








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (4): 77-84.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0870
Previous Articles Next Articles
Received:2023-09-11
Online:2024-04-26
Published:2024-04-30
SANG Sen-hua. Detection of NK Cell Cytotoxicity: Real-time Dynamic Imaging-Based Analysis[J]. Biotechnology Bulletin, 2024, 40(4): 77-84.
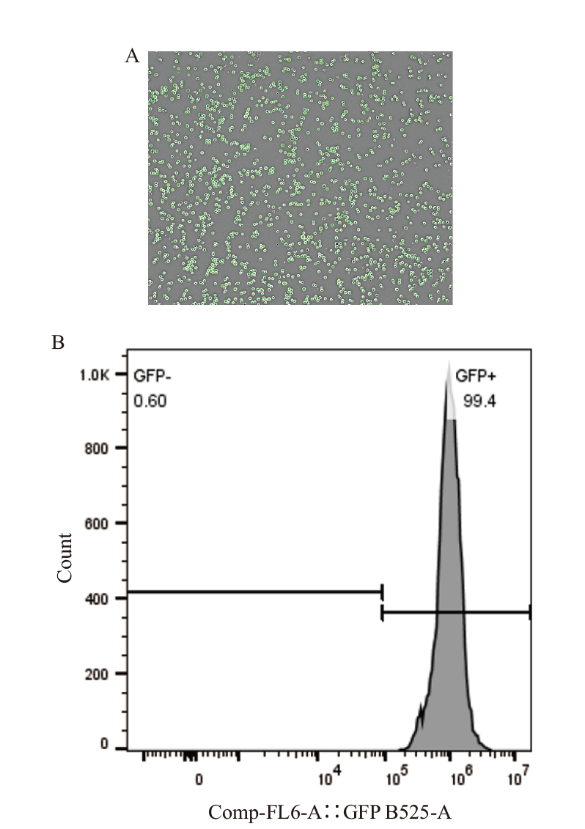
Fig. 1 Transfection efficiency and fluorescence intensity of K562-GFP cells A: The transfection of K562-GFP cells with GFP was observed by integrating the white light image with the fluorescence image. B: The green fluorescence intensity of K562-GFP cells was analyzed by flow cytometry
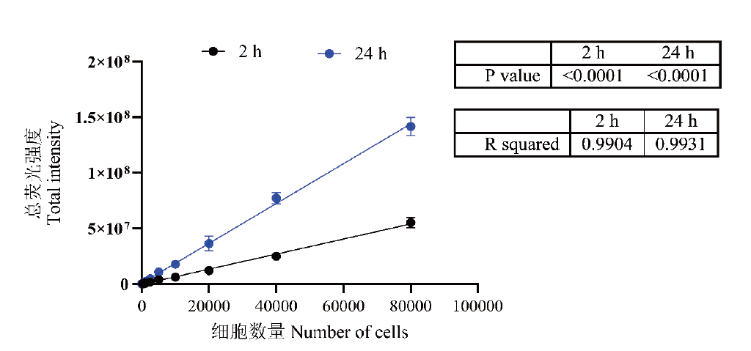
Fig. 3 Relationship between total intensity and number of cells There is a good linear relationship between the number of cells and the total fluorescence intensity at both the early stage(solid black line)and the growth stage(solid blue line)
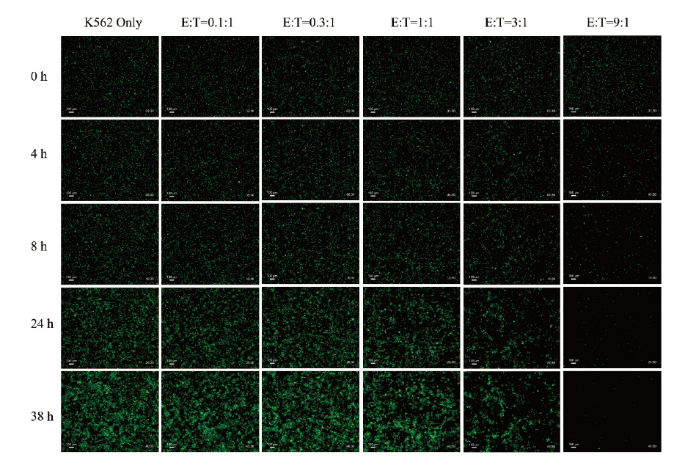
Fig. 4 Green fluorescence images of NK cells and K562-GFP cells at different co-culture moments Fluorescence images of different culture groups(columns), including the single culture group of K562 and the co-culture groups of NK and K562 at different efficiency target ratios(0.1, 0.3, 1, 3, and 9)at different co-culture times(rows), and co-culture time parameters captured included 0, 4, 8, 24, and 38 h
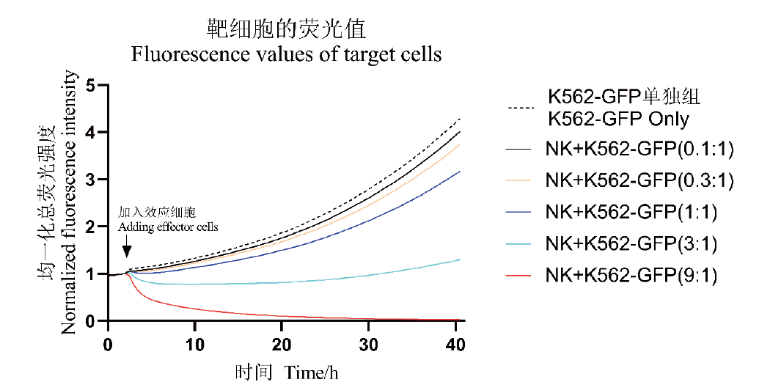
Fig. 5 Changing curves of NK cells-mediated cytotoxicity leading to K562-GFP cell lysis The proliferation curve of K562-GFP alone(dotted line)and the cytotoxic cleavage curve of K562-GFP cells mediated by NK cells under different effect target ratios(9∶1, 3∶1, 1∶1, 0.3∶1, and 0.1∶1)are monitored in real time within 40 h(solid line)

Fig. 6 Cytotoxicity mediated by NK cells to K562-GFP cells at different co-culture time points The 4PL curves corresponding to different effector target ratios(9∶1, 3∶1, 1∶1, 0.3∶1, 0.1∶1)obtained every 0.5 h during co-culture of effector cells and target cells, where the solid blue line is the 4PL curve of 24 h
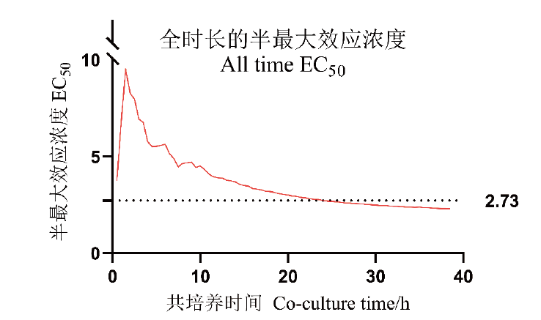
Fig. 7 EC50 of NK cells to K562-GFP cells in co-culture stage The real-time change curve of EC50 generated every 0.5 h during co-culture of effector cells and target cells(solid red line)and the results of EC50 coculture for 24 h

Fig. 8 Comparison of two different methods of calculating cytotoxicity A: Cytotoxicity of NK cells at co-culture for 2 h. B: Cytotoxicity of NK cells at co-culture for 4 h. C: Cytotoxicity of NK cells at co-culture for 6 h. D: Cytotoxicity of NK cells at co-culture for 24 h. Late apoptosis indicator 7AAD+ cell population for calculating the cytotoxicity of NK cells to K562-GFP cells(black), and GFP+ cell population for calculating the cytotoxicity of NK cells to K562-GFP cells(red)
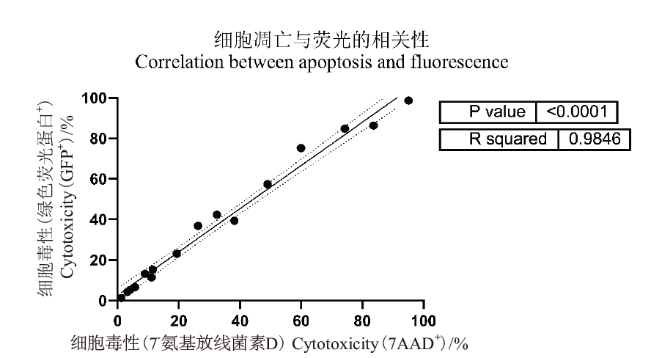
Fig. 9 Correlation between late apoptotic cells and green fluorescent protein degradation The horizontal coordinate is 7AAD+ cell population to calculate the cytotoxicity of NK cells to K562-GFP cells, and the vertical coordinate is GFP+ cell population to calculate the cytotoxicity of NK cells to K562-GFP cells
| [1] |
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017[J]. CA A Cancer J Clinicians, 2017, 67(3): 177-193.
doi: 10.3322/caac.v67.3 URL |
| [2] |
Yang XL, Cao WD, Wang XF, et al. Down-regulation of 14-3-3zeta reduces proliferation and increases apoptosis in human glioblastoma[J]. Cancer Gene Ther, 2020, 27(6): 399-411.
doi: 10.1038/s41417-019-0097-7 pmid: 31068674 |
| [3] | Chen ZW, Zhang PD, Xu Y, et al. Surgical stress and cancer progression: the twisted tango[J]. Mol Cancer, 2019, 18(1): 132. |
| [4] | Fokas E, Schlenska-Lange A, Polat B, et al. Chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for patients with locally advanced rectal cancer: long-term results of the CAO/ARO/AIO-12 randomized clinical trial[J]. JAMA Oncol, 2022, 8(1): e215445. |
| [5] |
Xu-Monette ZY, Zhou JF, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas[J]. Blood, 2018, 131(1): 68-83.
doi: 10.1182/blood-2017-07-740993 pmid: 29118007 |
| [6] |
Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012[J]. CA A Cancer J Clinicians, 2015, 65(2): 87-108.
doi: 10.3322/caac.21262 URL |
| [7] |
Sun Y. Translational horizons in the tumor microenvironment: harnessing breakthroughs and targeting cures[J]. Med Res Rev, 2015, 35(2): 408-436.
doi: 10.1002/med.21338 pmid: 25588753 |
| [8] | Haslauer T, Greil R, Zaborsky N, et al. CAR T-cell therapy in hematological malignancies[J]. Int J Mol Sci, 2021, 22(16): 8996. |
| [9] |
Kiesgen S, Messinger JC, Chintala NK, et al. Comparative analysis of assays to measure CAR T-cell-mediated cytotoxicity[J]. Nat Protoc, 2021, 16(3): 1331-1342.
doi: 10.1038/s41596-020-00467-0 pmid: 33589826 |
| [10] | Lisby AN, Carlson RD, Baybutt TR, et al. Evaluation of CAR-T cell cytotoxicity: real-time impedance-based analysis[J]. Methods Cell Biol, 2022, 167: 81-98. |
| [11] |
Cen H, Mao F, Aronchik I, et al. DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells[J]. FASEB J, 2008, 22(7): 2243-2252.
doi: 10.1096/fj.07-099234 pmid: 18263700 |
| [12] |
Komoriya A, Packard BZ, Brown MJ, et al. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates[J]. J Exp Med, 2000, 191(11): 1819-1828.
doi: 10.1084/jem.191.11.1819 pmid: 10839799 |
| [13] |
Liu LZ, Chahroudi A, Silvestri G, et al. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates[J]. Nat Med, 2002, 8(2): 185-189.
pmid: 11821904 |
| [14] |
Poreba M, Szalek A, Kasperkiewicz P, et al. Small molecule active site directed tools for studying human caspases[J]. Chem Rev, 2015, 115(22): 12546-12629.
doi: 10.1021/acs.chemrev.5b00434 pmid: 26551511 |
| [15] | Maalej KM, Merhi M, Inchakalody VP, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances[J]. Mol Cancer, 2023, 22(1): 20. |
| [16] |
Franks SE, Wolfson B, Hodge JW. Natural born killers: NK cells in cancer therapy[J]. Cancers, 2020, 12(8): 2131.
doi: 10.3390/cancers12082131 URL |
| [17] |
Wu XL, Zhang Y, Li YT, et al. Improvements in flow cytometry-based cytotoxicity assay[J]. Cytometry A, 2021, 99(7): 680-688.
doi: 10.1002/cyto.a.v99.7 URL |
| [18] |
Chen XZ, Gao AQ, Zhang F, et al. ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation[J]. Theranostics, 2021, 11(7): 3392-3416.
doi: 10.7150/thno.52435 pmid: 33537094 |
| [19] | Oberg HH, Peters C, Kabelitz D, et al. Real-time cell analysis(RTCA)to measure killer cell activity against adherent tumor cells in vitro[J]. Methods Enzymol, 2020, 631: 429-441. |
| [20] |
Maser T, Zagorski J, Kelly S, et al. The MDM2 inhibitor CGM097 combined with the BET inhibitor OTX015 induces cell death and inhibits tumor growth in models of neuroblastoma[J]. Cancer Med, 2020, 9(21): 8144-8158.
doi: 10.1002/cam4.v9.21 URL |
| [21] |
Wetzel A, Bonnefoy F, Chagué C, et al. Pro-resolving factor administration limits cancer progression by enhancing immune response against cancer cells[J]. Front Immunol, 2022, 12: 812171.
doi: 10.3389/fimmu.2021.812171 URL |
| [22] | Park DJ, Sung PS, Kim JH, et al. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1[J]. J Immunother Cancer, 2020, 8(1): e000301. |
| [23] |
Irelan JT, Wu MJ, Morgan J, et al. Rapid and quantitative assessment of cell quality, identity, and functionality for cell-based assays using real-time cellular analysis[J]. J Biomol Screen, 2011, 16(3): 313-322.
doi: 10.1177/1087057110397359 pmid: 21310850 |
| [24] | Meng JY, Peng J, Feng J, et al. Niraparib exhibits a synergistic anti-tumor effect with PD-L1 blockade by inducing an immune response in ovarian cancer[J]. J Transl Med, 2021, 19(1): 415. |
| [25] | Zhang D, Teng R, Lv N, et al. A novel CD2 staining-based flow cytometric assay for assessment of natural killer cell cytotoxicity[J]. J Clin Lab Anal, 2020, 34(12): e23519. |
| [26] |
Zhou L, Wang SS, Cao LN, et al. Lead acetate induces apoptosis in Leydig cells by activating PPARγ/caspase-3/PARP pathway[J]. Int J Environ Health Res, 2021, 31(1): 34-44.
doi: 10.1080/09603123.2019.1625034 URL |
| [27] |
Jahanian-Najafabadi A, Mirian M, Rohani F, et al. Novel palladium complex: cytotoxicity against cisplatin-resistant K562 cells[J]. Iran J Pharm Res, 2019, 18(3): 1323-1331.
doi: 10.22037/ijpr.2019.1100714 pmid: 32641942 |
| [28] |
Rezano A, Ridhayanti F, Rangkuti AR, et al. Cytotoxicity of simvastatin in human breast cancer MCF-7 and MDA-MB-231 cell lines[J]. Asian Pac J Cancer Prev, 2021, 22(S1): 33-42.
doi: 10.31557/APJCP.2021.22.S1.33 URL |
| [29] | Ismail NI, Othman I, Abas F, et al. The curcumin analogue, MS13(1, 5-bis(4-hydroxy-3- methoxyphenyl)-1, 4-pentadiene-3-one), inhibits cell proliferation and induces apoptosis in primary and metastatic human colon cancer cells[J]. Molecules, 2020, 25 (17): 3798. |
| [30] | Wurster S, Kumaresan PR, Albert ND, et al. Live monitoring and analysis of fungal growth, viability, and mycelial morphology using the IncuCyte NeuroTrack processing module[J]. mBio, 2019, 10(3): e00673-19. |
| [1] | LI Yan-wei, SONG Xing-hui, WANG Jia-jia, LIU Li, HUANG Ying-ying, GUO Chun. Establishment of the Real-time and Label-free Screening System for Tumor Cell Apoptosis [J]. Biotechnology Bulletin, 2019, 35(10): 220-226. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
