








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (9): 191-197.doi: 10.13560/j.cnki.biotech.bull.1985.2022-0570
Previous Articles Next Articles
SHI Guang-zhen( ), WANG Zhao-ye, SUN Qi, ZHU Xin-xia(
), WANG Zhao-ye, SUN Qi, ZHU Xin-xia( )
)
Received:2022-05-09
Online:2022-09-26
Published:2022-10-11
Contact:
ZHU Xin-xia
E-mail:1106517620@qq.com;302641316@qq.com
SHI Guang-zhen, WANG Zhao-ye, SUN Qi, ZHU Xin-xia. Cloning and Activity Analysis of SikCDPK1 Promoter from Saussurea involucrata[J]. Biotechnology Bulletin, 2022, 38(9): 191-197.
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| AD1 | NTCGASTWTSGWGTT | TAIL-PCR |
| AD2 | NGTCGASWGANAWGAAAA | TAIL-PCR |
| AD3 | WGTCNACWANCANACA | TAIL-PCR |
| AD4 | TGWGNAGWANCANAGA | TAIL-PCR |
| AD5 | AGWGNAGWANCAWAGG | TAIL-PCR |
| SP1 | CGTTATCCCAAACTGCCCTTGTCCTA | TAIL-PCR |
| SP2 | TCCGTTAGGGGTAGGTGGGGTATCTT | TAIL-PCR |
| SP3 | TTCGGTCCAACACAAGTATTCCCCAT | TAIL-PCR |
| pSikCDPK1-F1 | CCCAAGCTTCCTTAGCATCTATGAGGGTCG | 启动子克隆 Promoter cloning |
| pSikCDPK1-R1 | GACTAGTTCCAACACAAGTATTCCCCATG | 启动子克隆 Promoter cloning |
| pSikCDPK1-F2 | CCCAAGCTTCTCTTCTTATGGGTTCAAAGGGTCA | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F3 | CCCAAGCTTAAGCGTCATGCCAGTCAAGC | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F4 | CCCAAGCTTTTTCAATGGAGAAGCGACGAGC | 5'端缺失分析 5'-end deletion analysis |
Table 1 Primers used in this study
| 引物Primer name | 引物序列Primer sequence(5'-3') | 用途Purpose |
|---|---|---|
| AD1 | NTCGASTWTSGWGTT | TAIL-PCR |
| AD2 | NGTCGASWGANAWGAAAA | TAIL-PCR |
| AD3 | WGTCNACWANCANACA | TAIL-PCR |
| AD4 | TGWGNAGWANCANAGA | TAIL-PCR |
| AD5 | AGWGNAGWANCAWAGG | TAIL-PCR |
| SP1 | CGTTATCCCAAACTGCCCTTGTCCTA | TAIL-PCR |
| SP2 | TCCGTTAGGGGTAGGTGGGGTATCTT | TAIL-PCR |
| SP3 | TTCGGTCCAACACAAGTATTCCCCAT | TAIL-PCR |
| pSikCDPK1-F1 | CCCAAGCTTCCTTAGCATCTATGAGGGTCG | 启动子克隆 Promoter cloning |
| pSikCDPK1-R1 | GACTAGTTCCAACACAAGTATTCCCCATG | 启动子克隆 Promoter cloning |
| pSikCDPK1-F2 | CCCAAGCTTCTCTTCTTATGGGTTCAAAGGGTCA | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F3 | CCCAAGCTTAAGCGTCATGCCAGTCAAGC | 5'端缺失分析 5'-end deletion analysis |
| pSikCDPK1-F4 | CCCAAGCTTTTTCAATGGAGAAGCGACGAGC | 5'端缺失分析 5'-end deletion analysis |
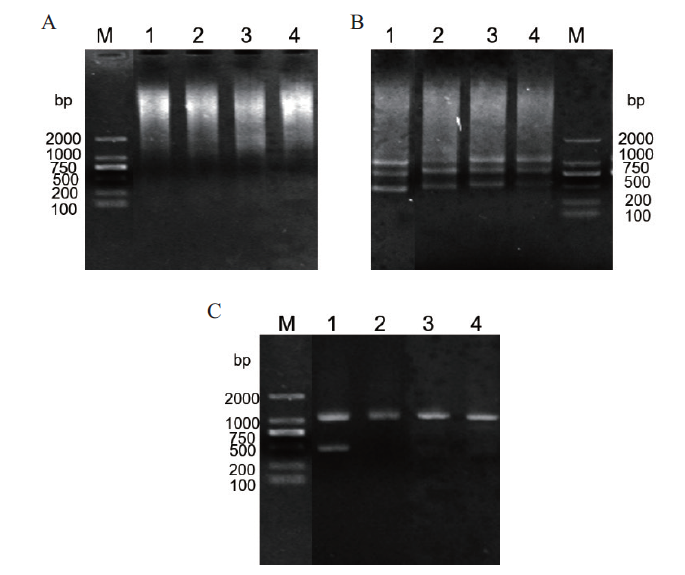
Fig. 2 Cloning of SikCDPK1 promoter A:The first round of PCR amplification. B:The second round of PCR amplification. C:The third round of PCR amplification. M:DNA2000 marker. 1:AD1. 2:AD2. 3:AD3. 4:AD4
| 顺式作用元件名称 Name of cis-acting element | 序列 Sequence(5'-3') | 功能 Function | 位置 Location/ nt |
|---|---|---|---|
| O2-site | GATGA(C/T)(A/G)TG(A/G)或GATGATGTGG | 玉米醇溶蛋白代谢调节 Zein metabolism regulation | -56 to -48,-596 to -587 |
| CGTCA-motif | CGTCA | 茉莉酸甲酯响应 MeJA-responsiveness | -94 to -89,-562 to -557,-479 to -474 |
| CBFHV | RYCGAC | 低温反应元件 Cis-acting element for cold | -103 to -97,-379 to -373,-672 to -666 |
| GT1GMSCAM4 | GAAAAA | 盐诱导响应相关元件 NaCl-induced | -159 to -153 |
| LTRECOREATCOR15 | CCGAC | 冷诱导低温反应元件 Cis-acting element for cold induction | -215 to -210,-459 to -454 |
| GC-motif | GCCCCC | 参与缺氧特异性诱导的类增强子Enhancer-like element involved in anoxic specific inducibility | -287 to -281 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应 MeJA-responsiveness | -310 to -305,-499 to -494 |
| ABRE | TACGGTC | ABA响应Abscisic acid responsiveness | -327 to -320 |
| W-box | TTGACC | WRKY转录因子的结合位点WRKY transcription factor binding site | -387 to -381 |
| box-S | AGCCACC | 无功能No function | -571 to -564 |
| MYC | CAATTG | 无功能No function | -533 to -539 |
| G-box | CACGTC | 光响应 Light responsiveness | -689 to -683 |
| MYB | CAACCA | MYB 顺式作用元件MYB Cis-acting element | -759 to -753 |
| OSE2ROOTNODULE | CTCTT | 在根瘤的感染细胞中被激活 Activated in infected cells of root nodules | -821 to -816 |
| MYB-Core | CGTTAG | 脱水胁迫反应元件Responsive to dehydration | -958 to -952 |
| DPBFCOREDCDC3 | ACACNNG | 脱落酸响应元件ABA-responsive elements | -975 to -967 |
| ACGTATERD1 | ACGT | 脱水诱导反应元件 Early responsive to dehydration | -1 038 to -1 034 |
Table 2 Cis-elements and functions in the promoter pSikCDPK1 sequence
| 顺式作用元件名称 Name of cis-acting element | 序列 Sequence(5'-3') | 功能 Function | 位置 Location/ nt |
|---|---|---|---|
| O2-site | GATGA(C/T)(A/G)TG(A/G)或GATGATGTGG | 玉米醇溶蛋白代谢调节 Zein metabolism regulation | -56 to -48,-596 to -587 |
| CGTCA-motif | CGTCA | 茉莉酸甲酯响应 MeJA-responsiveness | -94 to -89,-562 to -557,-479 to -474 |
| CBFHV | RYCGAC | 低温反应元件 Cis-acting element for cold | -103 to -97,-379 to -373,-672 to -666 |
| GT1GMSCAM4 | GAAAAA | 盐诱导响应相关元件 NaCl-induced | -159 to -153 |
| LTRECOREATCOR15 | CCGAC | 冷诱导低温反应元件 Cis-acting element for cold induction | -215 to -210,-459 to -454 |
| GC-motif | GCCCCC | 参与缺氧特异性诱导的类增强子Enhancer-like element involved in anoxic specific inducibility | -287 to -281 |
| TGACG-motif | TGACG | 茉莉酸甲酯响应 MeJA-responsiveness | -310 to -305,-499 to -494 |
| ABRE | TACGGTC | ABA响应Abscisic acid responsiveness | -327 to -320 |
| W-box | TTGACC | WRKY转录因子的结合位点WRKY transcription factor binding site | -387 to -381 |
| box-S | AGCCACC | 无功能No function | -571 to -564 |
| MYC | CAATTG | 无功能No function | -533 to -539 |
| G-box | CACGTC | 光响应 Light responsiveness | -689 to -683 |
| MYB | CAACCA | MYB 顺式作用元件MYB Cis-acting element | -759 to -753 |
| OSE2ROOTNODULE | CTCTT | 在根瘤的感染细胞中被激活 Activated in infected cells of root nodules | -821 to -816 |
| MYB-Core | CGTTAG | 脱水胁迫反应元件Responsive to dehydration | -958 to -952 |
| DPBFCOREDCDC3 | ACACNNG | 脱落酸响应元件ABA-responsive elements | -975 to -967 |
| ACGTATERD1 | ACGT | 脱水诱导反应元件 Early responsive to dehydration | -1 038 to -1 034 |
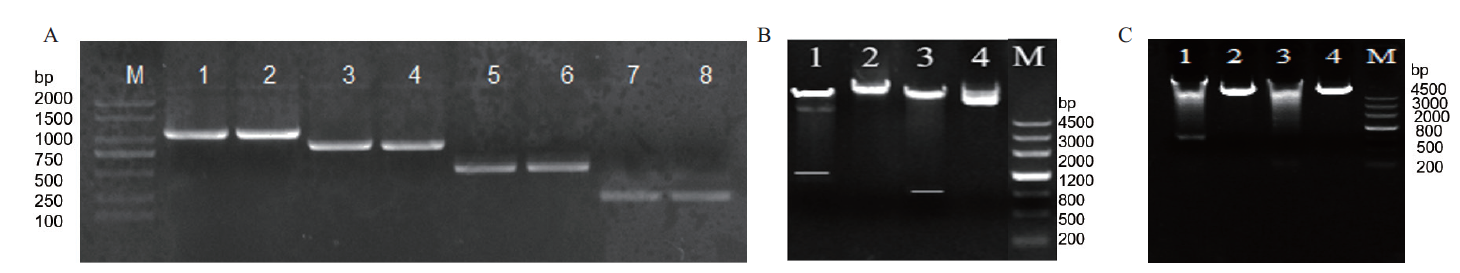
Fig. 3 PCR amplification of promoter SikCDPK1 and double restriction identification of expression vector A:PCR amplification SikCDPK1 promoter(M:DNA2000 marker. 1,2:PCR product of P0. 3,4:PCR product of P1. 5,6:PCR product of P2. 7,8:PCR product of P3). B,C:Double digestion identification of expression vector(B1:P0 double digestion. B2:P0 plasmid. B3:P1 double digestion. B4:P1 plasmid. C1:P2 double digestion. C2:P2 plasmid. C3:P3 double digestion. C4:P3 plasmid. M:DNA marker III)
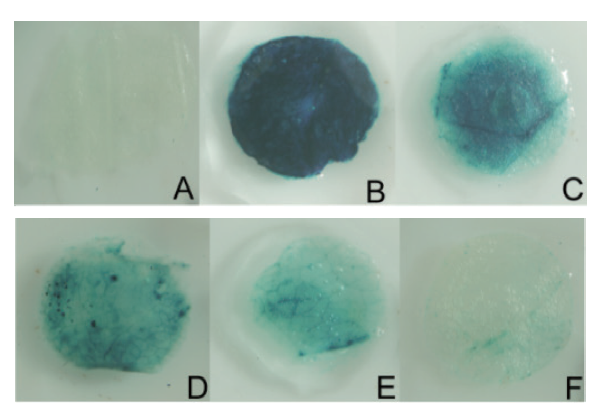
Fig. 4 GUS staining analysis of transient transformed tobacco with SikCDPK1 promoter A:Negative control(Non-transgenic). B:Positive control(35S∷GUS). C:P0. D:P1. E:P2. F:P3
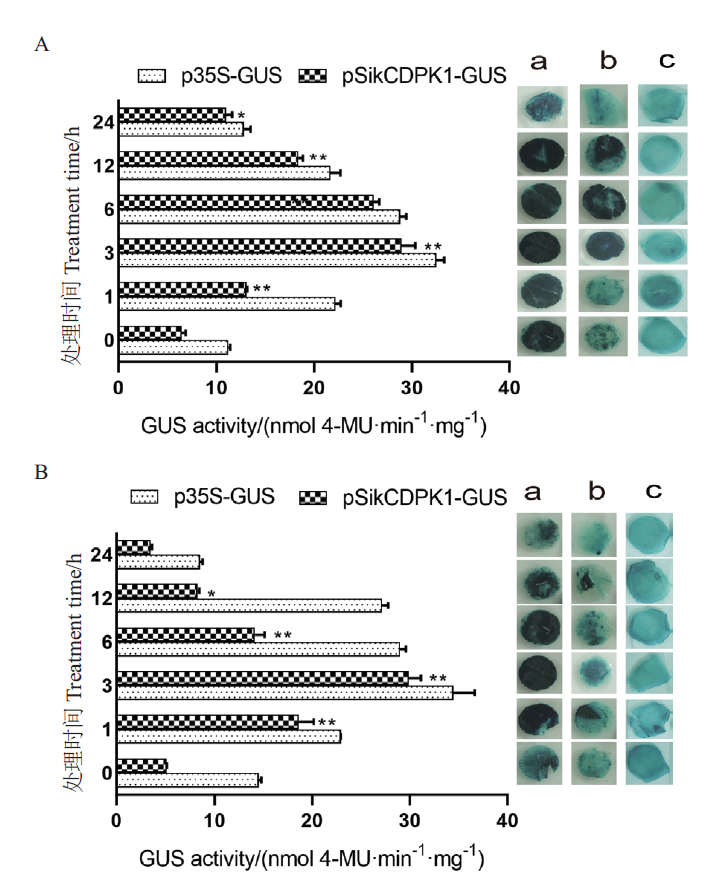
Fig. 5 Gus staining analysis and enzyme activity determin-ation under stress treatment A:Gus staining and enzyme activity after low temperature stress. B:Gus staining and enzyme activity after PEG stress. a:Positive control p35S-GUS-1304. b:Experimental group pSikCDPK1-GUS-1304. c:Experimental group control at room temperature
| [1] |
Li J, Liu HL, Xia WW, et al. De novo transcriptome sequencing and the hypothetical cold response mode of Saussurea involucrata in extreme cold environments[J]. Int J Mol Sci, 2017, 18(6):1155.
doi: 10.3390/ijms18061155 URL |
| [2] |
Singh A, Sagar S, Biswas DK. Calcium dependent protein kinase, a versatile player in plant stress management and development[J]. Crit Rev Plant Sci, 2017, 36(5/6):336-352.
doi: 10.1080/07352689.2018.1428438 URL |
| [3] |
Smale ST, Kadonaga JT. The RNA polymerase II core promoter[J]. Annu Rev Biochem, 2003, 72:449-479.
pmid: 12651739 |
| [4] | 田晓涵. 两种不同生境植物CDPK1基因的克隆及功能初探[D]. 石河子: 石河子大学, 2016. |
| Tian XH. Cloning and functional analysis of CDPK1 genes in two different habitats plants[D]. Shihezi: Shihezi University, 2016 | |
| [5] |
Liu YG, Chen YL. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences[J]. BioTechniques, 2007, 43(5):649-650, 652, 654 passim.
doi: 10.2144/000112601 URL |
| [6] |
Shi SJ, Li SG, Asim M, et al. The Arabidopsis calcium-dependent protein kinases(CDPKs)and their roles in plant growth regulation and abiotic stress responses[J]. Int J Mol Sci, 2018, 19(7):1900.
doi: 10.3390/ijms19071900 URL |
| [7] | Danino YM, Even D, Ideses D, et al. The core promoter:at the heart of gene expression[J]. Biochim Biophys Acta, 2015, 1849(8):1116-1131. |
| [8] | 李旭娟, 林秀琴, 字秋艳, 等. 甘蔗ScMOC1基因启动子的克隆与瞬时表达分析[J]. 植物遗传资源学报, 2019, 20(3):709-717. |
| Li XJ, Lin XQ, Zi QY, et al. Cloning and transient expression analysis of ScMOC1 promoter in sugarcane[J]. J Plant Genet Resour, 2019, 20(3):709-717. | |
| [9] |
Dang FF, Wang YN, She JJ, et al. Overexpression of CaWRKY27, a subgroup IIe WRKY transcription factor of Capsicum annuum, positively regulates tobacco resistance to Ralstonia solanacearum infection[J]. Physiol Plant, 2014, 150(3):397-411.
doi: 10.1111/ppl.12093 URL |
| [10] |
Dang FF, Wang YN, Yu L, et al. CaWRKY40, a WRKY protein of pepper, plays an important role in the regulation of tolerance to heat stress and resistance to Ralstonia solanacearum infection[J]. Plant Cell Environ, 2013, 36(4):757-774.
doi: 10.1111/pce.12011 URL |
| [11] |
Eulgem T. Dissecting the WRKY web of plant defense regulators[J]. PLoS Pathog, 2006, 2(11):e126.
doi: 10.1371/journal.ppat.0020126 pmid: 17121464 |
| [12] |
Eulgem T, Somssich IE. Networks of WRKY transcription factors in defense signaling[J]. Curr Opin Plant Biol, 2007, 10(4):366-371.
pmid: 17644023 |
| [13] |
Knoth C, Ringler J, Dangl JL, et al. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica[J]. Mol Plant Microbe Interact, 2007, 20(2):120-128.
doi: 10.1094/MPMI-20-2-0120 URL |
| [14] |
Wang YN, Dang FF, Liu ZQ, et al. CaWRKY58, encoding a group I WRKY transcription factor of Capsicum annuum, negatively regulates resistance to Ralstonia solanacearum infection[J]. Mol Plant Pathol, 2013, 14(2):131-144.
doi: 10.1111/j.1364-3703.2012.00836.x URL |
| [15] |
Bahn SC, Bae MS, Park YB, et al. Molecular cloning and characterization of a novel low temperature-induced gene, blti2, from barley(Hordeum vulgare L.)[J]. Biochim Biophys Acta, 2001, 1522(2):134-137.
pmid: 11750066 |
| [16] |
Chen A, Gusta LV, Brûlé-Babel A, et al. Varietal and chromosome 2H locus-specific frost tolerance in reproductive tissues of barley(Hordeum vulgare L. )detected using a frost simulation chamber[J]. Theor Appl Genet, 2009, 119(4):685-694.
doi: 10.1007/s00122-009-1079-1 URL |
| [17] |
Chen A, Reinheimer J, Brûlé-Babel A, et al. Genes and traits associated with chromosome 2H and 5H regions controlling sensitivity of reproductive tissues to frost in barley[J]. Theor Appl Genet, 2009, 118(8):1465-1476.
doi: 10.1007/s00122-009-0995-4 pmid: 19277599 |
| [18] |
Dunn MA, Goddard NJ, Zhang L, et al. Low-temperature-responsive barley genes have different control mechanisms[J]. Plant Mol Biol, 1994, 24(6):879-888.
pmid: 8204825 |
| [19] |
Ivashuta S, Naumkina M, Gau M, et al. Genotype-dependent transcriptional activation of novel repetitive elements during cold acclimation of alfalfa(Medicago sativa)[J]. Plant J, 2002, 31(5):615-627.
pmid: 12207651 |
| [20] |
Conforte AJ, Guimarães-Dias F, Neves-Borges AC, et al. Isolation and characterization of a promoter responsive to salt, osmotic and dehydration stresses in soybean[J]. Genet Mol Biol, 2017, 40(1 suppl 1):226-237.
doi: S1415-47572017000200226 pmid: 28350037 |
| [21] |
Alok A, Kaur J, Tiwari S. Functional characterization of wheat myo-inositol oxygenase promoter under different abiotic stress conditions in Arabidopsis thaliana[J]. Biotechnol Lett, 2020, 42(10):2035-2047.
doi: 10.1007/s10529-020-02967-1 URL |
| [22] |
Qian WJ, Xiao B, Wang L, et al. CsINV5, a tea vacuolar invertase gene enhances cold tolerance in transgenic Arabidopsis[J]. BMC Plant Biol, 2018, 18(1):228.
doi: 10.1186/s12870-018-1456-5 URL |
| [1] | LIU Yu-ling, WANG Meng-yao, SUN Qi, MA Li-hua, ZHU Xin-xia. Effect of RD29A Promoter on the Stress Resistance of Transgenic Tobacco with SikCDPK1 Gene from Saussurea involucrata [J]. Biotechnology Bulletin, 2023, 39(9): 168-175. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||