








Biotechnology Bulletin ›› 2023, Vol. 39 ›› Issue (9): 311-318.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0177
Previous Articles Next Articles
CHEN Zhong-yuan1( ), WANG Yu-hong2, DAI Wei-jun3, ZHANG Yan-min1, YE Qian1, LIU Xu-ping3, TAN Wen-Song1, ZHAO Liang1,3(
), WANG Yu-hong2, DAI Wei-jun3, ZHANG Yan-min1, YE Qian1, LIU Xu-ping3, TAN Wen-Song1, ZHAO Liang1,3( )
)
Received:2023-03-02
Online:2023-09-26
Published:2023-10-24
Contact:
ZHAO Liang
E-mail:614825923@qq.com;zhaoliang@ecust.edu.cn
CHEN Zhong-yuan, WANG Yu-hong, DAI Wei-jun, ZHANG Yan-min, YE Qian, LIU Xu-ping, TAN Wen-Song, ZHAO Liang. Mechanism Investigation of Ferric Ammonium Citrate on Transfection for Suspended HEK293 Cells[J]. Biotechnology Bulletin, 2023, 39(9): 311-318.
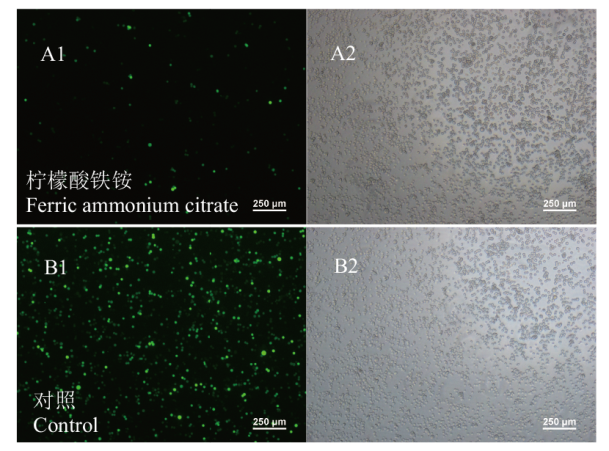
Fig. 1 Effects of adding ferric ammonium citrate on HEK293 cell transfection A: Transfection efficiency of cells in Celers101 medium containing ferric ammonium citrate. B: Transfection efficiency of cells in Celers101 medium without ferric ammonium citrate. 1: Fluorescence; 2: bright filed
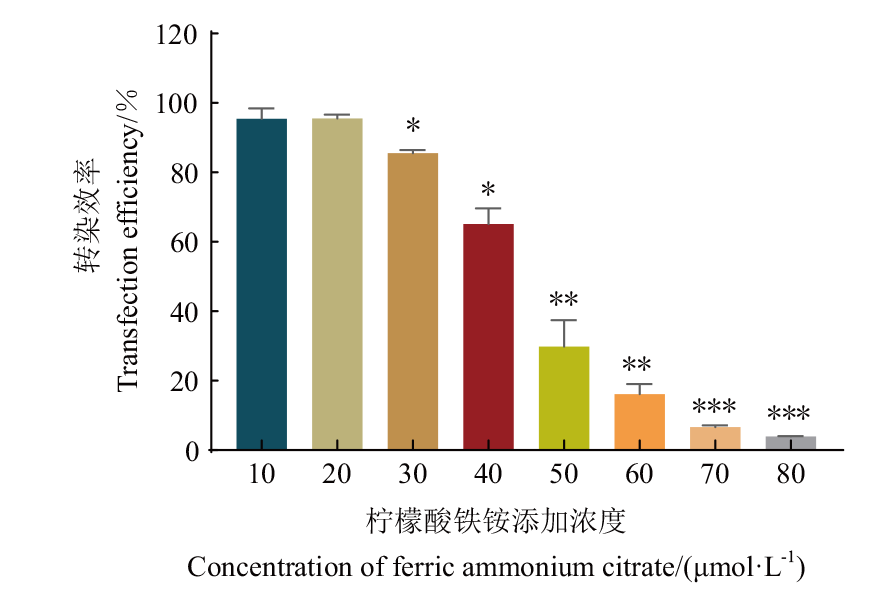
Fig. 2 Effect of ferric ammonium citrate concentration on transfection efficiency Compared with 10 μmol/L group, *P<0.05; **P<0.01; ***P<0.001, the same below
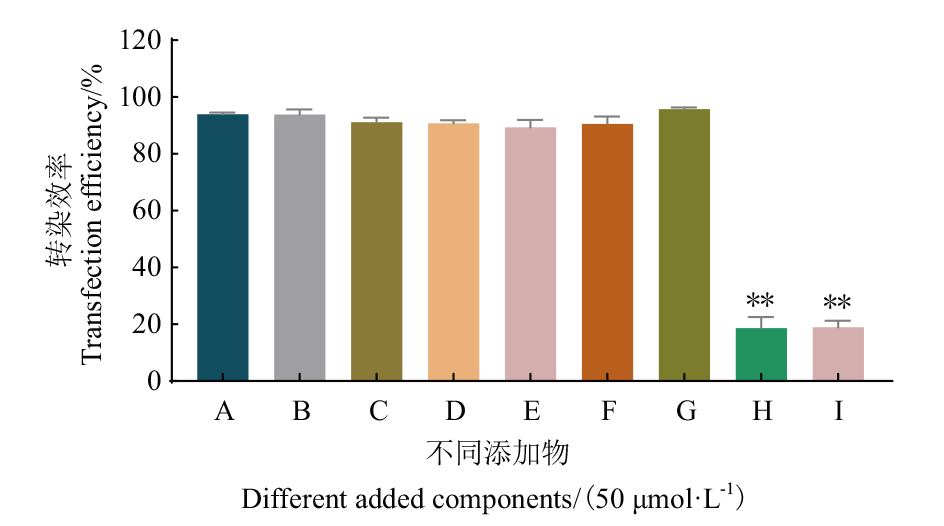
Fig. 3 Effects of different citrate donors and iron donors on transfection efficiency A: Control(Without iron). B: Transferrin. C: Sodium citrate. D: Citric acid. E: FeCl3. F: Potassium citrate. G: FeNaEDTA. H: Iron(III)citrate. I: Ferric ammonium citrate
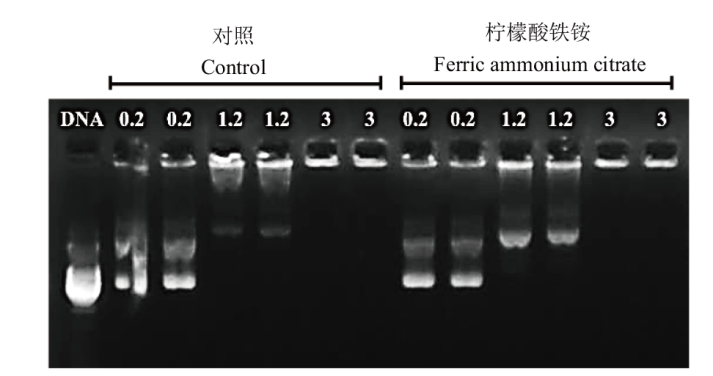
Fig. 5 Effects of ferric ammonium citrate on the binding of PEI to DNA DNA: PEI and DNA added ratio is 0∶1. 0.2: PEI and DNA added ratio is 0.2∶1. 1.2: PEI and DNA added ratio is 1.2∶1. 3: PEI and DNA added ratio is 3∶1
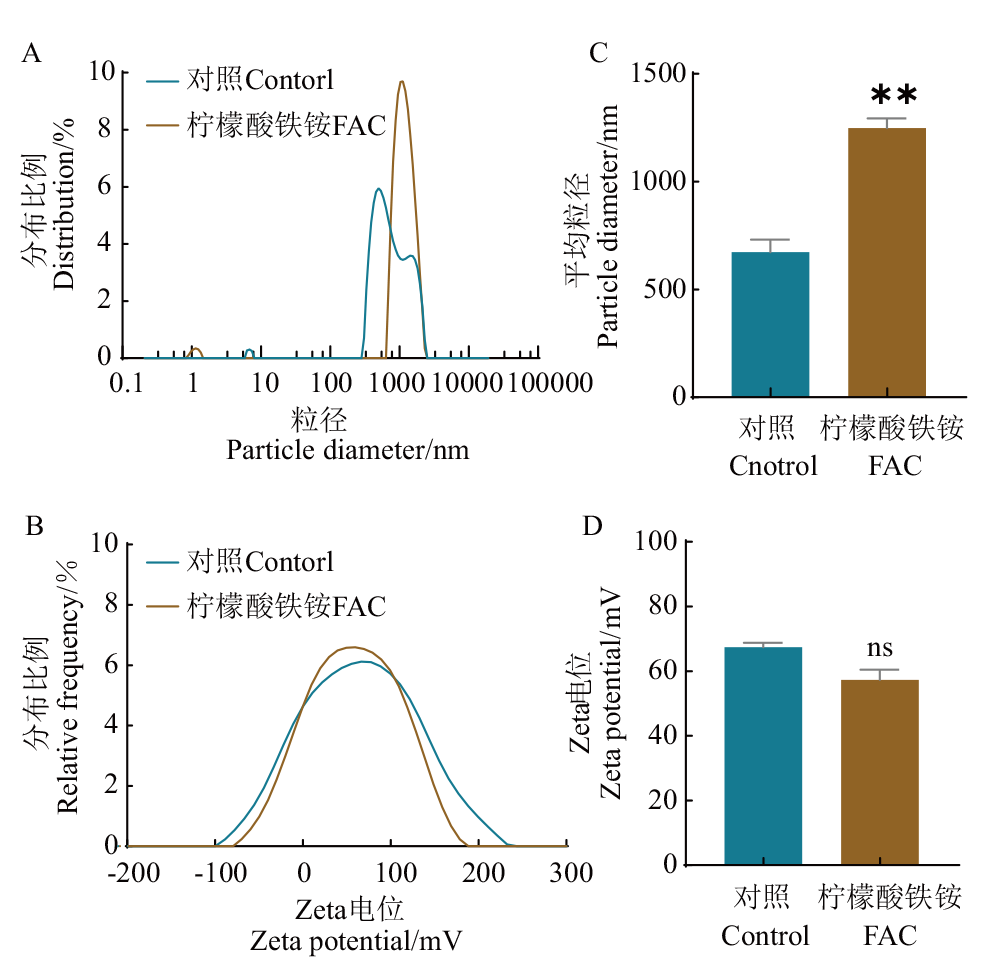
Fig. 6 Changes of particle size distribution(A), zeta potential distribution(B), average particle size(C)and average potential(D)after ferric ammonium citrate(FAC)added
| [1] |
Zhu JW. Mammalian cell protein expression for biopharmaceutical production[J]. Biotechnol Adv, 2012, 30(5): 1158-1170.
doi: 10.1016/j.biotechadv.2011.08.022 pmid: 21968146 |
| [2] |
Godbey WT, Wu KK, Mikos AG. Poly(ethylenimine)and its role in gene delivery[J]. J Control Release, 1999, 60(2/3): 149-160.
doi: 10.1016/S0168-3659(99)00090-5 URL |
| [3] |
Coll JL, Chollet P, Brambilla E, et al. In vivo delivery to tumors of DNA complexed with linear polyethylenimine[J]. Hum Gene Ther, 1999, 10(10): 1659-1666.
pmid: 10428211 |
| [4] |
Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells[J]. Nat Biotechnol, 2004, 22(11): 1393-1398.
doi: 10.1038/nbt1026 pmid: 15529164 |
| [5] |
Kim TK, Eberwine JH. Mammalian cell transfection: the present and the future[J]. Anal Bioanal Chem, 2010, 397(8): 3173-3178.
doi: 10.1007/s00216-010-3821-6 pmid: 20549496 |
| [6] |
Lai WF. In vivo nucleic acid delivery with PEI and its derivatives: current status and perspectives[J]. Expert Rev Med Devices, 2011, 8(2): 173-185.
doi: 10.1586/erd.10.83 URL |
| [7] |
Tan E, Chin CSH, Lim ZFS, et al. HEK293 cell line as a platform to produce recombinant proteins and viral vectors[J]. Front Bioeng Biotechnol, 2021, 9: 796991.
doi: 10.3389/fbioe.2021.796991 URL |
| [8] |
Gutiérrez-Granados S, Cervera L, Kamen AA, et al. Advancements in mammalian cell transient gene expression(TGE)technology for accelerated production of biologics[J]. Crit Rev Biotechnol, 2018, 38(6): 918-940.
doi: 10.1080/07388551.2017.1419459 pmid: 29295632 |
| [9] |
Torabfam GC, Yetisgin AA, Erdem C, et al. A feasibility study of different commercially available serum-free mediums to enhance lentivirus and adeno-associated virus production in HEK 293 suspension cells[J]. Cytotechnology, 2022, 74(6): 635-655.
doi: 10.1007/s10616-022-00551-1 pmid: 36389283 |
| [10] |
Cervera L, Gutiérrez-Granados S, Berrow NS, et al. Extended gene expression by medium exchange and repeated transient transfection for recombinant protein production enhancement[J]. Biotechnol Bioeng, 2015, 112(5): 934-946.
doi: 10.1002/bit.25503 pmid: 25421734 |
| [11] |
Dekevic G, Tasto L, Czermak P, et al. Statistical experimental designs to optimize the transient transfection of HEK 293T cells and determine a transfer criterion from adherent cells to larger-scale cell suspension cultures[J]. J Biotechnol, 2022, 346: 23-34.
doi: 10.1016/j.jbiotec.2022.01.004 URL |
| [12] |
Park JY, Lim BP, Lee K, et al. Scalable production of adeno-associated virus type 2 vectors via suspension transfection[J]. Biotechnol Bioeng, 2006, 94(3): 416-430.
pmid: 16622883 |
| [13] |
Abbott WM, Middleton B, Kartberg F, et al. Optimisation of a simple method to transiently transfect a CHO cell line in high-throughput and at large scale[J]. Protein Expr Purif, 2015, 116: 113-119.
doi: 10.1016/j.pep.2015.08.016 URL |
| [14] |
Mercedes SM, Alain G, Yves D, et al. Production of lentiviral vectors by large-scale transient transfection of suspension cultures and affinity chromatography purification[J]. Biotechnol Bioeng, 2007, 98(4): 789-99.
pmid: 17461423 |
| [15] |
Haldankar R, Li DQ, Saremi Z, et al. Serum-free suspensin large-scale transient transfection of CHO cells in WAVE bioreactors[J]. Mol Biotechnol, 2006, 34(2): 191-199.
pmid: 17172664 |
| [16] |
van Gaal EVB, van Eijk R, Oosting RS, et al. How to screen non-viral gene delivery systems in vitro?[J]. J Control Release, 2011, 154(3): 218-232.
doi: 10.1016/j.jconrel.2011.05.001 URL |
| [17] |
Pham PL, Perret S, Doan HC, et al. Large-scale transient transfection of serum-free suspension-growing HEK293 EBNA1 cells: peptone additives improve cell growth and transfection efficiency[J]. Biotechnol Bioeng, 2003, 84(3): 332-342.
pmid: 12968287 |
| [18] |
Vega MC. Advanced technologies for protein complex production and characterization[J]. Anticancer Research, 2016, 36(8): 4375.
pmid: 27466587 |
| [19] |
Eberhardy SR, Radzniak L, Liu Z. Iron(III)citrate inhibits polyethylenimine-mediated transient transfection of Chinese hamster ovary cells in serum-free medium[J]. Cytotechnology, 2009, 60(1): 1-9.
doi: 10.1007/s10616-009-9198-8 URL |
| [20] |
Yu Y, Kovacevic Z, Richardson DR. Tuning cell cycle regulation with an iron key[J]. Cell Cycle, 2007, 6(16): 1982-1994.
pmid: 17721086 |
| [21] |
Bai YL, Wu CJ, Zhao J, et al. Role of iron and sodium citrate in animal protein-free CHO cell culture medium on cell growth and monoclonal antibody production[J]. Biotechnol Prog, 2011, 27(1): 209-219.
doi: 10.1002/btpr.v27.1 URL |
| [22] |
Capella Roca B, Lao NT, Clynes M, et al. Investigation and circumvention of transfection inhibition by ferric ammonium citrate in serum-free media for Chinese hamster ovary cells[J]. Biotechnol Prog, 2020, 36(3): e2954.
doi: 10.1002/btpr.2954 URL |
| [23] |
Jorge AF, Röder R, Kos P, et al. Combining polyethylenimine and Fe(III)for mediating pDNA transfection[J]. Biochim Biophys Acta Gen Subj, 2015, 1850(6): 1325-1335.
doi: 10.1016/j.bbagen.2015.02.007 URL |
| [24] |
Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism[J]. Cell, 2004, 117(3): 285-297.
doi: 10.1016/s0092-8674(04)00343-5 pmid: 15109490 |
| [25] |
Prabha S, Zhou WZ, Panyam J, et al. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles[J]. Int J Pharm, 2002, 244(1/2): 105-115.
doi: 10.1016/S0378-5173(02)00315-0 URL |
| [26] |
van Asbeck AH, Beyerle A, McNeill H, et al. Molecular parameters of siRNA-cell penetrating peptide nano complexes for efficient cellular delivery[J]. ACS Nano, 2013, 7(5): 3797-3807.
doi: 10.1021/nn305754c URL |
| [1] | DENG Xiao-fen, YANG Xiao-jia, YI Tian-hong, FENG Ying, KE Xiao, LAI Wei-li. Optimization of Electrotransfection Conditions of Genes for Fusion Protein and Antibody to CHO-S Cells [J]. Biotechnology Bulletin, 2019, 35(4): 223-228. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||