








生物技术通报 ›› 2024, Vol. 40 ›› Issue (1): 231-242.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0690
常泸尹( ), 王中华, 李凤敏, 高梓源, 张辉红, 王祎, 李芳, 韩燕来, 姜瑛(
), 王中华, 李凤敏, 高梓源, 张辉红, 王祎, 李芳, 韩燕来, 姜瑛( )
)
收稿日期:2023-07-17
出版日期:2024-01-26
发布日期:2024-02-06
通讯作者:
姜瑛,女,博士,副教授,研究方向:土壤生态学;E-mail: JY27486@163.com作者简介:常泸尹,女,研究方向:农业资源与环境;E-mail: chang931162@163.com;王中华为共同第一作者
基金资助:
CHANG Lu-yin( ), WANG Zhong-hua, LI Feng-min, GAO Zi-yuan, ZHANG Hui-hong, WANG Yi, LI Fang, HAN Yan-lai, JIANG Ying(
), WANG Zhong-hua, LI Feng-min, GAO Zi-yuan, ZHANG Hui-hong, WANG Yi, LI Fang, HAN Yan-lai, JIANG Ying( )
)
Received:2023-07-17
Published:2024-01-26
Online:2024-02-06
摘要:
【目的】黄淮海平原小麦玉米生产区主要实行冬小麦-夏玉米轮作种植模式。砂质潮土是广泛分布于黄淮海地区的土壤,属性和衍生障碍较多,包括结构性较差、蓄水保肥能力较弱等。为了提高肥料利用率、改善土壤肥力,综合实现作物产量提升和品质优化,筛选了一株多功能促生菌,并在该轮作体系验证其广谱促生效应。【方法】从玉米根际砂质潮土中筛选多功能促生菌,测定其产吲哚乙酸(IAA)、溶有机磷、解钾能力。通过形态学、生理生化及16S rDNA序列分析方法对其种属进行鉴定。摇瓶条件下探究其产IAA的最适条件,通过玉米盆栽验证其促生能力,通过冬小麦-夏玉米大田试验验证其在轮作体系中的广谱性增产效果。【结果】(1)试验筛选得到一株命名为YM3的多功能根际促生菌枯草芽孢杆菌(Bacillus subtilis),其产IAA能力达到59.21 mg/L、解有机磷能力达到0.72 mg/L、解钾能力达到18.56 mg/L。当装液量为25 mL/250 mL,pH 6-8范围内,分别以麦芽糖、蛋白胨为碳、氮源时,YM3产IAA能力最佳。(2)玉米盆栽试验结果可见,与接种灭活菌相比,接种YM3菌水剂的土壤IAA、速效磷、速效钾含量分别显著提高75.00%、48.66%、20.00%。玉米幼苗的根长、根表面积、根体积、根尖数、根分枝数分别显著增加67.95%、59.21%、51.13%、71.34%、92.06%。玉米植株的鲜重、株高、相对叶绿素含量、全氮、全磷、全钾分别显著提高了39.86%、23.51%、18.27%、17.68%、52.26%和36.53%。(3)小麦玉米轮作大田试验结果表明,接种YM3菌剂的小麦大田土壤有效氮、速效磷、速效钾分别显著增加了9.08%、13.78%、16.66%,增产率达到42.18%。玉米大田土壤速效磷和速效钾含量分别显著增加了19.18%和15.95%,增产率达到13.22%。【结论】筛选得到的枯草芽孢杆菌YM3菌株兼具产IAA、溶有机磷、解钾功能,在黄淮海平原小麦玉米轮作体系土壤中适应性强,广谱性强,能够提升砂质潮土土壤肥力,提高冬小麦-夏玉米轮作体系的产量。
常泸尹, 王中华, 李凤敏, 高梓源, 张辉红, 王祎, 李芳, 韩燕来, 姜瑛. 玉米根际多功能促生菌的筛选及其对冬小麦-夏玉米轮作体系产量提升效果[J]. 生物技术通报, 2024, 40(1): 231-242.
CHANG Lu-yin, WANG Zhong-hua, LI Feng-min, GAO Zi-yuan, ZHANG Hui-hong, WANG Yi, LI Fang, HAN Yan-lai, JIANG Ying. Screening Multi-functional Rhizobacteria from Maize Rhizosphere and Their Ehancing Effects on Winter Wheat-Summer Maize Rotation System[J]. Biotechnology Bulletin, 2024, 40(1): 231-242.
| 土壤 Soil | 有机碳 Organic carbon/(g·kg-1) | 全磷 Total P/(g·kg-1) | 速效磷 Available P/(mg·kg-1) | 全钾 Total K/(g·kg-1) | 速效钾 Available K/(mg·kg-1) | pH |
|---|---|---|---|---|---|---|
| 砂质潮土 Sandy fluvo-aquic soil | 1.91 | 0.29 | 3.44 | 19.56 | 20.42 | 7.39 |
表1 供试土壤基本性质
Table 1 Basic properties of soil for testing
| 土壤 Soil | 有机碳 Organic carbon/(g·kg-1) | 全磷 Total P/(g·kg-1) | 速效磷 Available P/(mg·kg-1) | 全钾 Total K/(g·kg-1) | 速效钾 Available K/(mg·kg-1) | pH |
|---|---|---|---|---|---|---|
| 砂质潮土 Sandy fluvo-aquic soil | 1.91 | 0.29 | 3.44 | 19.56 | 20.42 | 7.39 |
| 项目Item | 结果Results | 项目Item | 结果 Results | |
|---|---|---|---|---|
| 革兰氏染色 Gram stain | + | 淀粉水解 Amylohydrolysis test | + | |
| 好氧性试验 Aerobic test | 兼性厌氧 Facultative anaerobic | 明胶液化 Gelatin liquefaction test | + | |
| 接触酶试验 Catalase | + | 硝酸盐还原 Nitrate reduction test | + | |
| 甲基红(M.R)反应 Methyl red test | - | 柠檬酸盐利用 Citrate utilization | + | |
| V-P试验 Voges-Proskauer test | + |
表2 YM3菌株的生理生化特性
Table 2 Physiological and biochemical characteristics of YM3 strain
| 项目Item | 结果Results | 项目Item | 结果 Results | |
|---|---|---|---|---|
| 革兰氏染色 Gram stain | + | 淀粉水解 Amylohydrolysis test | + | |
| 好氧性试验 Aerobic test | 兼性厌氧 Facultative anaerobic | 明胶液化 Gelatin liquefaction test | + | |
| 接触酶试验 Catalase | + | 硝酸盐还原 Nitrate reduction test | + | |
| 甲基红(M.R)反应 Methyl red test | - | 柠檬酸盐利用 Citrate utilization | + | |
| V-P试验 Voges-Proskauer test | + |

图2 YM3菌株16S rDNA序列的系统发育树 标尺代表每10 000 个核苷中有5个核苷替代
Fig. 2 Phylogenetic tree of 16S rDNA gene sequence of YM3 strain The scale represents 5 nucleoside substitutions per 10 000 nucleosides
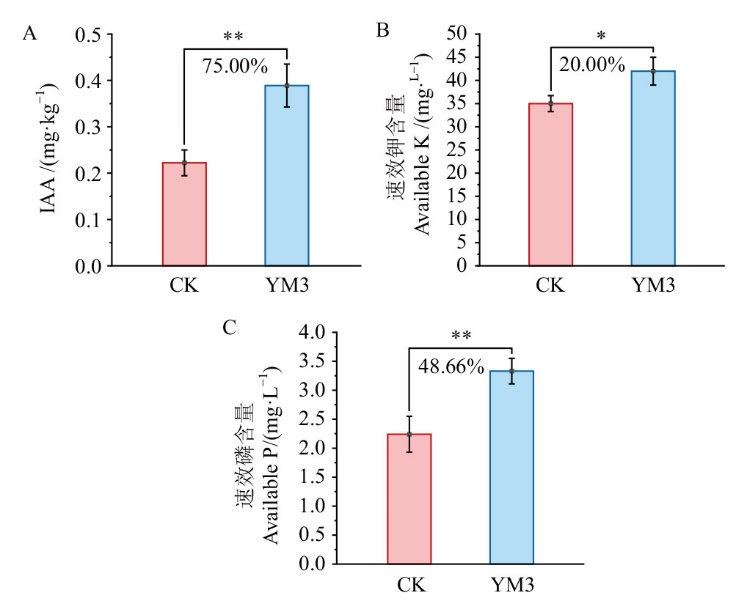
图4 接种菌株YM3培养30 d后土壤IAA及速效钾、速效磷含量 *和**分别表示处理间在0.05和0.01水平差异显著。下同
Fig. 4 Soil IAA, available potassium, and available phosphorus contents after 30 d of inoculation with strain YM3 * and ** indicate significant difference between treatments at 0.05 and 0.01 levels, respectively. The same below
| 项目 Item | CK | YM3 |
|---|---|---|
| 总根长RL/cm | 886.39±220.22 | 1 488.67±210.93* |
| 各分级总根长 RL of each class/cm | ||
| I/(RD 0.0-0.5 mm) | 691.11±165.95 | 1 192.04±157.68* |
| II/(RD 0.5-1.0 mm) | 125.53±46.42 | 203.13±62.03 |
| III/(RD 1.0-1.5 mm) | 46.56±8.97 | 59.59±3.35 |
| IV/(RD>1.5 mm) | 22.92±0.67 | 33.08±5.63 |
| 根表面积RSA/cm2 | 121.61±27.37 | 193.61±16.59* |
| 各分级根表面积 RSA of each class/cm2 | ||
| I/(RD 0.0-0.5 mm) | 46.43±13.62 | 74.04±4.67* |
| II/(RD 0.5-1.0 mm) | 27.97±10.88 | 45.50±14.92 |
| III/(RD 1.0-1.5 mm) | 17.41±3.43 | 22.41±0.99 |
| IV/(RD>1.5 mm) | 15.49±1.85 | 23.97±4.47 |
| 根体积RV/cm3 | 1.33±0.26 | 2.01±0.07* |
| 各分级根体积 RV of each class/cm3 | ||
| I/(RD 0.0-0.5 mm) | 14.26±3.19 | 23.57±1.49* |
| II/(RD 0.5-1.0 mm) | 8.90±3.46 | 14.48±4.75 |
| III/(RD 1.0-1.5 mm) | 5.54±1.09 | 7.13±0.32 |
| IV/(RD>1.5 mm) | 4.93±0.59 | 7.63±1.42 |
| 根平均直径RD/mm | 0.44±0.01 | 0.42±0.02 |
| 根尖数RT | 4 505.66±1 199.46 | 7 720.02±1 115.10* |
| 分枝数RF | 4 760.67±780.30 | 9 143.33±2 714.33* |
表3 接种菌株YM3对玉米幼苗根系结构和根系分级的影响
Table 3 Effects of inoculation with strain YM3 on the root system structure and root classification of maize seedlings
| 项目 Item | CK | YM3 |
|---|---|---|
| 总根长RL/cm | 886.39±220.22 | 1 488.67±210.93* |
| 各分级总根长 RL of each class/cm | ||
| I/(RD 0.0-0.5 mm) | 691.11±165.95 | 1 192.04±157.68* |
| II/(RD 0.5-1.0 mm) | 125.53±46.42 | 203.13±62.03 |
| III/(RD 1.0-1.5 mm) | 46.56±8.97 | 59.59±3.35 |
| IV/(RD>1.5 mm) | 22.92±0.67 | 33.08±5.63 |
| 根表面积RSA/cm2 | 121.61±27.37 | 193.61±16.59* |
| 各分级根表面积 RSA of each class/cm2 | ||
| I/(RD 0.0-0.5 mm) | 46.43±13.62 | 74.04±4.67* |
| II/(RD 0.5-1.0 mm) | 27.97±10.88 | 45.50±14.92 |
| III/(RD 1.0-1.5 mm) | 17.41±3.43 | 22.41±0.99 |
| IV/(RD>1.5 mm) | 15.49±1.85 | 23.97±4.47 |
| 根体积RV/cm3 | 1.33±0.26 | 2.01±0.07* |
| 各分级根体积 RV of each class/cm3 | ||
| I/(RD 0.0-0.5 mm) | 14.26±3.19 | 23.57±1.49* |
| II/(RD 0.5-1.0 mm) | 8.90±3.46 | 14.48±4.75 |
| III/(RD 1.0-1.5 mm) | 5.54±1.09 | 7.13±0.32 |
| IV/(RD>1.5 mm) | 4.93±0.59 | 7.63±1.42 |
| 根平均直径RD/mm | 0.44±0.01 | 0.42±0.02 |
| 根尖数RT | 4 505.66±1 199.46 | 7 720.02±1 115.10* |
| 分枝数RF | 4 760.67±780.30 | 9 143.33±2 714.33* |
| [1] | 冯倩倩, 韩惠芳, 张亚运, 等. 耕作方式对麦-玉轮作农田固碳、保水性能及产量的影响[J]. 植物营养与肥料学报, 2018, 24(4): 869-879. |
| Feng QQ, Han HF, Zhang YY, et al. Effects of tillage methods on soil carbon sequestration and water holding capacity and yield in wheat-maize rotation[J]. J Plant Nutr Fertil, 2018, 24(4): 869-879. | |
| [2] | 穆文强, 康慎敏, 李平兰. 根际促生菌对植物的生长促进作用及机制研究进展[J]. 生命科学, 2022, 34(2): 118-127. |
| Mu WQ, Kang SM, Li PL. Advances in rhizosphere growth-promoting bacteria function on plant growth facilitation and their mechanisms[J]. Chin Bull Life Sci, 2022, 34(2): 118-127. | |
| [3] |
Pantoja-Guerra M, Valero-Valero N, Ramírez CA. Total auxin level in the soil-plant system as a modulating factor for the effectiveness of PGPR inocula: a review[J]. Chem Biol Technol Agric, 2023, 10(1): 6.
doi: 10.1186/s40538-022-00370-8 |
| [4] |
Ha-Tran DM, Nguyen TTM, Hung SH, et al. Roles of plant growth-promoting rhizobacteria(PGPR)in stimulating salinity stress defense in plants: a review[J]. Int J Mol Sci, 2021, 22(6): 3154.
doi: 10.3390/ijms22063154 URL |
| [5] |
Ali S, Khan N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants[J]. Microbiol Res, 2021, 249: 126771.
doi: 10.1016/j.micres.2021.126771 URL |
| [6] |
Tariq M. Antagonistic features displayed by plant growth promoting rhizobacteria(PGPR): a review[J]. J Plant Sci Phytopathol, 2017, 1(1): 38-43.
doi: 10.29328/journal.jpsp URL |
| [7] | 周益帆, 白寅霜, 岳童, 等. 植物根际促生菌促生特性研究进展[J]. 微生物学通报, 2023, 50(2): 644-666. |
| Zhou YF, Bai YS, Yue T, et al. Research progress on the growth-promoting characteristics of plant growth-promoting rhizobacteria[J]. Microbiol China, 2023, 50(2): 644-666. | |
| [8] |
Beneduzi A, Ambrosini A, Passaglia LMP. Plant growth-promoting rhizobacteria(PGPR): their potential as antagonists and biocontrol agents[J]. Genet Mol Biol, 2012, 35(4 suppl): 1044-1051.
doi: 10.1590/S1415-47572012000600020 URL |
| [9] |
Shabaan M, Asghar HN, Zahir ZA, et al. Salt-tolerant PGPR confer salt tolerance to maize through enhanced soil biological health, enzymatic activities, nutrient uptake and antioxidant defense[J]. Front Microbiol, 2022, 13: 901865.
doi: 10.3389/fmicb.2022.901865 URL |
| [10] |
Adedayo AA, Babalola OO, Prigent-Combaret C, et al. The application of plant growth-promoting rhizobacteria in Solanum lycopersicum production in the agricultural system: a review[J]. PeerJ, 2022, 10: e13405.
doi: 10.7717/peerj.13405 URL |
| [11] | Libbert E, Risch H. Interactions between plants and epiphytic bacteria regarding their auxin metabolism. V. isolation and identification of the IAA-producing and destroying bacteria from pea plants[J]. Physiol Plant, 1969, 22(1): 51-58. |
| [12] |
Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria[J]. Appl Environ Microbiol, 1995, 61(2): 793-796.
doi: 10.1128/aem.61.2.793-796.1995 URL |
| [13] | 赵小蓉, 林启美, 孙焱鑫, 等. 细菌解磷能力测定方法的研究[J]. 微生物学通报, 2001, 28(1): 1-4. |
| Zhao XR, Lin QM, Sun YX, et al. The methods for quantifying capacity of bacteria in dissolving p compounds[J]. Microbiology, 2001, 28(1): 1-4. | |
| [14] | 中国科学院南京土壤研究所微生物室. 土壤微生物研究法[M]. 北京: 科学出版社, 1985. |
| Department of Microbiology, Nanjing Institute of Soil Research, Chinese Academy of Sciences. Soil microorganism research method[M]. Beijing: Science Press, 1985. | |
| [15] | 东秀珠, 蔡妙英. 常见细菌系统鉴定手册[M]. 北京: 科学出版社, 2001. |
| Dong XZ, Cai MY. Handbook of identification of common bacterial systems[M]. Beijing: Science Press, 2001. | |
| [16] | 郭素枝. 扫描电镜技术及其应用[M]. 厦门: 厦门大学出版社, 2006. |
| Guo SZ. Scanning electron microscope technology and its application[M]. Xiamen, China: Xiamen University Press, 2006. | |
| [17] |
Saghai-Maroof MA, Soliman KM, Jorgensen RA, et al. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics[J]. Proc Natl Acad Sci USA, 1984, 81(24): 8014-8018.
doi: 10.1073/pnas.81.24.8014 pmid: 6096873 |
| [18] |
Jiang Y, Wu Y, Hu N, et al. Interactions of bacterial-feeding nematodes and indole-3-acetic acid(IAA)-producing bacteria promotes growth of Arabidopsis thaliana by regulating soil auxin status[J]. Appl Soil Ecol, 2020, 147: 103447.
doi: 10.1016/j.apsoil.2019.103447 URL |
| [19] | 鲍士旦. 土壤农化分析[M]. 3版. 北京: 中国农业出版社, 2000. |
| Bao SD. Soil and agricultural chemistry analysis[M]. 3rd ed. Beijing: China Agriculture Press, 2000. | |
| [20] |
Shi GR, Xia SL, Ye J, et al. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology[J]. Environ Exp Bot, 2015, 111: 127-134.
doi: 10.1016/j.envexpbot.2014.11.008 URL |
| [21] |
Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria(PGPR): emergence in agriculture[J]. World J Microbiol Biotechnol, 2012, 28(4): 1327-1350.
doi: 10.1007/s11274-011-0979-9 URL |
| [22] |
Ma SY, Lin YB, Qin YQ, et al. Microbial diversity characteristics of Areca palm rhizosphere soil at different growth stages[J]. Plants, 2021, 10(12): 2706.
doi: 10.3390/plants10122706 URL |
| [23] |
Ullah A, Akbar A, Luo QQ, et al. Microbiome diversity in cotton rhizosphere under normal and drought conditions[J]. Microb Ecol, 2019, 77(2): 429-439.
doi: 10.1007/s00248-018-1260-7 pmid: 30196314 |
| [24] | de O Nunes PS, de Oliveira TS, et al. Bacillus subtilis and Bacillus licheniformis promote tomato growth[J]. Publ Braz Soc Microbiol, 2023, 54(1): 397-406. |
| [25] | 王舒, 张林平, 郝菲菲, 等. 油茶根际高效溶磷细菌的筛选、鉴定及其安全性测试[J]. 林业科学研究, 2015, 28(2): 166-172. |
| Wang S, Zhang LP, Hao FF, et al. Screening, identification and security test of Camellia oleifera rhizosphere phosphate-solubilizing bacteria[J]. For Res, 2015, 28(2): 166-172. | |
| [26] | 徐文思, 姜瑛, 李引, 等. 一株植物促生菌的筛选、鉴定及其对花生的促生效应研究[J]. 土壤, 2014, 46(1): 119-125. |
| Xu WS, Jiang Y, Li Y, et al. Isolation, identification of plant growth-promoting bacteria and its promoting effects on peanuts[J]. Soils, 2014, 46(1): 119-125. | |
| [27] |
Liang CY, Tian J, Liao H. Proteomics dissection of plant responses to mineral nutrient deficiency[J]. Proteomics, 2013, 13(3/4): 624-636.
doi: 10.1002/pmic.v13.3-4 URL |
| [28] |
Keswani C, Singh SP, Cueto L, et al. Auxins of microbial origin and their use in agriculture[J]. Appl Microbiol Biotechnol, 2020, 104(20): 8549-8565.
doi: 10.1007/s00253-020-10890-8 pmid: 32918584 |
| [29] |
Niu YF, Jin GL, Li X, et al. Phosphorus and magnesium interactively modulate the elongation and directional growth of primary roots in Arabidopsis thaliana(L.) Heynh[J]. J Exp Bot, 2015, 66(13): 3841-3854.
doi: 10.1093/jxb/erv181 URL |
| [30] | Tian XL, Wang GW, Zhu R, et al. Conditions and indicators for screening cotton(Gossypium hirsutum L.) varieties tolerant to low potassium[J]. Acta Agron Sin, 2008, 34(8): 1435-1443. |
| [31] |
He AL, Zhao LY, Ren W, et al. A volatile producing Bacillus subtilis strain from the rhizosphere of Haloxylon ammodendron promotes plant root development[J]. Plant Soil, 2023, 486(1): 661-680.
doi: 10.1007/s11104-023-05901-2 |
| [32] | 勾宇春, 王宗抗, 张志鹏, 等. 植物根际促生菌作用机制研究进展[J]. 应用与环境生物学报, 2023, 29(2): 495-506. |
| Gou YC, Wang ZK, Zhang ZP, et al. Advance in role mechanisms of plant growth-promoting rhizobacteria[J]. Chin J Appl Environ Biol, 2023, 29(2): 495-506. | |
| [33] |
李刚强, 王楠, 李永斌, 等. 两种固氮芽孢杆菌菌剂在小麦—玉米轮作区大田试验效果评价[J]. 中国农业科技导报, 2020, 22(4): 147-152.
doi: 10.13304/j.nykjdb.2019.0556 |
| Li GQ, Wang N, Li YB, et al. Effect evaluation of field experiment of two Paenibacillus sp. agents in wheat-maize rotation area[J]. J Agric Sci Technol, 2020, 22(4): 147-152. | |
| [34] |
Sapre S, Gontia-Mishra I, Tiwari S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings(Avena sativa)[J]. Microbiol Res, 2018, 206: 25-32.
doi: 10.1016/j.micres.2017.09.009 URL |
| [35] |
Elsharkawy MM, Elsawy MM, Ismail IA. Mechanism of resistance to Cucumber mosaic virus elicited by inoculation with Bacillus subtilis subsp. subtilis[J]. Pest Manag Sci, 2022, 78(1): 86-94.
doi: 10.1002/ps.v78.1 URL |
| [36] |
Xie DS, Cai XW, Yang CP, et al. Studies on the control effect of Bacillus subtilis on wheat powdery mildew[J]. Pest Manag Sci, 2021, 77(10): 4375-4382.
doi: 10.1002/ps.v77.10 URL |
| [37] |
Meena M, Swapnil P, Divyanshu K, et al. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives[J]. J Basic Microbiol, 2020, 60(10): 828-861.
doi: 10.1002/jobm.v60.10 URL |
| [1] | 张道磊, 甘雨军, 乐亮, 普莉. 玉米产量性状的表观遗传调控机制和育种应用[J]. 生物技术通报, 2023, 39(8): 31-42. |
| [2] | 罗义, 张丽娟, 黄伟, 王宁, 吾尔丽卡·买提哈斯木, 施宠, 王玮. 一株耐铀菌株的鉴定及其促生特性研究[J]. 生物技术通报, 2023, 39(5): 286-296. |
| [3] | 江美彦, 周杨, 刘仁浪, 姚菲, 杨云舒, 侯凯, 冯冬菊, 吴卫. 白芷根际促生菌的筛选及其促生效果研究[J]. 生物技术通报, 2022, 38(8): 167-178. |
| [4] | 张昊鑫, 王中华, 牛兵, 郭慷, 刘璐, 姜瑛, 张仕祥. 产IAA兼具溶磷解钾高效促生菌的筛选、鉴定及其广谱性应用[J]. 生物技术通报, 2022, 38(5): 100-111. |
| [5] | 李毅丹, 单晓辉. 赤霉素代谢调控与绿色革命[J]. 生物技术通报, 2022, 38(2): 195-204. |
| [6] | 山琦, 贾惠舒, 姚文博, 刘伟灿, 李海燕. 植物miR396-GRF模块的生物学功能及其潜在应用价值[J]. 生物技术通报, 2022, 38(10): 34-44. |
| [7] | 李和平, 李积铭, 李爱国, 武军艳, 宋聪敏, 申彦平, 杨莉. 播期对河北省超采水区白菜型冬油菜越冬率及产量的影响[J]. 生物技术通报, 2021, 37(4): 35-46. |
| [8] | 付严松, 李宇聪, 徐志辉, 邵佳慧, 刘云鹏, 宣伟, 张瑞福. 根际促生菌调控植物根系发育的信号与分子机制研究进展[J]. 生物技术通报, 2020, 36(9): 42-48. |
| [9] | 潘晶, 黄翠华, 彭飞, 尤全刚, 刘斐耀, 薛娴. 植物根际促生菌诱导植物耐盐促生作用机制[J]. 生物技术通报, 2020, 36(9): 75-87. |
| [10] | 宫伟, 余健源, 张曦, 单晓昳. 硝酸根调控植物开花和产量分子机制的研究进展[J]. 生物技术通报, 2020, 36(8): 162-172. |
| [11] | 马军, 徐通达. 植物非经典生长素信号转导通路解析[J]. 生物技术通报, 2020, 36(7): 15-22. |
| [12] | 万水霞, 王静, 李帆, 蒋光月, 徐文静, 刘祚军. 玉米根际高效溶磷菌的筛选、鉴定及促生效应研究[J]. 生物技术通报, 2020, 36(5): 98-103. |
| [13] | 杨茉, 高婷, 李滟璟, 魏崇瑶, 高淼, 马莲菊. 辣椒根际促生菌的分离筛选及抗病促生特性研究[J]. 生物技术通报, 2020, 36(5): 104-109. |
| [14] | 郭宇泽, 丁雪敏, 姚岚, 许德敏, 赵雨洁, 冯福应, 孟建宇. 马西利亚菌B260的分离鉴定及促进育苗的效果[J]. 生物技术通报, 2019, 35(9): 144-149. |
| [15] | 孙培, 王罡, 张亚楠, 李倩, 季静, 杨丹, 袁东, 王畅, 王昱蓉, 王萍. 一种耐盐促生菌筛选、鉴定及对玉米幼苗生长的影响[J]. 生物技术通报, 2019, 35(8): 27-33. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||