








生物技术通报 ›› 2024, Vol. 40 ›› Issue (8): 275-287.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0110
马小翔1( ), 马泽源1, 刘亚月1,2,3, 周龙建1,2,3, 和羿帆4, 张翼1,2,3(
), 马泽源1, 刘亚月1,2,3, 周龙建1,2,3, 和羿帆4, 张翼1,2,3( )
)
收稿日期:2024-01-31
出版日期:2024-08-26
发布日期:2024-07-02
通讯作者:
张翼,男,博士,教授,研究方向:海洋天然产物;E-mail: hubeizhangyi@163.com作者简介:马小翔,女,硕士研究生,研究方向:海洋天然产物;E-mail: ma.xiao.xiang@163.com基金资助:
MA Xiao-xiang1( ), MA Ze-yuan1, LIU Ya-yue1,2,3, ZHOU Long-jian1,2,3, HE Yi-fan4, ZHANG Yi1,2,3(
), MA Ze-yuan1, LIU Ya-yue1,2,3, ZHOU Long-jian1,2,3, HE Yi-fan4, ZHANG Yi1,2,3( )
)
Received:2024-01-31
Published:2024-08-26
Online:2024-07-02
摘要:
【目的】丁内酯I是土曲霉(Aspergillus terreus)产生的一种具有多样生物活性的小分子天然产物,对其结构多样性拓展的探索具有重要意义。以一株高产丁内酯I的海洋来源土曲霉C23-3作为基础菌株,研究丁内酯前体小分子类似物和前体合成酶抑制剂对其次生代谢的影响。【方法】以海水马铃薯液体培养基为基础培养基,以3种前体小分子类似物和3种对羟基苯丙酮酸的合成酶抑制剂作为化学诱导剂,在静置发酵条件下进行菌株的化学调控培养。采用高效液相色谱、高效液相色谱-离子阱质谱联用及基于质谱的分子网络和数据库挖掘分析次生代谢产物的产量和多样性。【结果】多数前体小分子类似物和合成酶抑制剂对丁内酯I产量均有不同程度抑制,而高浓度的前体小分子类似物3,4-二羟基苯丙酮酸、两种合成酶抑制剂联用及这三者的联用表现出了较强的抑制效果;并且在丁内酯I的合成受到强烈抑制条件下,丁内酯类、土震素类、洛伐他汀类等多类次生代谢产物的合成也下降,但一种环肽类化合物产量超过10倍的大幅提升,可能是菌体对作为群体感应信号及全局性转录因子lae A诱导子的丁内酯I合成受到压制胁迫的一种应激反应,具体机制有待深入研究。【结论】丁内酯I的前体确为对羟基苯丙酮酸,3,4-二羟基苯丙酮酸及对羟基苯丙酮酸合成酶抑制剂可用作抑制丁内酯I合成的小分子工具,以用于仿突变生物合成的进一步探索以获得多样化丁内酯衍生物,也可诱导产生环肽类化合物。
马小翔, 马泽源, 刘亚月, 周龙建, 和羿帆, 张翼. 仿突变生物合成调控对土曲霉C23-3次生代谢产物的影响[J]. 生物技术通报, 2024, 40(8): 275-287.
MA Xiao-xiang, MA Ze-yuan, LIU Ya-yue, ZHOU Long-jian, HE Yi-fan, ZHANG Yi. Effects of Simulated Mutational Biosynthetic Regulation on the Secondary Metabolites of Aspergillus terreus C23-3[J]. Biotechnology Bulletin, 2024, 40(8): 275-287.
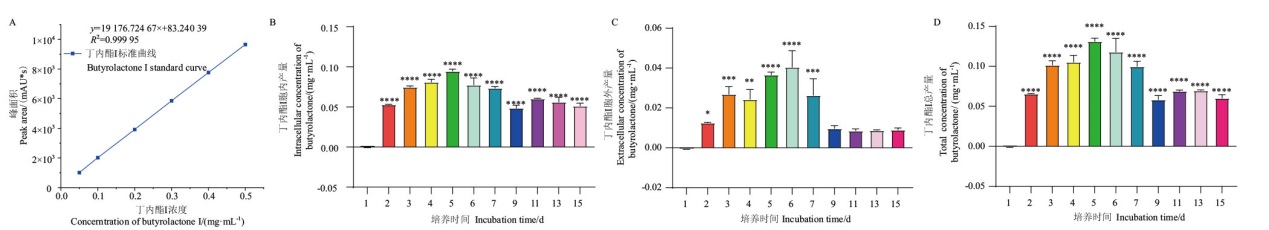
图2 不同培养时间菌株 C23-3 的胞内、胞外及总丁内酯I含量 A:用于计算提取物中丁内酯I含量的标准曲线;B-D:分别为胞内、胞外和总的丁内酯I含量,培养时间从接种后开始计算。*, **, ***, ****指的是其他天数与第1天对比的差异性,分别是P值<0.05、0.01、0.001和0.000 1。组间差异分析用的是Ordinary one-way ANOVA multiple comparisons
Fig. 2 Extracellular, intracellular, and total contents of butyrolactone I of strain C23-3 at different culture time A: Standard curve used to calculate the content of butyrolactone I in the extracts. B-D: Extracellular, intracellular, and total contents of butyrolactone I, the incubation time is calculated from the time after inoculation. *, **, ***, **** refers to the difference between the other days and the first day, which are P values < 0.05, 0.01, 0.001, and 0.000 1, respectively. Ordinary one-way ANOVA multiple comparisons were used for difference analysis between groups

图3 不同酶抑制剂或诱导剂作用下菌株C23-3的丁内酯I总产量(A)与总提取物量(B) ADA:酶抑制剂1,3-金刚烷二乙酸;NTSP:酶抑制剂N-(对甲苯磺酰)-L-苯丙氨酸;PS:酶抑制剂苯乙肼硫酸盐;HT:诱导剂3-羟基-L-酪氨酸;DHPPA:诱导剂3,4-二羟基对苯丙酮酸;Far:诱导剂金合欢醇;DMSO:溶剂DMSO对照组;blank:空白组。缩写后的数字表示浓度,单位为mmol/L。*, **, ***指的是其他组与blank对比的差异性,分别是P值<0.05、0.01和0.001。组间差异分析用的是Ordinary one-way ANOVA multiple comparisons
Fig. 3 Butyrolactone I contents(A)and total extract mass(B)of strain C23-3 under the effect of different enzyme inhibitors or inducers ADA: Enzyme inhibitor 1,3-adamantane diacetic acid; NTSP: enzyme inhibitor N-(p-toluenesulfonyl)-L-phenylalanine; PS: enzyme inhibitor phenelzine sulfate; HT: inducer 3-hydroxy-L-tyrosine; DHPPA: inducer 3,4-dihydroxy-p-phenylpyruvate; Far: inducer farnesol; DMSO: the DMSO control group; blank: the blank group. The number after the abbreviations indicate the concentrations, and the unit is mmol/L. *, **, *** refer to the differences between the other groups and blank, which were P values < 0.05, 0.01, and 0.001, respectively. Ordinary one-way ANOVA multiple comparisons were used for difference analysis between groups
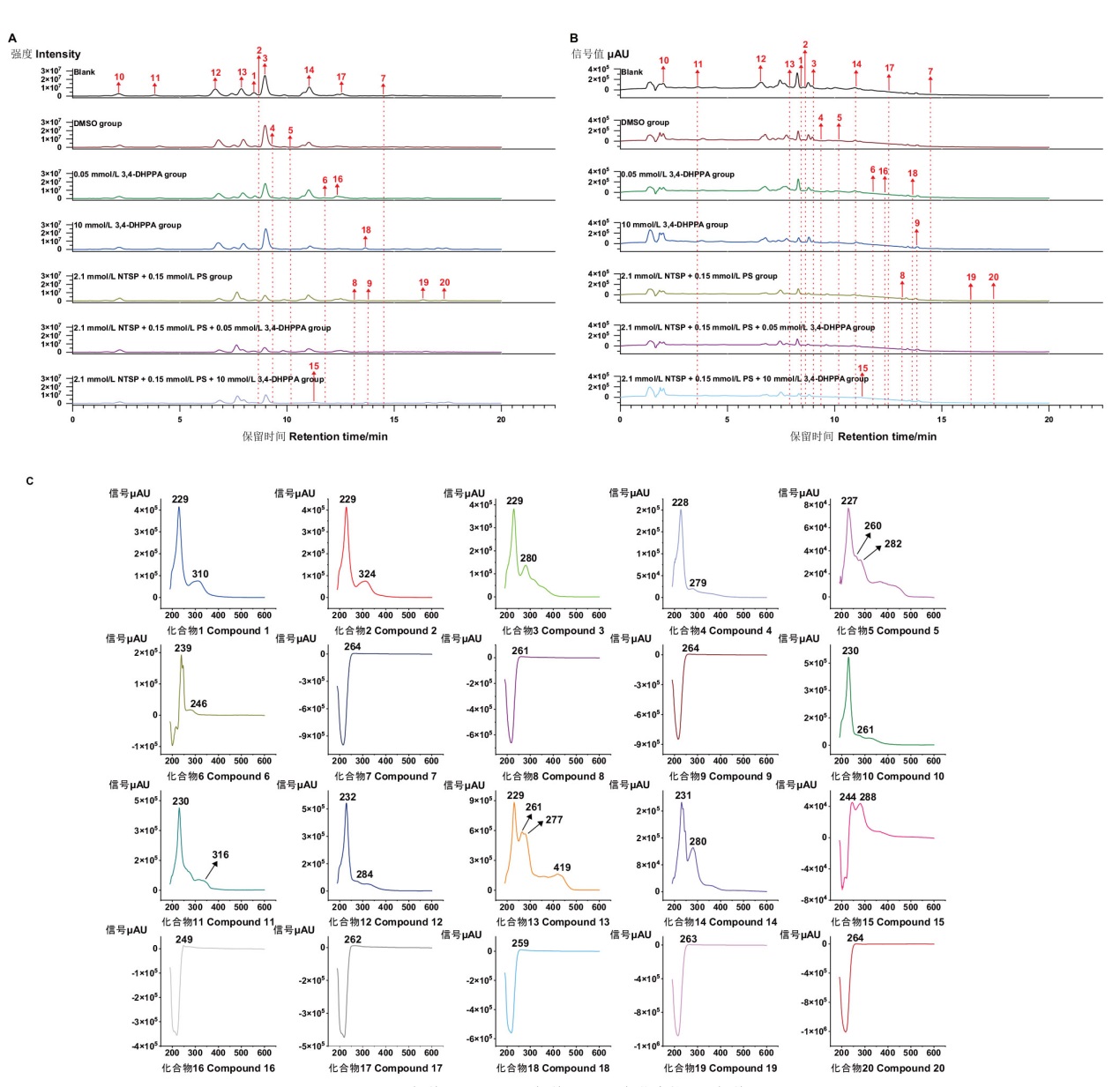
图4 不同培养条件下菌株Aspergillus terreus C23-3提取物LC-MS/MS-PDA色谱图 A:BPC色谱图;B:PDA色谱图;C:各化合物UV色谱图
Fig. 4 LC-MS/MS-PDA chromatogram of Aspergillus terreus C23-3 extracts under different cultural conditions A: BPC chromatogram; B: PDA chromatogram; C: UV chromatogram of each compound
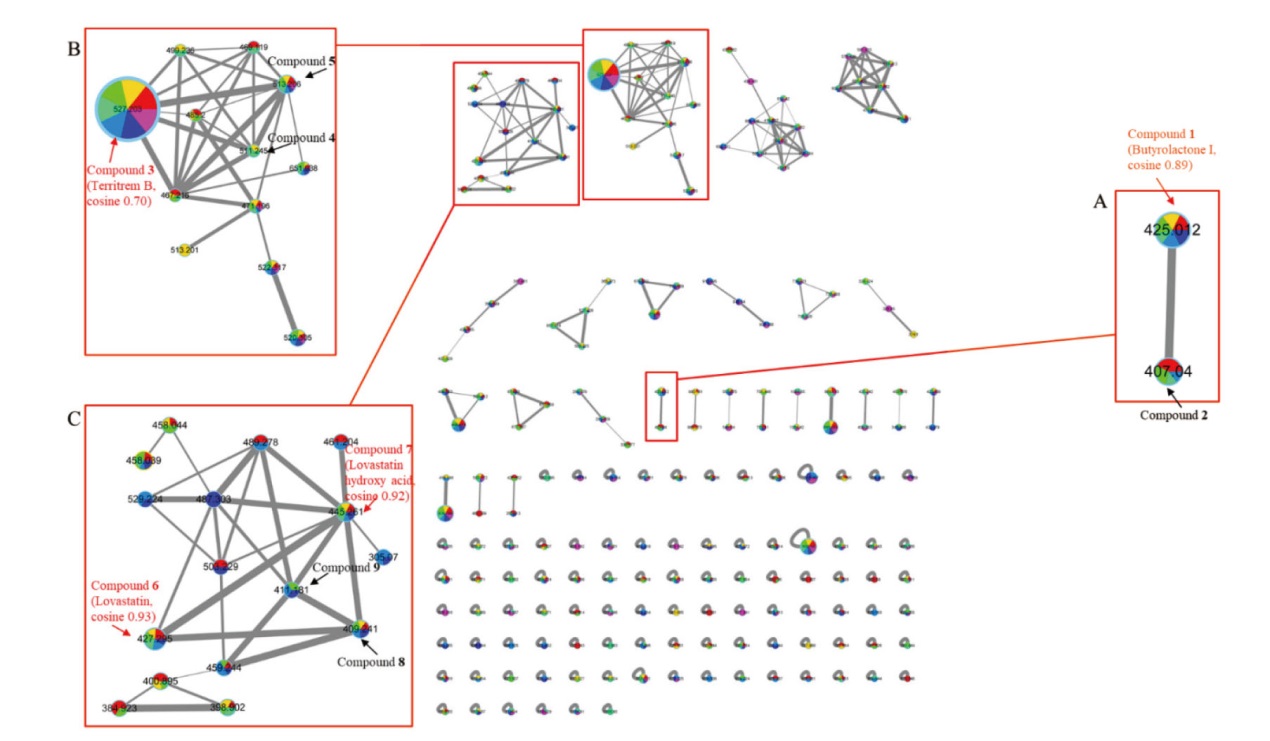
图5 菌株C23-3不同培养条件下代谢产物基于二级质谱联系的分子网络图及局部放大图 A-C:分别是化合物丁内酯I、土震素B、洛伐他汀同系物的代谢物簇放大图。Compounds 1、3、6、7:分别是丁内酯I、土震素B、洛伐他汀、洛伐他汀羟酸;Compounds 2、4、5、8、9:已知化合物的可能衍生物,分别是[M(丁内酯I-H2O)+H]+、[M(土震素B-O)+H]+、[M(土震素B-CH2)+H]+、[M(洛伐他汀-H2O)+Na]+、[M(洛伐他汀-O)+Na]+。分子网络图中的节点包含7种颜色, 分别表示化合物的7个样品来源:依次是空白组、溶剂DMSO对照组、添加3,4-二羟基苯丙酮酸(0.05 mmol/L、10 mmol/L)、添加N-(对甲苯磺酰)-L-苯丙氨酸(2.1 mmol/L)和苯乙肼硫酸盐(0.15 mmol/L)、添加3,4-二羟基苯丙酮酸(0.05 mmol/L)和N-(对甲苯磺酰)-L-苯丙氨酸(2.1 mmol/L)和苯乙肼硫酸盐(0.15 mmol/L)、添加3,4-二羟基苯丙酮酸(10 mmol/L)和N-(对甲苯磺酰)-L-苯丙氨酸(2.1 mmol/L)和苯乙肼硫酸盐(0.15 mmol/L)实验组
Fig. 5 Molecular network and its partial enlarged diagram of the metabolites of strain C23-3 under different cultural conditions based on MS2 relationships A-C:Enlarged images of metabolites clusters of butyrolactone I, territrem B and lovastatin congeners. Compounds 1, 3, 6, 7: Butyrolactone I,territrem B,lovastatin,and lovastatin hydroxy acid. Compounds 2, 4, 5, 8, 9:Possible derivatives of known compounds,i.e.,[M(butyrolactone I isomer)+ H]+,[M(butyrolactone I-2H)+ H]+,[M(butyrolactone I isomer + O)+ H]+,[M(butyrolactone I - H2O)+ H]+,[M(territrem B-O)+ H]+,[M(territrem B - CH2)+ H]+,[M(lovastatin - H2O)+ H]+,[M(lovastatin - O)+ H]+,and[M(lovastatin + 2H)+ H]+,respectively. The nodes in the molecular network are marked in 7 colors, , sequentially representing the 7 sample sources of compounds: blank group, control group with solvent DMSO; groups with the addition of 3,4-dihydroxyphenylpyruvate(0.05, 10 mmol/L), N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L), 3,4-dihydroxyphenylpyruvate(0.05 mmol/L)+ N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L), 3,4-dihydroxyphenylpyruvate(10 mmol/L)+N-(p-toluenesulfonyl)-L-phenylalanine(2.1 mmol/L)+ phenelzine sulfate(0.15 mmol/L)respectively
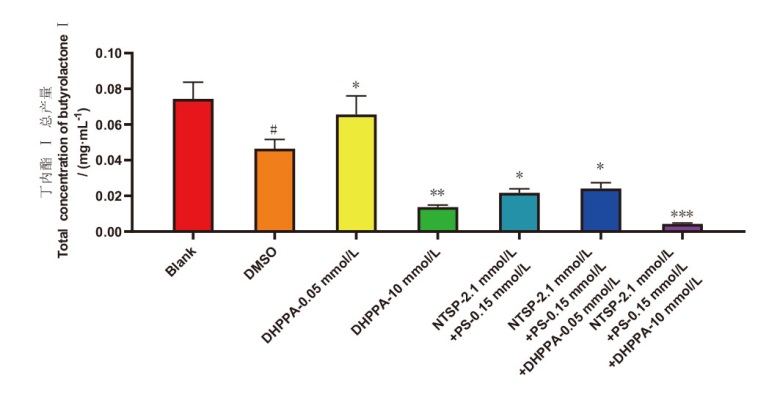
图7 菌株C23-3丁内酯I总产量随酶抑制剂和诱导剂添加的变化 Blank:空白组;DMSO:溶剂DMSO对照组;NTSP:N-(对甲苯磺酰)-L-苯丙氨酸;PS:苯乙肼硫酸盐。#P<0.05,DMSO对照组比空白组;*P<0.05,实验组比DMSO对照组;**P<0.01,样品组比对照组,***P<0.001,样品组比对照组
Fig. 7 Butyrolactone I content variation of strain C23-3 with the addition of enzyme inhibitors and inducers Blank: Blank group; DMSO: the solvent DMSO control group; NTSP: N-(p-toluenesulfonyl)-L-phenylalanine, PS: phenelzine sulfate. # P<0.05, DMSO control group vs blank group; * P<0.05, experimental group vs DMSO Control group; ** P<0.01, the sample group vs the control group, *** P<0.001, the sample group vs the control group
| 化合物序号Compound No. | 化合物注释 Compound note | 不同培养条件下的产物产量变化幅度 Changes in product yield under different cultivation conditions/% | |||||
|---|---|---|---|---|---|---|---|
| DMSO group | 0.05 mmol/L 3,4-DHPPA group | 10 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mmol/L PS group | 2.1 mmol/L NTSP + 0.15 mM PS + 0.05 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mM PS + 10 mmol/L 3,4-DHPPA group | ||
| 1 | 丁内酯I Butyrolactone I | ↓62.50 | ↓32.71 | ↓90.06 | ↓74.04 | ↓68.76 | ↓92.19 |
| 2 | 丁内酯I脱水产物 Butyrolactone I dehydrated product | ↓71.53 | ↓34.28 | ↓78.36 | ↓100.00 | ↓67.42 | ↓100.00 |
| 3 | 土震素B Territrem B | ↑4.40 | ↓26.45 | ↑9.58 | ↓73.15 | ↓78.94 | ↓57.47 |
| 4 | 土震素B脱氧产物 Territrem B deoxylation products | ↑65.52 | ↓100.00 | ↑38.07 | ↓80.67 | ↓84.58 | ↓100.00 |
| 5 | 土震素B降碳产物 Territrem B carbon reduction products | ↑50.57 | ↓56.52 | ↓33.36 | ↓100.00 | ↓100.00 | ↓86.79 |
| 土震素B类化合物总产量 Total production of territrem B compound | ↑5.12 | ↓27.10 | ↑9.36 | ↓73.42 | ↓79.14 | ↓57.95 | |
| 6 | 洛伐他汀Lovastatin | ↑6.60 | ↓28.74 | ↓29.12 | ↑86.14 | ↑46.16 | ↓100.00 |
| 7 | 洛伐他汀羟酸 Lovastatin hydroxy acid | ↓91.26 | ↓85.64 | ↓88.04 | ↓62.17 | ↓83.70 | ↓85.34 |
| 8 | 洛伐他汀脱水产物 Lovastatin dehydrated products | ↓34.19 | ↑10.05 | ↓57.14 | ↑50.70 | ↑15.97 | ↓98.61 |
| 9 | 洛伐他脱氧产物 Lovastatin deoxygenation products | ↓64.96 | ↓100.00 | ↓100.00 | ↑95.58 | ↑12.68 | ↓100.00 |
| 洛伐他汀类化合物总产量 Total production of lovastatin compounds | ↓43.48 | ↓48.02 | ↓60.20 | ↑16.07 | ↓15.59 | ↓93.41 | |
| 10 | N/A | ↓25.36 | ↓12.73 | ↓16.03 | ↓6.82 | ↓17.87 | ↓34.47 |
| 11 | Aspernolide C | ↑30.46 | ↓20.96 | ↑24.90 | ↓79.36 | ↓80.96 | ↓55.49 |
| 12 | Isochromophilone VI脱氢产物 Isochromophilone VI dehydrogenation product | ↓1.59 | ↓29.31 | ↓6.46 | ↓71.79 | ↓75.73 | ↓58.11 |
| 13 | Epi-aszonaleni A | ↑11.30 | ↓24.58 | ↓9.34 | ↓66.76 | ↓70.52 | ↓40.02 |
| 14 | N/A | ↓28.33 | ↑0.59 | ↓54.44 | ↓24.12 | ↓53.74 | ↓90.22 |
| 15 | Asterriquinone CT3 | ↓21.98 | ↓31.05 | ↑34.49 | ↓81.50 | ↓84.16 | ↓11.36 |
| 16 | Perinadine A | ↓43.49 | ↑38.06 | ↓66.44 | ↓15.69 | ↓46.11 | ↓88.34 |
| 17 | 12a-dehydroxyisoterreulactone A or Terreulactone C | ↓67.24 | ↓26.58 | ↓85.57 | ↑6.36 | ↓22.89 | ↓100.00 |
| 18 | Teraspiridole B | ↑8.11 | ↑14.79 | ↑148.27 | ↓13.45 | ↓38.63 | ↑94.77 |
| 19 | N/A | ↓26.37 | ↑1.29 | ↑13.45 | ↑50.55 | ↓3.21 | ↑19.44 |
| 20 | N/A | ↑111.26 | ↓81.63 | ↑1208.42 | ↑570.07 | ↑181.73 | ↑1399.99 |
表1 化合物1-20在不同培养条件下的含量(峰面积积分)相对于空白对照的变化
Table 1 Variation of compounds 1-20's contents(integrated peak areas)under different cultural conditions compared to CK
| 化合物序号Compound No. | 化合物注释 Compound note | 不同培养条件下的产物产量变化幅度 Changes in product yield under different cultivation conditions/% | |||||
|---|---|---|---|---|---|---|---|
| DMSO group | 0.05 mmol/L 3,4-DHPPA group | 10 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mmol/L PS group | 2.1 mmol/L NTSP + 0.15 mM PS + 0.05 mmol/L 3,4-DHPPA group | 2.1 mmol/L NTSP + 0.15 mM PS + 10 mmol/L 3,4-DHPPA group | ||
| 1 | 丁内酯I Butyrolactone I | ↓62.50 | ↓32.71 | ↓90.06 | ↓74.04 | ↓68.76 | ↓92.19 |
| 2 | 丁内酯I脱水产物 Butyrolactone I dehydrated product | ↓71.53 | ↓34.28 | ↓78.36 | ↓100.00 | ↓67.42 | ↓100.00 |
| 3 | 土震素B Territrem B | ↑4.40 | ↓26.45 | ↑9.58 | ↓73.15 | ↓78.94 | ↓57.47 |
| 4 | 土震素B脱氧产物 Territrem B deoxylation products | ↑65.52 | ↓100.00 | ↑38.07 | ↓80.67 | ↓84.58 | ↓100.00 |
| 5 | 土震素B降碳产物 Territrem B carbon reduction products | ↑50.57 | ↓56.52 | ↓33.36 | ↓100.00 | ↓100.00 | ↓86.79 |
| 土震素B类化合物总产量 Total production of territrem B compound | ↑5.12 | ↓27.10 | ↑9.36 | ↓73.42 | ↓79.14 | ↓57.95 | |
| 6 | 洛伐他汀Lovastatin | ↑6.60 | ↓28.74 | ↓29.12 | ↑86.14 | ↑46.16 | ↓100.00 |
| 7 | 洛伐他汀羟酸 Lovastatin hydroxy acid | ↓91.26 | ↓85.64 | ↓88.04 | ↓62.17 | ↓83.70 | ↓85.34 |
| 8 | 洛伐他汀脱水产物 Lovastatin dehydrated products | ↓34.19 | ↑10.05 | ↓57.14 | ↑50.70 | ↑15.97 | ↓98.61 |
| 9 | 洛伐他脱氧产物 Lovastatin deoxygenation products | ↓64.96 | ↓100.00 | ↓100.00 | ↑95.58 | ↑12.68 | ↓100.00 |
| 洛伐他汀类化合物总产量 Total production of lovastatin compounds | ↓43.48 | ↓48.02 | ↓60.20 | ↑16.07 | ↓15.59 | ↓93.41 | |
| 10 | N/A | ↓25.36 | ↓12.73 | ↓16.03 | ↓6.82 | ↓17.87 | ↓34.47 |
| 11 | Aspernolide C | ↑30.46 | ↓20.96 | ↑24.90 | ↓79.36 | ↓80.96 | ↓55.49 |
| 12 | Isochromophilone VI脱氢产物 Isochromophilone VI dehydrogenation product | ↓1.59 | ↓29.31 | ↓6.46 | ↓71.79 | ↓75.73 | ↓58.11 |
| 13 | Epi-aszonaleni A | ↑11.30 | ↓24.58 | ↓9.34 | ↓66.76 | ↓70.52 | ↓40.02 |
| 14 | N/A | ↓28.33 | ↑0.59 | ↓54.44 | ↓24.12 | ↓53.74 | ↓90.22 |
| 15 | Asterriquinone CT3 | ↓21.98 | ↓31.05 | ↑34.49 | ↓81.50 | ↓84.16 | ↓11.36 |
| 16 | Perinadine A | ↓43.49 | ↑38.06 | ↓66.44 | ↓15.69 | ↓46.11 | ↓88.34 |
| 17 | 12a-dehydroxyisoterreulactone A or Terreulactone C | ↓67.24 | ↓26.58 | ↓85.57 | ↑6.36 | ↓22.89 | ↓100.00 |
| 18 | Teraspiridole B | ↑8.11 | ↑14.79 | ↑148.27 | ↓13.45 | ↓38.63 | ↑94.77 |
| 19 | N/A | ↓26.37 | ↑1.29 | ↑13.45 | ↑50.55 | ↓3.21 | ↑19.44 |
| 20 | N/A | ↑111.26 | ↓81.63 | ↑1208.42 | ↑570.07 | ↑181.73 | ↑1399.99 |
| [1] | 张偲, 张长生, 田新朋, 等. 中国海洋微生物多样性研究[J]. 中国科学院院刊, 2010, 25(6): 651-658. |
| Zhang S, Zhang CS, Tian XP, et al. The study of diversities of marine microbes in China[J]. Bull Chin Acad Sci, 2010, 25(6): 651-658. | |
| [2] |
马丽丽, 田新朋, 李桂菊, 等. 海洋微生物来源天然产物研究现状与态势[J]. 热带海洋学报, 2021, 40(5): 134-146.
doi: 10.11978/2020104 |
| Ma LL, Tian XP, Li GJ, et al. Research status and development trends of natural products from marine microorganisms[J]. J Trop Oceanogr, 2021, 40(5): 134-146. | |
| [3] |
Carroll AR, Copp BR, Davis RA, et al. Marine natural products[J]. Nat Prod Rep, 2020, 37(2): 175-223.
doi: 10.1039/c9np00069k pmid: 32025684 |
| [4] |
Weist S, Süssmuth RD. Mutational biosynthesis—a tool for the generation of structural diversity in the biosynthesis of antibiotics[J]. Appl Microbiol Biotechnol, 2005, 68(2): 141-150.
pmid: 15702315 |
| [5] | Rinehart KL Jr. Biosynthesis and mutasynthesis of aminocyclitol antibiotics[J]. Jpn J Antibiot, 1979, 32 Suppl: S32-S46. |
| [6] |
An X, Feng BM, Chen G, et al. Isolation and identification of phase I metabolites of butyrolactone I in rats[J]. Xenobiotica, 2017, 47(3): 236-244.
doi: 10.3109/00498254.2016.1172280 pmid: 27604497 |
| [7] |
Ibrahim SRM, Mohamed GA, Khedr AIM. γ-butyrolactones from Aspergillus species: structures, biosynthesis, and biological activities[J]. Nat Prod Commun, 2017, 12(5): 791-800.
pmid: 30496667 |
| [8] | Jiang M, Zhang HR. Engineering the shikimate pathway for biosynthesis of molecules with pharmaceutical activities in E. coli[J]. Curr Opin Biotechnol, 2016, 42: 1-6. |
| [9] | 江晶洁, 刘涛, 林双君. 基于莽草酸途径微生物合成芳香族化合物及其衍生物的研究进展[J]. 生命科学, 2019, 31(5): 430-448. |
| Jiang JJ, Liu T, Lin SJ. Research progress on the biosynthesis of aromatic compounds by microorganisms[J]. Chin Bull Life Sci, 2019, 31(5): 430-448. | |
| [10] | Pittard J, Yang J. Biosynthesis of the aromatic amino acids[J]. EcoSal Plus, 2008, 3(1): 10.1128/ecosalplus.3.6.1.8. |
| [11] |
Chen RD, Gao BQ, Liu X, et al. Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase[J]. Nat Chem Biol, 2017, 13(2): 226-234.
doi: 10.1038/nchembio.2263 pmid: 27992881 |
| [12] | Hühner E, Öqvist K, Li SM. Design of α-keto carboxylic acid dimers by domain recombination of nonribosomal peptide synthetase(NRPS)-like enzymes[J]. Org Lett, 2019, 21(2): 498-502. |
| [13] |
van Dijk JWA, Wang CCC. Expanding the chemical space of nonribosomal peptide synthetase-like enzymes by domain and tailoring enzyme recombination[J]. Org Lett, 2018, 20(17): 5082-5085.
doi: 10.1021/acs.orglett.8b01581 pmid: 30106305 |
| [14] | Zheng YY, Ma ZL, Wu JS, et al. Induction of secondary metabolite biosynthesis by deleting the histone deacetylase HdaA in the marine-derived fungus Aspergillus terreus RA2905[J]. J Fungi, 2022, 8(10): 1024. |
| [15] | Fan H, Wei X, Si-Tu MX, et al. γ-aromatic butenolides of microbial source-A review of their structures, biological activities and biosynthesis[J]. Chem Biodivers, 2022, 19(6): e202200208. |
| [16] | 蔡彩虹, 郑浩, 盖翠娟, 等. 一株藤壶内生真菌的次生代谢产物[J]. 中国药科大学学报, 2023, 54(1): 62-67. |
| Cai CH, Zheng H, Gai CJ, et al. Secondary metabolites of the endophytic fungus Aspergillus sp. Dq-25 from barnacle[J]. J China Pharm Univ, 2023, 54(1): 62-67. | |
| [17] | 刘冰, 唐祉娟, 陈宁, 等. 海洋来源曲霉丁内酯类次级代谢产物研究进展[J]. 中国海洋药物, 2021, 40(5): 59-70. |
| Liu B, Tang ZJ, Chen N, et al. Research progress of butyrolactones isolated from marine-derived Aspergillus sp[J]. Chin J Mar Drugs, 2021, 40(5): 59-70. | |
| [18] | 杨静明, 杨文聪, 刘亚月, 等. 化学诱导对一株海洋来源土曲霉C23-3次生代谢产物及其生物活性的影响[J]. 微生物学通报, 2019, 46(3): 441-452. |
| Yang JM, Yang WC, Liu YY, et al. Influence of chemical induction on the secondary metabolites and biological activities of a marine-derived fungal strain Aspergillus terreus C23-3[J]. Microbiol China, 2019, 46(3): 441-452. | |
| [19] |
马小翔, 刘亚月, 聂影影, 等. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-1398 |
| Ma XX, Liu YY, Nie YY, et al. LC-MS/MS based molecular network analysis of the effects of chemical regulation on the secondary metabolites and biological activities of a fungal strain Aspergillus terreus C23-3[J]. Biotechnol Bull, 2021, 37(8): 95-110. | |
| [20] |
Andrews PR, Cain EN, Rizzardo E, et al. Rearrangement of chorismate to prephenate. Use of chorismate mutase inhibitors to define the transition state structure[J]. Biochemistry, 1977, 16(22): 4848-4852.
pmid: 911795 |
| [21] |
Smith GD, Roberts DV, Daday A. Affinity chromatography and inhibition of chorismate mutase-prephenate dehydrogenase by derivatives of phenylalanine and tyrosine[J]. Biochem J, 1977, 165(1): 121-126.
pmid: 889568 |
| [22] |
Dyck LE, Dewar KM. Inhibition of aromatic L-amino acid decarboxylase and tyrosine aminotransferase by the monoamine oxidase inhibitor phenelzine[J]. J Neurochem, 1986, 46(6): 1899-1903.
pmid: 2871132 |
| [23] | Guo CJ, Knox BP, Sanchez JF, et al. Application of an efficient gene targeting system linking secondary metabolites to their biosynthetic genes in Aspergillus terreus[J]. Org Lett, 2013, 15(14): 3562-3565. |
| [24] |
Palonen EK, Neffling MR, Raina S, et al. Butyrolactone I quantification from lovastatin producing Aspergillus terreus using tandem mass spectrometry-evidence of signalling functions[J]. Microorganisms, 2014, 2(2): 111-127.
doi: 10.3390/microorganisms2020111 pmid: 27682234 |
| [25] | Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp[J]. Eukaryot Cell, 2004, 3(2): 527-535. |
| [26] | Palonen EK, Raina S, Brandt A, et al. Transcriptomic complexity of Aspergillus terreus velvet gene family under the influence of butyrolactone I[J]. Microorganisms, 2017, 5(1): 12. |
| [27] | Raina S, De Vizio D, Palonen EK, et al. Is quorum sensing involved in lovastatin production in the filamentous fungus Aspergillus terreus?[J]. Process Biochem, 2012, 47(5): 843-852. |
| [28] |
奚萌宇, 胡逸灵, 顾玉城, 等. 基因组挖掘指导天然药物分子的发现[J]. 合成生物学, 2024, 5(3): 447-473.
doi: 10.12211/2096-8280.2023-086 |
| Xi MY, Hu YL, Gu YC, et al. Genome mining-directed discovery for natural medicinal products[J]. Synth Biol J, 2024, 5(3): 447-473. | |
| [29] | Wang XH, Lin MY, Xu D, et al. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides[J]. Molecules, 2017, 22(12): 2069. |
| [30] | Wang XH, Li YY, Zhang XP, et al. Structural diversity and biological activities of the cyclodipeptides from fungi[J]. Molecules, 2017, 22(12): 2026. |
| [1] | 姚近东, 汤华妹, 杨文霄, 张丽珊, 林向民. 恩诺沙星胁迫下嗜水气单胞菌的比较蛋白质组学研究[J]. 生物技术通报, 2023, 39(4): 288-296. |
| [2] | 孙淑芳, 骆永丽, 李春辉, 金敏, 胥倩. UPLC-MS/MS测定小麦茎秆木质素单体交联结构的方法[J]. 生物技术通报, 2022, 38(10): 66-72. |
| [3] | 马小翔, 刘亚月, 聂影影, 黎燕媚, 王远, 薛欣怡, 洪鹏志, 张翼. 基于质谱的分子网络分析化学调控对土曲霉C23-3次生代谢产物及生物活性的影响[J]. 生物技术通报, 2021, 37(8): 95-110. |
| [4] | 刘珊, 叶伟, 朱牧孜, 李赛妮, 邓张双, 章卫民. 一种新型酰基转移酶GPAT的克隆、表达与酶学性质研究[J]. 生物技术通报, 2021, 37(11): 257-266. |
| [5] | 孟丽娜, 彭春莹, 李铁栋, 李博生. 基于蛋白质组学对螺旋藻砷胁迫响应机制的研究[J]. 生物技术通报, 2020, 36(4): 107-116. |
| [6] | 甘崇琨, 周慧文, 陈荣发, 范业赓, 丘立杭, 黄杏, 李杨瑞, 卢星高, 吴建明. 化学调控在甘蔗生产上的研究应用[J]. 生物技术通报, 2019, 35(2): 163-170. |
| [7] | 黄自磊, 章卫民, 叶伟, 李赛妮, 李浩华, 朱牧孜. 深海真菌Dichotomomyces cejpii胶霉毒素生物合成基因启动子的克隆和功能鉴定[J]. 生物技术通报, 2018, 34(4): 144-150. |
| [8] | 胡晟源, 刘姝, 王淑军, 焦豫良, 来蒋丽, 顾张慧, 房耀维,. 一株产黑色素海洋真菌的分离、鉴定及色素性质的研究[J]. 生物技术通报, 2017, 33(12): 138-143. |
| [9] | 杨小岚, 陈玉婵, 李浩华, 章卫民. 23株海洋真菌的分子鉴定及其抗植物病原真菌和细胞毒活性研究[J]. 生物技术通报, 2014, 30(8): 132-137. |
| [10] | 李浩华 陈玉婵 王磊 章卫民. 海洋真菌梅花状青霉FS83 抗菌抗肿瘤活性研究[J]. 生物技术通报, 2013, 0(1): 151-155. |
| [11] | . 抗生素和干扰素[J]. , 1996, 0(05): 64-66. |
| [12] | E.R.Ward. 植物转基因表达的化学调控[J]. , 1994, 0(01): 1-4. |
| [13] | 李思经;. 转基因植物中基因表达的化学调节[J]. , 1993, 0(01): 18-19. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||