








生物技术通报 ›› 2023, Vol. 39 ›› Issue (10): 292-303.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0281
张傲洁1( ), 李青云1,2(
), 李青云1,2( ), 宋文红1, 颜少慧1, 唐爱星1,2, 刘幽燕1,2
), 宋文红1, 颜少慧1, 唐爱星1,2, 刘幽燕1,2
收稿日期:2023-03-27
出版日期:2023-10-26
发布日期:2023-11-28
通讯作者:
李青云,女,博士,讲师,研究方向:环境微生物学;E-mail: qyli@gxu.edu.cn作者简介:张傲洁,女,硕士研究生,研究方向:化学工程; E-mail: zhangaojieao@163.com
基金资助:
ZHANG Ao-jie1( ), LI Qing-yun1,2(
), LI Qing-yun1,2( ), SONG Wen-hong1, YAN Shao-hui1, TANG Ai-xing1,2, LIU You-yan1,2
), SONG Wen-hong1, YAN Shao-hui1, TANG Ai-xing1,2, LIU You-yan1,2
Received:2023-03-27
Published:2023-10-26
Online:2023-11-28
摘要:
粪产碱杆菌属对芳香族类物质表现出广泛的降解能力,本研究对1株粪产碱杆菌(Alcaligenes faecalis)JF101开展苯酚降解基因解析,并进一步挖掘其潜在功能。通过对菌株JF101全基因组测序、组装和功能注释,分析其降解苯酚的功能基因,并与5株近缘菌株开展比较基因组学研究。结果表明,JF101基因组大小为4 143 816 bp,GC含量为 57.44%,含有3 804个编码蛋白基因和55个tRNA、9个rRNA、5个sRNA,在COG、GO、KEGG数据库中分别注释到3 040、2 529、2 415个基因。鉴定了11个苯酚降解基因。比较基因组学表明,菌株JF101没有质粒,与同属的5株菌株有2 008个共有同源基因家族和5个特异基因家族。此外发现菌株JF101含有与相容性溶质转运相关的Trk、Kdp等基因,实验测定了其耐盐能力。通过对菌株JF101全基因组的解析,为进一步阐释其苯酚降解机制及发展实际工程应用奠定了基础。
张傲洁, 李青云, 宋文红, 颜少慧, 唐爱星, 刘幽燕. 基于苯酚降解的粪产碱杆菌Alcaligenes faecalis JF101的全基因组分析[J]. 生物技术通报, 2023, 39(10): 292-303.
ZHANG Ao-jie, LI Qing-yun, SONG Wen-hong, YAN Shao-hui, TANG Ai-xing, LIU You-yan. Whole Genome Sequencing Analysis of a Phenol-degrading Strain Alcaligenes faecalis JF101[J]. Biotechnology Bulletin, 2023, 39(10): 292-303.
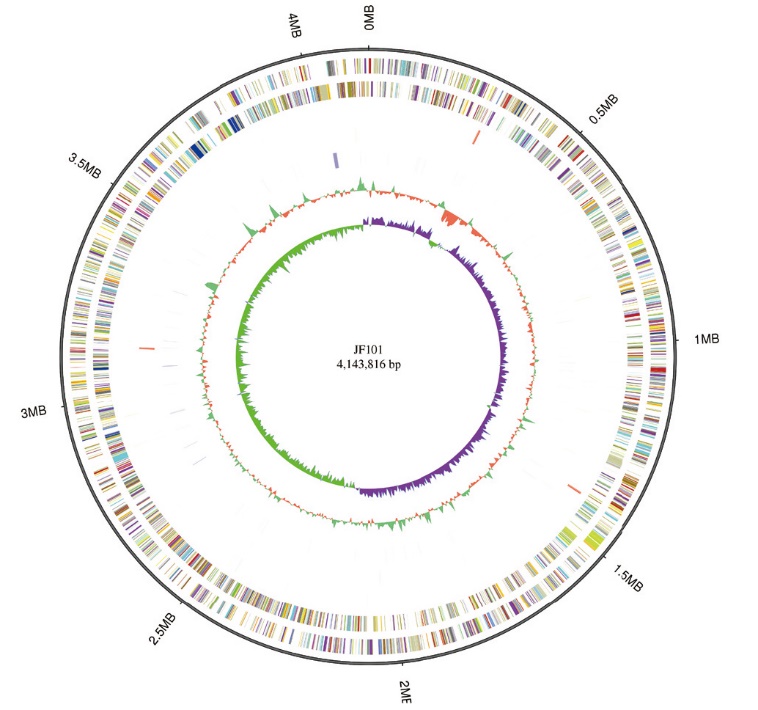
图1 粪产碱杆菌JF101基因组 最外面一圈为基因组大小;第二圈和第三圈分别为正链基因和负链基因;第四圈和五圈为正链ncRNA和反链ncRNA;第六圈为重复序列;第七圈为 GC 含量;最内圈是 GC-SKEW
Fig. 1 Circular representation of Alcaligenes faecalis JF101 genome From the outside to the inside, the circle chart shows the genome size, forward strand gene, reverse strand gene, forward strand ncRNA, reverse strand ncRNA, repeat sequence, GC share, and GC offset
| Protein | Amino acid | Proposed function | Sequence similarrty | Identity similarity/% | Accession No |
|---|---|---|---|---|---|
| GL-1751 | 396 | Muconate cycloisomerase | catB, Alcaligenes faecalis strain ZD02 | 97.0 | ALO39907.1 |
| GL-1752 | 311 | Catechol 1,2-dioxygenase | catA, Alcaligenes faecalis strain ZD02 | 99.7 | ALO39908.1 |
| GL-1755 | 267 | 3-oxoadipate enol-lactonase | pcaD, Alcaligenes aquatilis strain QD168 | 98.5 | AYN20737.1 |
| GL-1756 | 91 | Muconolactone D-isomerase | catC, Alcaligenes faecalis strain ZD02 | 100 | ALO39912.1 |
| GL-1849 | 334 | Nif-specific regulatory protein | MOPR, Sediminispirochaeta smaragdinae DSM 11293 | 47.9 | ADK82111.1 |
| GL-1850 | 82 | Phenol hydroxylase P0 protein | dmpK, Alcaligenes faecalis strain JQ135 | 97.6 | ASR89481.1 |
| GL-1851 | 332 | Phenol hydroxylase P1 protein | dmpL, Alcaligenes faecalis strain ZD02 | 99.1 | ALO39961.1 |
| GL-1852 | 90 | Phenol hydroxylase P2 protein | dmpM, Alcaligenes faecalis strain ZD02 | 100 | ALO39962.1 |
| GL-1853 | 498 | Phenol hydroxylase P3 protein | dmpN, Psychrobacter sp. P2G3 | 86.7 | AMN49709.1 |
| GL-1854 | 120 | Phenol hydroxylase P4 protein | dmpO, Alcaligenes faecalis strain ZD02 | 98.3 | ALO39964.1 |
| GL-1855 | 353 | Phenol hydroxylase P5 protein | dmpP, Alcaligenes faecalis strain ZD02 | 98.9 | ALO39965.1 |
表1 粪产碱杆菌JF101苯酚降解相关基因与其他细菌同源基因的同源性比较
Table 1 Homology comparison of phenol degradation-related genes of A. faecalis JF101 and homologous genes in other bacteria
| Protein | Amino acid | Proposed function | Sequence similarrty | Identity similarity/% | Accession No |
|---|---|---|---|---|---|
| GL-1751 | 396 | Muconate cycloisomerase | catB, Alcaligenes faecalis strain ZD02 | 97.0 | ALO39907.1 |
| GL-1752 | 311 | Catechol 1,2-dioxygenase | catA, Alcaligenes faecalis strain ZD02 | 99.7 | ALO39908.1 |
| GL-1755 | 267 | 3-oxoadipate enol-lactonase | pcaD, Alcaligenes aquatilis strain QD168 | 98.5 | AYN20737.1 |
| GL-1756 | 91 | Muconolactone D-isomerase | catC, Alcaligenes faecalis strain ZD02 | 100 | ALO39912.1 |
| GL-1849 | 334 | Nif-specific regulatory protein | MOPR, Sediminispirochaeta smaragdinae DSM 11293 | 47.9 | ADK82111.1 |
| GL-1850 | 82 | Phenol hydroxylase P0 protein | dmpK, Alcaligenes faecalis strain JQ135 | 97.6 | ASR89481.1 |
| GL-1851 | 332 | Phenol hydroxylase P1 protein | dmpL, Alcaligenes faecalis strain ZD02 | 99.1 | ALO39961.1 |
| GL-1852 | 90 | Phenol hydroxylase P2 protein | dmpM, Alcaligenes faecalis strain ZD02 | 100 | ALO39962.1 |
| GL-1853 | 498 | Phenol hydroxylase P3 protein | dmpN, Psychrobacter sp. P2G3 | 86.7 | AMN49709.1 |
| GL-1854 | 120 | Phenol hydroxylase P4 protein | dmpO, Alcaligenes faecalis strain ZD02 | 98.3 | ALO39964.1 |
| GL-1855 | 353 | Phenol hydroxylase P5 protein | dmpP, Alcaligenes faecalis strain ZD02 | 98.9 | ALO39965.1 |
| 菌株 Strain | 基因组大小 Genome size/Mb | 编码蛋白 Protein coding | rRNA数量 rRNA genes | tRNA数量 tRNA genes | GC含量 GC/% | 质粒数 Number of plasmids |
|---|---|---|---|---|---|---|
| MUB14 | 4.35 | 3973 | 9 | 58 | 56.74 | 2(0.04 Mb, 0.06 Mb) |
| P156 | 4.04 | 3616 | 9 | 57 | 56.7 | 0 |
| ZD02 | 4.23 | 3752 | 9 | 57 | 56.81 | 1(0.01 Mb) |
| c16 | 4.30 | 3847 | 9 | 57 | 56.3 | 0 |
| ASM96730v2 | 4.23 | 3770 | 9 | 57 | 56.8 | 1(0.01 Mb) |
| JF101 | 4.14 | 3804 | 9 | 55 | 57.44 | 0 |
表2 粪产碱杆菌基因组的基本特征
Table 2 Basic characteristics of the genome of A. faecalis
| 菌株 Strain | 基因组大小 Genome size/Mb | 编码蛋白 Protein coding | rRNA数量 rRNA genes | tRNA数量 tRNA genes | GC含量 GC/% | 质粒数 Number of plasmids |
|---|---|---|---|---|---|---|
| MUB14 | 4.35 | 3973 | 9 | 58 | 56.74 | 2(0.04 Mb, 0.06 Mb) |
| P156 | 4.04 | 3616 | 9 | 57 | 56.7 | 0 |
| ZD02 | 4.23 | 3752 | 9 | 57 | 56.81 | 1(0.01 Mb) |
| c16 | 4.30 | 3847 | 9 | 57 | 56.3 | 0 |
| ASM96730v2 | 4.23 | 3770 | 9 | 57 | 56.8 | 1(0.01 Mb) |
| JF101 | 4.14 | 3804 | 9 | 55 | 57.44 | 0 |
| 相容性溶质转运相关基因 Compatible solutes transport genes | 基因名称 Name | 基因位置 Locus tag | 基因长度 Length/bp | 基因描述 Description |
|---|---|---|---|---|
| K+ transport system | Trk system Kdp system | GL-1953、GL-1954 GL-1574、GL-1575、GL-1576 | 455、217 568、237、687 | Trk系统钾摄取蛋白 K+转运ATP酶A、B、C链 |
| Proline and betaine transport | ProP system | GL-0834、GL-1501、GL-2239、GL-2295、GL-3438、GL-3579 | 452、436、565、423、425、510 | MFS转运体,MHS家族,脯氨酸/甜菜碱转运体 |
| Choline/glycine/proline betaine transport genes | betT、betS | GL-2836 | 706 | 胆碱/甘氨酸/脯氨酸甜菜碱转运蛋白 |
表3 菌株JF101与相容性溶质转运相关的耐盐基因
Table 3 Salt-tolerant genes related to compatible solute transport in strain JF101
| 相容性溶质转运相关基因 Compatible solutes transport genes | 基因名称 Name | 基因位置 Locus tag | 基因长度 Length/bp | 基因描述 Description |
|---|---|---|---|---|
| K+ transport system | Trk system Kdp system | GL-1953、GL-1954 GL-1574、GL-1575、GL-1576 | 455、217 568、237、687 | Trk系统钾摄取蛋白 K+转运ATP酶A、B、C链 |
| Proline and betaine transport | ProP system | GL-0834、GL-1501、GL-2239、GL-2295、GL-3438、GL-3579 | 452、436、565、423、425、510 | MFS转运体,MHS家族,脯氨酸/甜菜碱转运体 |
| Choline/glycine/proline betaine transport genes | betT、betS | GL-2836 | 706 | 胆碱/甘氨酸/脯氨酸甜菜碱转运蛋白 |
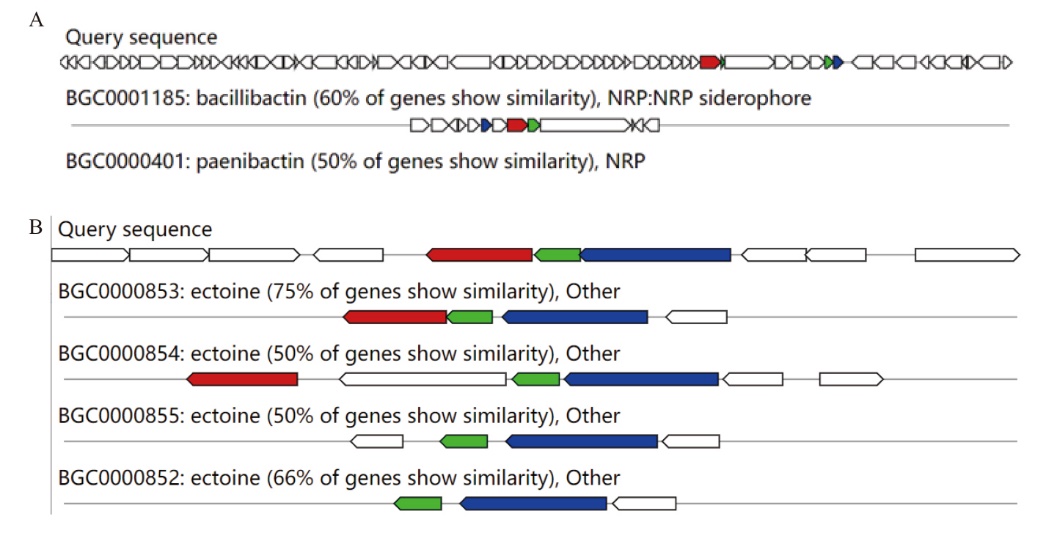
图7 粪产碱杆菌JF101次级代谢产物合成基因簇的序列比较 A:细菌素基因簇,B:萜类合成基因簇
Fig. 7 Sequence comparison of the JF101 secondary metabolite synthesis gene cluster in A. faecalis JF101 A: Bacteriocin gene cluste. B: Terpene synthesis gene cluste
| [1] |
Pazarlioğlu NK, Telefoncu A. Biodegradation of phenol by Pseudomonas putida immobilized on activated pumice particles[J]. Process Biochem, 2005, 40(5): 1807-1814.
doi: 10.1016/j.procbio.2004.06.043 URL |
| [2] |
Amor L, Eiroa M, Kennes C, et al. Phenol biodegradation and its effect on the nitrification process[J]. Water Res, 2005, 39(13): 2915-2920.
pmid: 15998531 |
| [3] |
Li XY, Cui YH, Feng YJ, et al. Reaction pathways and mechanisms of the electrochemical degradation of phenol on different electrodes[J]. Water Res, 2005, 39(10): 1972-1981.
doi: 10.1016/j.watres.2005.02.021 URL |
| [4] |
Liu L, Si L, Yang JH, et al. Biodegradation and process optimization of phenol and formaldehyde by Aspergillus nomius SGFA1[J]. Int Biodeterior Biodegrad, 2023, 182: 105630.
doi: 10.1016/j.ibiod.2023.105630 URL |
| [5] |
Saravanan P, Pakshirajan K, Saha P. Growth kinetics of an indigenous mixed microbial consortium during phenol degradation in a batch reactor[J]. Bioresour Technol, 2008, 99(1): 205-209.
doi: 10.1016/j.biortech.2006.11.045 URL |
| [6] |
Wang CC, Lee CM, Lu CJ, et al. Biodegradation of 2, 4, 6-trichlorophenol in the presence of primary substrate by immobilized pure culture bacteria[J]. Chemosphere, 2000, 41(12): 1873-1879.
pmid: 11061309 |
| [7] |
Prieto MB, Hidalgo A, Rodríguez-Fernández C, et al. Biodegradation of phenol in synthetic and industrial wastewater by Rhodococcus erythropolis UPV-1 immobilized in an air-stirred reactor with clarifier[J]. Appl Microbiol Biotechnol, 2002, 58(6): 853-859.
pmid: 12021809 |
| [8] |
Santos VL, Linardi VR. Biodegradation of phenol by a filamentous fungi isolated from industrial effluents—identification and degradation potential[J]. Process Biochem, 2004, 39(8): 1001-1006.
doi: 10.1016/S0032-9592(03)00201-2 URL |
| [9] |
Arutchelvan V, Kanakasabai V, Elangovan R, et al. Kinetics of high strength phenol degradation using Bacillus brevis[J]. J Hazard Mater, 2006, 129(1/2/3): 216-222.
doi: 10.1016/j.jhazmat.2005.08.040 URL |
| [10] |
Jiang Y, Wen JP, Bai J, et al. Biodegradation of phenol at high initial concentration by Alcaligenes faecalis[J]. J Hazard Mater, 2007, 147(1/2): 672-676.
doi: 10.1016/j.jhazmat.2007.05.031 URL |
| [11] |
Rehfuss M, Urban J. Alcaligenes faecalis subsp. phenolicus subsp. nov. a phenol-degrading, denitrifying bacterium isolated from a graywater bioprocessor[J]. Syst Appl Microbiol, 2005, 28(5): 421-429.
pmid: 16094869 |
| [12] |
Kumar A, Bhunia B, Dasgupta D, et al. Optimization of culture condition for growth and phenol degradation by Alcaligenes faecalisJF339228 using Taguchi Methodology[J]. Desalin Water Treat, 2013, 51(16/17/18): 3153-3163.
doi: 10.1080/19443994.2012.749021 URL |
| [13] |
Deveryshetty J, Phale PS. Biodegradation of phenanthrene by Alcaligenes sp. strain PPH: partial purification and characterization of 1-hydroxy-2-naphthoic acid hydroxylase[J]. FEMS Microbiol Lett, 2010, 311(1): 93-101.
doi: 10.1111/j.1574-6968.2010.02079.x pmid: 20727010 |
| [14] |
Bharali P, Das S, Konwar BK, et al. Crude biosurfactant from thermophilic Alcaligenes faecalis: feasibility in petro-spill bioremediation[J]. Int Biodeterior Biodegrad, 2011, 65(5): 682-690.
doi: 10.1016/j.ibiod.2011.04.001 URL |
| [15] |
John RC, Essien JP, Akpan SB, et al. Polycyclic aromatic hydrocarbon-degrading bacteria from aviation fuel spill site at Ibeno, Nigeria[J]. Bull Environ Contam Toxicol, 2012, 88(6): 1014-1019.
doi: 10.1007/s00128-012-0598-7 URL |
| [16] |
Shah PD, Dave SR, Rao MS. Enzymatic degradation of textile dye Reactive Orange 13 by newly isolated bacterial strain Alcaligenes faecalis PMS-1[J]. Int Biodeterior Biodegrad, 2012, 69: 41-50.
doi: 10.1016/j.ibiod.2012.01.002 URL |
| [17] |
Siripattanakul S, Wirojanagud W, McEvoy J, et al. Atrazine degradation by stable mixed cultures enriched from agricultural soil and their characterization[J]. J Appl Microbiol, 2009, 106(3): 986-992.
doi: 10.1111/j.1365-2672.2008.04075.x pmid: 19191954 |
| [18] | Kong LF, Zhu SY, Zhu LS, et al. Biodegradation of organochlorine pesticide endosulfan by bacterial strain Alcaligenes faecalis JBW4[J]. J Environ Sci(China), 2013, 25(11): 2257-2264. |
| [19] |
Pandeeti EVP, Siddavattam D. Purification and characterization of catechol 1, 2-dioxygenase from Acinetobacter sp. DS002 and cloning, sequencing of partial catA gene[J]. Indian J Microbiol, 2011, 51(3): 312-318.
doi: 10.1007/s12088-011-0123-4 URL |
| [20] |
Tuan NN, Hsieh HC, Lin YW, et al. Analysis of bacterial degradation pathways for long-chain alkylphenols involving phenol hydroxylase, alkylphenol monooxygenase and catechol dioxygenase genes[J]. Bioresour Technol, 2011, 102(5): 4232-4240.
doi: 10.1016/j.biortech.2010.12.067 URL |
| [21] | 陈健峰, 徐天虹, 梁谏婷, 等. 2, 4-二氯苯酚羟化酶在大肠杆菌中的表达及靛蓝的生物合成[J]. 药物生物技术, 2015, 22(6): 476-479. |
| Chen JF, Xu TH, Liang JT, et al. Cloning and expression of 2, 4-dichlorophenol hydroxylase and indigo biosynthesis in Escherichia coli[J]. Pharm Biotechnol, 2015, 22(6): 476-479. | |
| [22] |
Zhou WG, Guo WB, Zhou HB, et al. Phenol degradation by Sulfobacillus acidophilus TPY via the meta-pathway[J]. Microbiol Res, 2016, 190: 37-45.
doi: 10.1016/j.micres.2016.05.005 URL |
| [23] |
Trivedi VD, Jangir PK, Sharma R, et al. Erratum: insights into functional and evolutionary analysis of carbaryl metabolic pathway from Pseudomonas sp. strain C5pp[J]. Sci Rep, 2017, 7: 40899.
doi: 10.1038/srep40899 pmid: 28358360 |
| [24] |
Silva CC, Hayden H, Sawbridge T, et al. Phylogenetic and functional diversity of metagenomic libraries of phenol degrading sludge from petroleum refinery wastewater treatment system[J]. AMB Express, 2012, 2(1): 18.
doi: 10.1186/2191-0855-2-18 pmid: 22452812 |
| [25] |
Shingler V, Powlowski J, Marklund U. Nucleotide sequence and functional analysis of the complete phenol/3, 4-dimethylphenol catabolic pathway of Pseudomonas sp. strain CF600[J]. J Bacteriol, 1992, 174(3): 711-724.
pmid: 1732207 |
| [26] |
Teramoto M, Ohnishi K, Harayama S, et al. An AraC/XylS family member at a high level in a hierarchy of regulators for phenol-metabolizing enzymes in Comamonas testosteroni R5[J]. J Bacteriol, 2002, 184(14): 3941-3946.
doi: 10.1128/JB.184.14.3941-3946.2002 pmid: 12081966 |
| [27] |
Powlowski J, Shingler V. In vitro analysis of polypeptide requirements of multicomponent phenol hydroxylase from Pseudomonas sp. strain CF600[J]. J Bacteriol, 1990, 172(12): 6834-6840.
pmid: 2254259 |
| [28] | 苏佳岐, 张红兵. 苯酚羟化酶基因的研究进展[J]. 产业与科技论坛, 2017, 16(14): 48-50. |
| Su JQ, Zhang HB. Research progress of phenol hydroxylase gene[J]. Ind Sci Tribune, 2017, 16(14): 48-50. | |
| [29] |
Lagesen K, Hallin P, Rødland EA, et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes[J]. Nucleic Acids Res, 2007, 35(9): 3100-3108.
doi: 10.1093/nar/gkm160 pmid: 17452365 |
| [30] |
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence[J]. Nucleic Acids Res, 1997, 25(5): 955-964.
doi: 10.1093/nar/25.5.955 pmid: 9023104 |
| [31] |
Nawrocki EP, Eddy SR. Infernal 1.1: 100-fold faster RNA homology searches[J]. Bioinformatics, 2013, 29(22): 2933-2935.
doi: 10.1093/bioinformatics/btt509 pmid: 24008419 |
| [32] |
Nawrocki EP, Burge SW, Bateman A, et al. Rfam 12.0: updates to the RNA families database[J]. Nucleic Acids Res, 2015, 43(Database issue): D130-D137.
doi: 10.1093/nar/gku1063 URL |
| [33] |
Benson G. Tandem repeats finder: a program to analyze DNA sequences[J]. Nucleic Acids Res, 1999, 27(2): 573-580.
doi: 10.1093/nar/27.2.573 pmid: 9862982 |
| [34] |
Nandi T, Ong C, Singh AP, et al. A genomic survey of positive selection in Burkholderia pseudomallei provides insights into the evolution of accidental virulence[J]. PLoS Pathog, 2010, 6(4): e1000845.
doi: 10.1371/journal.ppat.1000845 URL |
| [35] |
Konuk HB, Ergüden B. Phenolic-OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity[J]. Folia Microbiol, 2020, 65(4): 775-783.
doi: 10.1007/s12223-020-00787-4 |
| [36] |
Park KH, Kim S, Lee SJ, et al. Tetrameric architecture of an active phenol-bound form of the AAA+ transcriptional regulator DmpR[J]. Nat Commun, 2020, 11(1): 2728.
doi: 10.1038/s41467-020-16562-5 |
| [37] |
Suvorova IA, Gelfand MS. Comparative genomic analysis of the regulation of aromatic metabolism in betaproteobacteria[J]. Front Microbiol, 2019, 10: 642.
doi: 10.3389/fmicb.2019.00642 pmid: 30984152 |
| [38] |
Yu HY, Peng ZX, Zhan YH, et al. Novel regulator MphX represses activation of phenol hydroxylase genes caused by a XylR/DmpR-type regulator MphR in Acinetobacter calcoaceticus[J]. PLoS One, 2011, 6(3): e17350.
doi: 10.1371/journal.pone.0017350 URL |
| [39] | 彭子欣, 于海英, 战嵛华, 等. 苯酚羟化酶的功能与调控研究进展[J]. 中国农业科技导报, 2010, 12(5): 36-41. |
| Peng ZX, Yu HY, Zhan YH, et al. Advances in function and regulation of phenol hydroxylase[J]. J Agric Sci Technol, 2010, 12(5): 36-41. | |
| [40] |
Arai H, Akahira S, Ohishi T, et al. Adaptation of Comamonas testosteroni TA441 to utilization of phenol by spontaneous mutation of the gene for a trans-acting factor[J]. Mol Microbiol, 1999, 33(6): 1132-1140.
pmid: 10510228 |
| [41] |
Qu YY, Shi SN, Zhou H, et al. Characterization of a novel phenol hydroxylase in indoles biotransformation from a strain Arthrobacter sp. W1[J]. PLoS One, 2012, 7(9): e44313.
doi: 10.1371/journal.pone.0044313 URL |
| [42] |
Sela I, Wolf YI, Koonin EV. Assessment of assumptions underlying models of prokaryotic pangenome evolution[J]. BMC Biology, 2021, 19(1): 27.
doi: 10.1186/s12915-021-00960-2 pmid: 33563283 |
| [43] | 卢源达, 陈玲, 杜云龙, 等. 不同禾本科作物中ZmWRKY79同源基因的鉴定与分析[J]. 西南农业学报, 2023, 36(3): 522-531. |
| Lu YD, Chen L, Du YL, et al. Identification and analysis of ZmWRKY79 homologous genes in different gramineal crops[J]. Southwest China J Agric Sci, 2023, 36(3): 522-531. | |
| [44] |
Majewski P, Majewska P, Gutowska A, et al. Molecular characterisation of clinical pandrug-resistant Alcaligenes faecalis strain MUB14[J]. Int J Antimicrob Agents, 2020, 55(6): 105939.
doi: 10.1016/j.ijantimicag.2020.105939 URL |
| [45] |
Hu CH, Zhao SX, Li KR, et al. Microbial degradation of nicotinamide by a strain Alcaligenes sp. P156[J]. Sci Rep, 2019, 9(1): 3647.
doi: 10.1038/s41598-019-40199-0 |
| [46] |
Ju SY, Zheng JS, Lin J, et al. The complete genome sequence of Alcaligenes faecalis ZD02, a novel potential bionematocide[J]. J Biotechnol, 2016, 218: 73-74.
doi: 10.1016/j.jbiotec.2015.12.001 URL |
| [47] |
Kong LF, Zhu SY, Zhu LS, et al. Colonization of Alcaligenes faecalis strain JBW4 in natural soils and its detoxification of endosulfan[J]. Appl Microbiol Biotechnol, 2014, 98(3): 1407-1416.
doi: 10.1007/s00253-013-5033-4 URL |
| [48] |
Liu YX, Wang Y, Li Y, et al. Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis C16[J]. Chin J Chem Eng, 2015, 23(5): 827-834.
doi: 10.1016/j.cjche.2014.04.005 URL |
| [49] |
Finks SS, Martiny JBH. Plasmid-encoded traits vary across environments[J]. mBio, 2023, 14(1): e0319122.
doi: 10.1128/mbio.03191-22 URL |
| [50] |
Alva VA, Peyton BM. Phenol and catechol biodegradation by the haloalkaliphile Halomonas campisalis: influence of pH and salinity[J]. Environ Sci Technol, 2003, 37(19): 4397-4402.
doi: 10.1021/es0341844 URL |
| [51] |
Li H, Meng FP, Duan WY, et al. Biodegradation of phenol in saline or hypersaline environments by bacteria: a review[J]. Ecotoxicol Environ Saf, 2019, 184: 109658.
doi: 10.1016/j.ecoenv.2019.109658 URL |
| [52] |
Tanudjaja E, Hoshi N, Yamamoto K, et al. Two Trk/Ktr/HKT-type potassium transporters, TrkG and TrkH, perform distinct functions in Escherichia coli K-12[J]. J Biol Chem, 2023, 299(2): 102846.
doi: 10.1016/j.jbc.2022.102846 URL |
| [53] |
Malek AA, Chen CL, Wargo MJ, et al. Roles of three transporters, CbcXWV, BetT1, and BetT3, in Pseudomonas aeruginosa choline uptake for catabolism[J]. J Bacteriol, 2011, 193(12): 3033-3041.
doi: 10.1128/JB.00160-11 pmid: 21478341 |
| [54] | Souii A, Gorrab A, Ouertani R, et al. Sustainable bioethanol production from enzymatically hydrolyzed second-generation Posidonia oceanica waste using stable Microbacterium metallidurans carbohydrate-active enzymes as biocatalysts[J]. Biomass Convers Biorefin, 2022: 1-20. |
| [55] |
Mei RW, Zhou M, Xu LN, et al. Characterization of a pH-tolerant strain Cobetia sp. SASS1 and its phenol degradation performance under salinity condition[J]. Front Microbiol, 2019, 10: 2034.
doi: 10.3389/fmicb.2019.02034 URL |
| [56] |
Gong Y, Ding P, Xu MJ, et al. Biodegradation of phenol by a halotolerant versatile yeast Candida tropicalis SDP-1 in wastewater and soil under high salinity conditions[J]. J Environ Manage, 2021, 289: 112525.
doi: 10.1016/j.jenvman.2021.112525 URL |
| [57] |
Zhang SS, An ZJ, Su XM, et al. Phenol degradation at high salinity by a resuscitated strain Pseudomonas sp. SAS26: kinetics and pathway[J]. J Environ Chem Eng, 2023, 11(4): 110182.
doi: 10.1016/j.jece.2023.110182 URL |
| [1] | 王腾辉, 葛雯冬, 罗雅方, 范震宇, 王玉书. 基于极端混合池(BSA)全基因组重测序的羽衣甘蓝白色叶基因定位[J]. 生物技术通报, 2023, 39(9): 176-182. |
| [2] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [3] | 郭少华, 毛会丽, 刘征权, 付美媛, 赵平原, 马文博, 李旭东, 关建义. 一株鱼源致病性嗜水气单胞菌XDMG的全基因组测序及比较基因组分析[J]. 生物技术通报, 2023, 39(8): 291-306. |
| [4] | 张志霞, 李天培, 曾虹, 朱稀贤, 杨天雄, 马斯楠, 黄磊. 冰冷杆菌PG-2的基因组测序及生物信息学分析[J]. 生物技术通报, 2023, 39(3): 290-300. |
| [5] | 和梦颖, 刘文彬, 林震鸣, 黎尔彤, 汪洁, 金小宝. 一株抗革兰阳性菌的戈登氏菌WA4-43全基因组测序与分析[J]. 生物技术通报, 2023, 39(2): 232-242. |
| [6] | 王帅, 吕鸿睿, 张昊, 吴占文, 肖翠红, 孙冬梅. 解磷菌PSB-R全基因组测序鉴定及其解磷特性分析[J]. 生物技术通报, 2023, 39(1): 274-283. |
| [7] | 张泽颖, 范清锋, 邓云峰, 韦廷舟, 周正富, 周建, 王劲, 江世杰. 一株高产脂肪酶菌株WCO-9全基因组测序及比较基因组分析[J]. 生物技术通报, 2022, 38(10): 216-225. |
| [8] | 薛清, 杜虹锐, 薛会英, 王译浩, 王暄, 李红梅. 苜蓿滑刃线虫线粒体基因组及其系统发育研究[J]. 生物技术通报, 2021, 37(7): 98-106. |
| [9] | 陈体强, 徐晓兰, 石林春, 钟礼义. 紫芝栽培品种‘武芝2号’(‘紫芝S2’)全基因组测序及分析[J]. 生物技术通报, 2021, 37(11): 42-56. |
| [10] | 郭鹤宝, 王星, 何山文, 张晓霞. 表型特征结合基因组分析鉴定不同菌落形态Bacillus velezensis ACCC 19742[J]. 生物技术通报, 2020, 36(2): 142-148. |
| [11] | 李晓凯 ,王贵 ,乔贤 ,范一星 ,张磊 ,马宇浩 ,聂瑞雪 ,王瑞军 ,何利兵 ,苏蕊. 全基因组测序在重要家畜上的研究进展[J]. 生物技术通报, 2018, 34(6): 11-21. |
| [12] | 向沙, 刘明学, 张格格, 罗浪, 魏红福,董发勤. 光电子响应微生物的筛选鉴定及生长代谢特征研究[J]. 生物技术通报, 2017, 33(4): 205-213. |
| [13] | 王兴文, 王加启, 赵圣国, 李发弟, 卜登攀. 未培养技术在瘤胃产甲烷菌群研究中的应用[J]. 生物技术通报, 2014, 0(6): 67-74. |
| [14] | . 环境保护及农业废物利用[J]. , 1996, 0(02): 104-109. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||