SDS-degrading bacteria attach to riverine sediment in response to the surfactant or its primary biodegradation product dodecan-1-ol[J]. Microbiol Read Engl, 1994, 140(Pt 11):2999-3006.
doi: 10.1099/13500872-140-11-2999 URL |
| [48] |
John EM, Rebello S, Asok AK, et al. Pseudomonas plecoglossicida S5, a novel nonpathogenic isolate for sodium dodecyl sulfate degradation[J]. Environ Chem Lett, 2015, 13(1):117-123.
doi: 10.1007/s10311-015-0493-7 URL |
| [1] | 饶紫环, 谢志雄. 一株Olivibacter jilunii 纤维素降解菌株的分离鉴定与降解能力分析[J]. 生物技术通报, 2023, 39(8): 283-290. |
| [2] | 游子娟, 陈汉林, 邓辅财. 鱼皮生物活性肽的提取及功能活性研究进展[J]. 生物技术通报, 2023, 39(7): 91-104. |
| [3] | 潘虎, 周子琼, 田云. 三株异菌脲高效降解菌株的筛选、鉴定及其降解特性分析[J]. 生物技术通报, 2023, 39(6): 298-307. |
| [4] | 车永梅, 郭艳苹, 刘广超, 叶青, 李雅华, 赵方贵, 刘新. 菌株C8和B4的分离鉴定及其耐盐促生效果和机制[J]. 生物技术通报, 2023, 39(5): 276-285. |
| [5] | 王凤婷, 王岩, 孙颖, 崔文婧, 乔凯彬, 潘洪玉, 刘金亮. 耐盐碱土曲霉SYAT-1的分离鉴定及抑制植物病原真菌特性研究[J]. 生物技术通报, 2023, 39(2): 203-210. |
| [6] | 王亚军, 司运美. 除磷菌CP-7的筛选及其降解特性研究[J]. 生物技术通报, 2022, 38(7): 258-268. |
| [7] | 祖雪, 周瑚, 朱华珺, 任佐华, 刘二明. 枯草芽孢杆菌K-268的分离鉴定及对水稻稻瘟病的防病效果[J]. 生物技术通报, 2022, 38(6): 136-146. |
| [8] | 古丽加马力·艾萨, 邢军, 李安, 张瑞. 开菲尔粒中微生物对苯并(α)芘的非靶向代谢组学分析[J]. 生物技术通报, 2022, 38(5): 123-135. |
| [9] | 王春艳, 腊贵晓, 苏秀红, 李萌, 董诚明. 地黄不同时期内生促生细菌的筛选及其促生特性分析[J]. 生物技术通报, 2022, 38(4): 242-252. |
| [10] | 马青云, 江旭, 李情情, 宋金龙, 周义清, 阮志勇. 烟嘧磺隆降解菌Chryseobacterium sp. LAM-M5的分离、鉴定及其降解机理研究[J]. 生物技术通报, 2022, 38(2): 113-122. |
| [11] | 张功友, 王一涵, 郭敏, 张婷婷, 王兵, 刘红美. 重楼中一株产纤维素酶内生真菌的分离及鉴定[J]. 生物技术通报, 2022, 38(2): 95-104. |
| [12] | 崔欣雨, 李荣荣, 蔡瑞, 王妍, 郑猛虎, 徐春城. 苜蓿青贮中乳酸降解菌的分离、鉴定及降解性能研究[J]. 生物技术通报, 2021, 37(9): 58-67. |
| [13] | 汪颖, 陈永静, 孙庆业, 杨梦瑶, 吴盾. Inquilinus sp. P6-4菌株的生理生化特征及萘降解特性[J]. 生物技术通报, 2021, 37(6): 117-126. |
| [14] | 沈聪, 刘爽, 王春霞, 严雪梅, 代金霞. 盐池采油区污染土壤石油降解菌的筛选鉴定及其降解特性[J]. 生物技术通报, 2021, 37(6): 127-135. |
| [15] | 李瑾, 彭可为, 潘求一, 朱哲远, 彭迪. 解淀粉芽胞杆菌HR-2的分离鉴定及对水稻稻瘟病菌的拮抗效果[J]. 生物技术通报, 2021, 37(3): 27-34. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||