








生物技术通报 ›› 2023, Vol. 39 ›› Issue (12): 200-208.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0653
收稿日期:2023-07-10
出版日期:2023-12-26
发布日期:2024-01-11
通讯作者:
曹学丽,女,博士,教授,研究方向:植物资源的开发和利用;E-mail: caoxl@th.btbu.edu.cn;作者简介:韩潆仪,女,博士研究生,实验师,研究方向:生物分离分析及应用;E-mail: hanyi@btbu.edu.cn
基金资助:
HAN Ying-yi( ), LI Zhang-han, CAO Xue-li(
), LI Zhang-han, CAO Xue-li( ), PEI Hai-run(
), PEI Hai-run( )
)
Received:2023-07-10
Published:2023-12-26
Online:2024-01-11
摘要:
芍药苷是一种在传统草药芍药中发现的糖苷类化合物,对心脑血管系统有保护作用。然而,口服芍药苷的生物利用度较低,直接吸收的很少,极大地限制了其临床应用。已有研究表明,芍药苷的药理作用可能是由其代谢产物产生的,且主要在肠内菌中被代谢。本文研究了芍药苷转化酶G6046在大肠杆菌BL21 DE3中的诱导表达和纯化,并通过优化诱导条件提高酶的产量,发现在诱导表达的最佳条件(16℃,诱导24 h)下,菌体的产酶量可达到5.34 mg/g,产酶量提高51.69%。基于此诱导条件,本研究对G6046与芍药苷的反应条件(pH和温度)进行优化,发现G6046在pH 9.0、45℃时反应最快,酶活性最高,比酶活为14.56 U/mg。在此优化条件下扩大反应体系,将反应规模从G6046-底物(1/1, mg/mg)扩大到G6046-底物(1/20,mg/mg),转化产物的产量显著提升,为分离芍药苷转化产物奠定了基础。
韩潆仪, 李章涵, 曹学丽, 裴海闰. 芍药苷转化酶G6046的异源表达及酶活鉴定[J]. 生物技术通报, 2023, 39(12): 200-208.
HAN Ying-yi, LI Zhang-han, CAO Xue-li, PEI Hai-run. Heterologous Expression of Paeoniflorin Converting Enzyme G6046 and the Identification of Its Enzymatic Activities[J]. Biotechnology Bulletin, 2023, 39(12): 200-208.
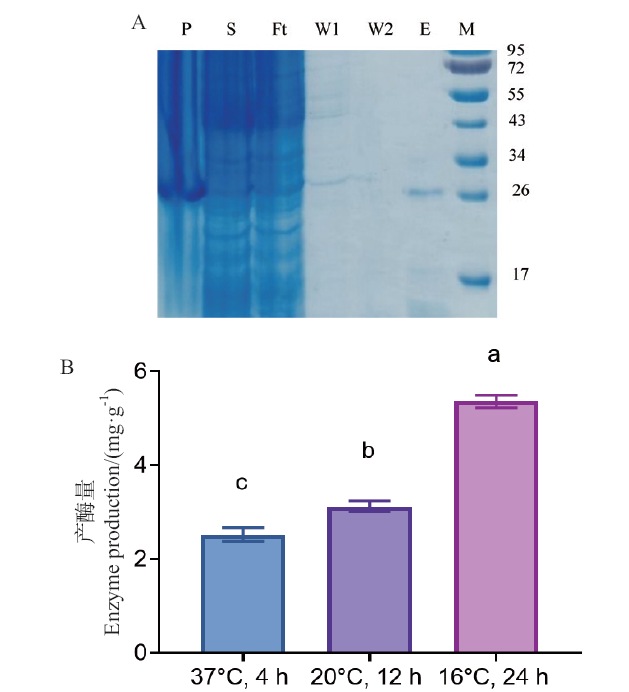
图1 G6046-NΔ82-22b表达及条件优化 A:G6046-NΔ82-22b表达纯化的SDS-PAGE电泳图(P:G6046-NΔ82-22b的沉淀;S:G6046-NΔ82-22b的上清液;Ft:G6046-NΔ82-22b流出液;W1和W2:缓冲液冲洗的样品;E:洗脱的样品;M:蛋白分子量标记物);B:不同诱导条件(温度、时间)下每克菌体产酶量,不同小写字母表示在P < 0.05水平差异显著
Fig. 1 Expressions of G6046-NΔ82-22b and condition optimization A: SDS-PAGE analysis of expression and purification of G6046-NΔ82-22b(P: the precipitation of G6046-NΔ82-22b; lane S: the supernatant of G6046-NΔ82-22b; lane Ft: the flow through of G6046-NΔ82-22b; W1 and W2: samples from the buffer wash; E: the eluted samples from the resin; M: molecular weight marker). B: Enzyme production per gram of bacterium under different induction conditions(temperature, time). Different lower letters indicate significant differences at P < 0.05 level

图2 芍药苷转化酶G6046的pH-酶活曲线 温度为25℃,反应时间为3 h,差异显著性分析结果显示pH 9和pH 7差异显著(P = 0.018 4),pH 9和pH 8差异显著(P = 0.040 4),其他没有显著差异性
Fig. 2 pH-enzyme activity curve of paeoniflorin converting enzyme G6046 Temperature:25℃;time:3 h; significant test results showed significant differences between pH 9 and pH 7(P = 0.018 4), pH 9 and pH 8(P = 0.040 4), and no significant differences among others

图3 芍药苷转化酶G6046在pH 9.0,不同温度条件下酶反应曲线
Fig. 3 Enzyme reaction curves of paeoniflorin converting enzyme G6046 at pH 9.0 and different temperatures A:15℃; B:25℃; C:35℃; D:45℃; E:55℃; F:65℃

图4 G6046在不同温度下反应曲线 A:G6046在不同温度下前3 h反应曲线,pH 9.0。B:G6046的温度-酶活曲线,pH 9.0,差异显著性分析结果显示45℃和55℃差异显著(P=0.017 0),45℃和65℃差异显著(P=0.001 6),其他没有显著差异性
Fig. 4 G6046 reaction curves at different temperatures A:G6046 reaction curves in the first 3 h at different temperatures,pH 9.0. B: Temperature-enzymatic activity curve of paeoniflorin converting enzyme G6046,pH 9.0,significant test results showed significant differences between 45℃ and 55℃(P=0.017 0), 45℃ and 65℃(P=0.001 6), and no significant differences among others

图5 芍药苷和不同条件下芍药苷转化反应后的HPLC色谱图 A:芍药苷标准品;B:pH 8.0, 25℃,酶-芍药苷 = 1∶10,mg/mg条件下酶反应96 h的液相色谱图;C:pH 9.0,25℃,酶-芍药苷 = 1∶10,mg/mg条件下酶反应48 h的液相色谱图;D:pH 9.0,45℃,酶-芍药苷=1∶10,mg/mg条件下酶反应28 h的液相色谱图
Fig. 5 HPLC analysis of paeoniflorin and paeoniflorin after conversion reaction under different conditions A:Standard of paeoniflorin;B: liquid analysis of enzyme reaction for 96 h at pH 8.0,and 25℃,enzyme-paeoniflorin = 1∶10,mg/mg;C:liquid analysis of enzyme reaction at pH 9.0 and 25℃,enzyme-paeoniflorin = 1∶10,mg/mg for 48 h;D:liquid analysisi of enzyme reaction for 28 h at pH 9.0 and 45℃,under the condition of enzyme-paeoniflorin = 1∶10,mg/mg
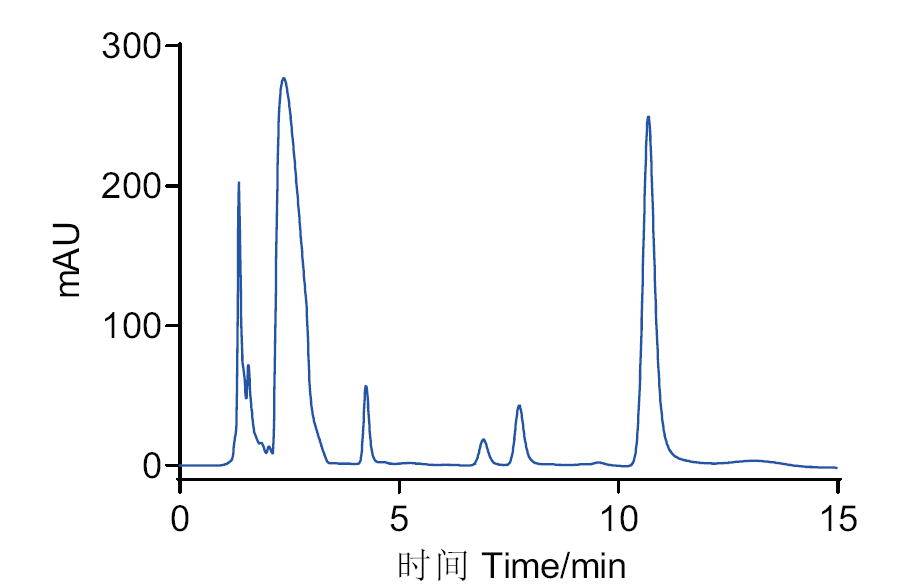
图6 G6046转化芍药苷36 h后HPLC检测图 反应体系:G6046-芍药苷(1∶20, mg/mg),反应条件: pH 9.0、45℃
Fig. 6 HPLC analysis of 36 h conversion of paeoniflorin by G6046 Reaction system:G6046-paeoniflorin(1∶20, mg/mg),reaction conditions: pH 9.0, 45℃

图7 G6046-芍药苷反应体系逐步放大示意图 A:原反应条件(pH 8.0, 室温)下芍药苷转化反应量,反应规模:1 mg芍药苷;B:优化反应条件(pH 9.0,45℃)下芍药苷转化反应量,反应规模:10 mg芍药苷加入1 mg G6046;C:优化反应条件(pH 9.0,45℃)下芍药苷转化反应量,反应规模:100 mg芍药苷加入5 mg G6046
Fig. 7 Schematic of G6046-paeoniflorin reaction system by step-by-step amplification A:The reaction volume of paeoniflorin conversion under the original reaction conditions(pH 8.0, room temperature),reaction scale:1 mg paeoniflorin. B:The reaction volume of paeoniflorin conversion under the optimized reaction conditions(pH 9.0,45℃), reaction scale:10 mg paeoniflorin added to 1 mg G6046. C:The reaction volume of paeoniflorin conversion under the optimized reaction conditions(pH 9.0,45℃), reaction scale:100 mg paeoniflorin was added to 5 mg of G6046
| [1] | 黄山君, 王瑞, 等. 硫磺熏制白芍的安全性评价初步研究[J]. 药学学报, 2012, 47(4): 486-491. |
| Huang SJ, Wang R, et al. Primary safety evaluation of sulfated Paeoniae Radix Alba[J]. Acta Pharm Sin, 2012, 47(4): 486-491. | |
| [2] |
Li YB, Sun YX, Ma XL, et al. Effects of Sini San used alone and in combination with fluoxetine on central and peripheral 5-HT levels in a rat model of depression[J]. J Tradit Chin Med, 2013, 33(5): 674-681.
pmid: 24660595 |
| [3] |
Zhu XX, Jing LL, Chen C, et al. Danzhi Xiaoyao San ameliorates depressive-like behavior by shifting toward serotonin via the downregulation of hippocampal indoleamine 2, 3-dioxygenase[J]. J Ethnopharmacol, 2015, 160: 86-93.
doi: 10.1016/j.jep.2014.11.031 URL |
| [4] | Ma X, Zhao YL, Zhu Y, et al. Paeonia lactiflora Pall. protects against ANIT-induced cholestasis by activating Nrf2 via PI3K/Akt signaling pathway[J]. Drug Des Devel Ther, 2015, 9: 5061-5074. |
| [5] | Wang JS, Huang Y, Zhang SP, et al. A protective role of paeoniflorin in fluctuant hyperglycemia-induced vascular endothelial injuries through antioxidative and anti-inflammatory effects and reduction of PKC β 1[J]. Oxid Med Cell Longev, 2019, 2019: 5647219. |
| [6] | 张燕丽, 田园, 付起凤, 等. 白芍的化学成分和药理作用研究进展[J]. 中医药学报, 2021, 49(2): 104-109. |
| Zhang YL, Tian Y, Fu QF, et al. Research progress of chemical constituents and pharmacological action of Paeonia tactilora pall[J]. Acta Chin Med Pharmacol, 2021, 49(2): 104-109. | |
| [7] |
Cheng J, Chen M, Wan HQ, et al. Paeoniflorin exerts antidepressant-like effects through enhancing neuronal FGF-2 by microglial inactivation[J]. J Ethnopharmacol, 2021, 274: 114046.
doi: 10.1016/j.jep.2021.114046 URL |
| [8] |
Wang K, Zhu L, Zhu X, et al. Protective effect of paeoniflorin on Aβ25-35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction[J]. Cell Mol Neurobiol, 2014, 34(2): 227-234.
doi: 10.1007/s10571-013-0006-9 pmid: 24263411 |
| [9] |
Gu XS, Wang F, Zhang CY, et al. Neuroprotective effects of paeoniflorin on 6-OHDA-lesioned rat model of Parkinson's disease[J]. Neurochem Res, 2016, 41(11): 2923-2936.
doi: 10.1007/s11064-016-2011-0 URL |
| [10] |
Zheng MZ, Liu CM, Fan YJ, et al. Neuroprotection by Paeoniflorin in the MPTP mouse model of Parkinson's disease[J]. Neuropharmacology, 2017, 116: 412-420.
doi: S0028-3908(17)30009-6 pmid: 28093210 |
| [11] |
Nizamutdinova IT, Jin YC, Kim JS, et al. Paeonol and paeoniflorin, the main active principles of Paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats[J]. Planta Med, 2008, 74(1): 14-18.
doi: 10.1055/s-2007-993775 pmid: 18203054 |
| [12] |
Chen C, Du P, Wang JJ. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways[J]. Mol Med Rep, 2015, 12(3): 3937-3943.
doi: 10.3892/mmr.2015.3870 pmid: 26035555 |
| [13] |
Li P, Li ZH. Neuroprotective effect of paeoniflorin on H2O2-induced apoptosis in PC12 cells by modulation of reactive oxygen species and the inflammatory response[J]. Exp Ther Med, 2015, 9(5): 1768-1772.
doi: 10.3892/etm.2015.2360 URL |
| [14] |
Yu JB, Zhao ZX, Peng R, et al. Gut microbiota-based pharmacokinetics and the antidepressant mechanism of paeoniflorin[J]. Front Pharmacol, 2019, 10: 268.
doi: 10.3389/fphar.2019.00268 URL |
| [15] |
Lin YT, Huang WS, Tsai HY, et al. In vivo microdialysis and in vitro HPLC analysis of the impact of paeoniflorin on the monoamine levels and their metabolites in the rodent brain[J]. BioMedicine, 2019, 9(2): 11.
doi: 10.1051/bmdcn/2019090211 URL |
| [16] |
Zhao ZX, Fu J, Ma SR, et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin[J]. Theranostics, 2018, 8(21): 5945-5959.
doi: 10.7150/thno.28068 URL |
| [17] | 刘玉峰, 孙珊珊, 等. 赤芍中芍药苷和芍药内酯苷的代谢及药动学研究进展[J]. 辽宁大学学报: 自然科学版, 2018, 45(4): 296-303. |
| Liu YF, Sun SS, et al. Research progress on the metabolism and pharmacokinetics of paeoniflorin and albiflorin in Radix paeoniae Rubra[J]. J Liaoning Univ Nat Sci Ed, 2018, 45(4): 296-303. | |
| [18] |
Heikal OA, Akao T, Takeda S, et al. Pharmacokinetic study of paeonimetabolin I, a major metabolite of paeoniflorin from paeony roots[J]. Biol Pharm Bull, 1997, 20(5): 517-521.
pmid: 9178932 |
| [19] |
Akao T, Shu YZ, Matsuda Y, et al. Metabolism of paeoniflorin and related compounds by human intestinal bacteria. IV. Formation and structures of adducts of a metabolic intermediate with sulfhydryl compounds by Lactobacillus brevis[J]. Chem Pharm Bull, 1988, 36(8): 3043-3048.
doi: 10.1248/cpb.36.3043 URL |
| [20] |
He JX, Goto E, Akao T, et al. Interaction between Shaoyao-Gancao-Tang and a laxative with respect to alteration of paeoniflorin metabolism by intestinal bacteria in rats[J]. Phytomedicine, 2007, 14(7-8): 452-459.
doi: 10.1016/j.phymed.2006.09.014 URL |
| [21] |
Takeda S, Isono T, et al. Absorption and excretion of paeoniflorin in rats[J]. J Pharm Pharmacol, 1995, 47(12A): 1036-1040.
pmid: 8932691 |
| [22] |
Gao H, Zhang LS, Song JN, et al. Absorption and biotransformation of four compounds in the Guizhi Decoction in the gastrointestinal tracts of rats[J]. J Tradit Chin Med, 2019, 39(3): 332-338.
pmid: 32186005 |
| [23] | Zhan JX, Guo HZ, Dai JG, et al. Microbial transformations of artemisinin by Cunninghamella echinulata and Aspergillus niger[J]. Tetrahedron Lett, 2002, 43(25): 4519-4521. |
| [24] |
Gonçalves MD, Tomiotto-Pellissier F, de Matos RLN, et al. Recent advances in biotransformation by Cunninghamella species[J]. Curr Drug Metab, 2021, 22(13): 1035-1064.
doi: 10.2174/1389200222666211126100023 pmid: 34825868 |
| [25] | 刘鑫鑫, 马骁驰, 霍长虹, 等. 芍药苷和芍药内酯苷的微生物转化[J]. 中国中药杂志, 2010, 35(7): 872-875. |
| Liu XX, Ma XC, Huo CH, et al. Microbiological transformation of paeoniflorin and albiflorin[J]. China J Chin Mater Med, 2010, 35(7): 872-875. | |
| [26] | 施敏, 马晓彤, 等. 基于短刺小克银汉霉的芍药苷转化芍药内酯苷研究[J]. 中国医药生物技术, 2018, 13(2): 178-184. |
| Shi M, Ma XT, et al. Study on the transformation of paeoniflorin from paeoniflorin to paeoniflorin based on Hyphantria brevispi-nosa[J]. Chin Med Biotechnol, 2018, 13(2): 178-184. | |
| [27] |
Ye YH, Pei HR, Cao XL, et al. The study of a novel paeoniflorin-converting enzyme from Cunninghamella blakesleeana[J]. Molecules, 2023, 28(3): 1289.
doi: 10.3390/molecules28031289 URL |
| [28] |
Buchan DWA, Jones DT. The PSIPRED protein analysis workbench: 20 years on[J]. Nucleic Acids Res, 2019, 47(W1): W402-W407.
doi: 10.1093/nar/gkz297 |
| [29] |
Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis[J]. Nat Protoc, 2015, 10(6): 845-858.
doi: 10.1038/nprot.2015.053 pmid: 25950237 |
| [30] |
Mao ZJ, Yu L, et al. Expression and immunogenicity analysis of two iron-regulated outer membrane proteins of Vibrio parahaemolyti-cus[J]. Acta Biochim Biophys Sin, 2007, 39(10): 763-769.
doi: 10.1111/j.1745-7270.2007.00339.x URL |
| [31] |
Wu WP, Shen QY, Zhang RL, et al. The structure of the MICU1-MICU2 complex unveils the regulation of the mitochondrial calcium uniporter[J]. EMBO J, 2020, 39(19): e104285.
doi: 10.15252/embj.2019104285 URL |
| [32] |
Lu ZF, Wang BY, et al. YdfD, a Lysis protein of the Qin prophage, is a specific inhibitor of the IspG-catalyzed step in the MEP pathway of Escherichia coli[J]. Int J Mol Sci, 2022, 23(3): 1560.
doi: 10.3390/ijms23031560 URL |
| [33] |
Pena-Francesch A, Demirel MC. Squid-inspired tandem repeat proteins: functional fibers and films[J]. Front Chem, 2019, 7: 69.
doi: 10.3389/fchem.2019.00069 pmid: 30847338 |
| [34] |
Wang WB, Liu JX, Guo SS, et al. Identification of Vibrio para-haemolyticus and Vibrio spp. specific outer membrane proteins by reverse vaccinology and surface proteome[J]. Front Microbiol, 2021, 11: 625315.
doi: 10.3389/fmicb.2020.625315 URL |
| [35] | 曲均革, 姚晓敏, 等. 产纤维素酶海洋细菌的筛选鉴定和产酶条件优化[J]. 上海海洋大学学报, 2012, 21(6): 1053-1057. |
| Qu JG, Yao XM, et al. Screening and identification of a cellulase-producing marine bacterium and optimization of its fermentation conditions[J]. J Shanghai Ocean Univ, 2012, 21(6): 1053-1057. | |
| [36] | 张大智, 詹儒林, 柳凤, 等. 芒果细菌性角斑病菌细胞壁降解酶反应条件优化[J]. 广东农业科学, 2015, 42(8): 72-76. |
| Zhang DZ, Zhan RL, Liu F, et al. Reaction conditions optimization of cell wall degrading enzymes from mango bacterial leaf spot pathogen[J]. Guangdong Agric Sci, 2015, 42(8): 72-76. |
| [1] | 孔谦, 黄文洁, 吴绍文, 李坤, 张名位, 晏石娟. 一种同时测定十种类胡萝卜素的液相色谱方法的建立[J]. 生物技术通报, 2022, 38(11): 80-89. |
| [2] | 蔡国磊, 陆小凯, 娄水珠, 杨海英, 杜刚. 芽孢杆菌LM基于全基因组的分类鉴定及抑菌原理的研究[J]. 生物技术通报, 2021, 37(8): 176-185. |
| [3] | 叶洲辰, 吴友根, 于靖, 张军锋, 杨东梅, 胡新文. 不同产地油茶籽油提取物的抗氧化活性比较分析及其营养评价[J]. 生物技术通报, 2019, 35(10): 80-88. |
| [4] | 赵杰, 岳华, 苟学磊, 周金燕, 谭红. 伊枯草菌素A发酵过程中游离氨基酸的HPLC分析[J]. 生物技术通报, 2018, 34(8): 151-158. |
| [5] | 刘建兵, 戚梦, 杜苑如, 傅俊生, 胡开辉. 拆分网络分析22株蛹虫草亲缘关系及高产虫草素菌株初筛[J]. 生物技术通报, 2018, 34(4): 133-138. |
| [6] | 乔玉玲, 黄铮, 秦海艳, 宋兰兰, 陈继军, 安晨, 叶星, 毛晓燕. 抗CD52单克隆抗体HPLC-肽图分析方法的建立[J]. 生物技术通报, 2018, 34(11): 216-222. |
| [7] | 许燕, 张宜涛, 叶贵子, 陆海荣, 黄青山. 重组溶葡萄球菌酶成品蛋白含量测定方法的建立[J]. 生物技术通报, 2016, 32(7): 54-58. |
| [8] | 张洋, 刘爱忠. 蓖麻种子油脂累积与可溶性糖变化的关系[J]. 生物技术通报, 2016, 32(6): 120-129. |
| [9] | 乔文婕, 雷荣, 蒋弘山, 胡帆, 李志红, 朱水芳. 离子对高效液相色谱法分析黄瓜花叶病毒侵染烟草的总RNA 水平变化[J]. 生物技术通报, 2013, 0(4): 90-95. |
| [10] | 佟新伟 杨泓喆 李玉 路福平. 大肠杆菌sdaA、sdaB、pgpB 基因的敲除及其对磷脂酰丝氨酸合成的影响[J]. 生物技术通报, 2013, 0(4): 123-128. |
| [11] | 布威海丽且姆阿巴拜科日, 木塔力甫艾买提, 阿米尼姑丽买买提, 尼砸木艾海提, 依米提热合曼. 新疆蜂胶提取物抗氧化活性及槲皮素和白杨素含量测定[J]. 生物技术通报, 2013, 0(2): 163-171. |
| [12] | 王少蓉;郁昂;. 产河豚毒素菌株的分离鉴定[J]. , 2010, 0(06): 231-233. |
| [13] | 曲直;阮继生;洪葵;. 高效液相色谱和气相色谱在放线菌分类鉴定中的应用[J]. , 2009, 0(S1): 79-82. |
| [14] | 曲直;洪葵;. 高效液相色谱法测定红树林放线菌DNA的(G+C)mol%含量[J]. , 2009, 0(S1): 209-214. |
| [15] | 惠芸华;冯兵;张晓玲;于慧娟;. 高效液相色谱法同时测定17种磺胺类药物的研究[J]. , 2009, 0(S1): 284-286. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||