








Biotechnology Bulletin ›› 2024, Vol. 40 ›› Issue (2): 48-54.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0844
Previous Articles Next Articles
SHAO Xue-hua1( ), LI Gui-lan2, XIAO Wei-qiang1, LAI Duo1, ZHUANG Qing-li1, QIN Jian1(
), LI Gui-lan2, XIAO Wei-qiang1, LAI Duo1, ZHUANG Qing-li1, QIN Jian1( )
)
Received:2023-08-29
Online:2024-02-26
Published:2024-03-13
Contact:
QIN Jian
E-mail:sxh19831017@163.com;qinjian@gdaas.cn
SHAO Xue-hua, LI Gui-lan, XIAO Wei-qiang, LAI Duo, ZHUANG Qing-li, QIN Jian. Establishment and Application of Ploidy Method for the Identification of Psidium guajava by Flow Cytometry[J]. Biotechnology Bulletin, 2024, 40(2): 48-54.
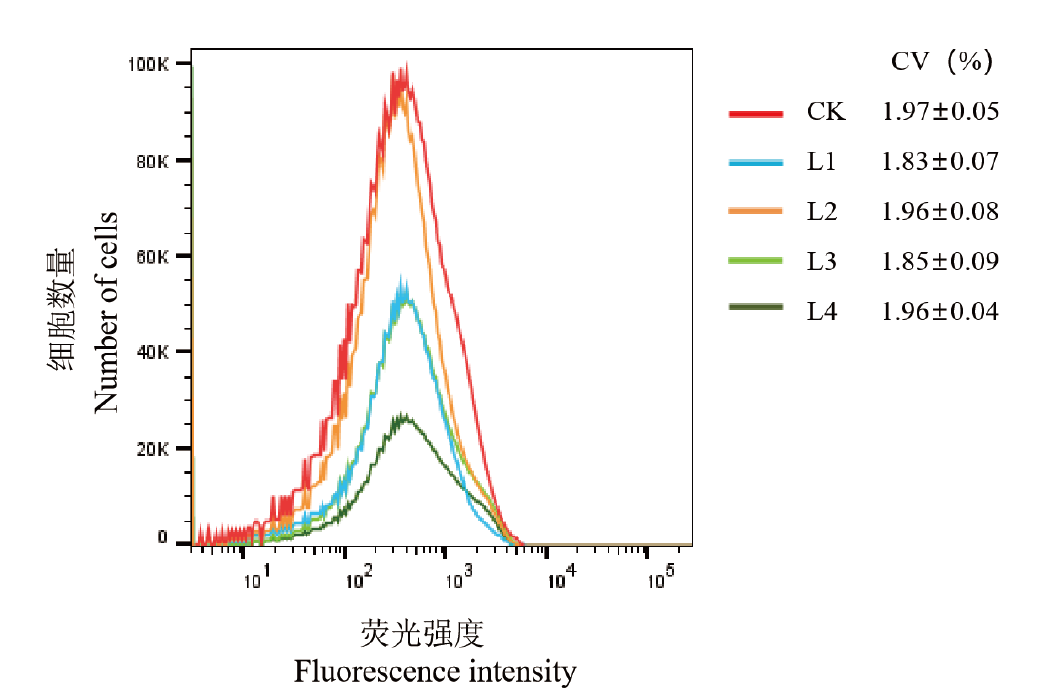
Fig. 2 Comparison of different centrifugation methods of guava L1: 1 000 r/min centrifuge for 5 min; L2: 1 000 r/min centrifuge for 10 min; L3: 2 000 r/min centrifuge for 5 min; L4: 2 000 r/min centrifuge for 10 min; CK: no centrifugation with filtration through 400 mesh membrane
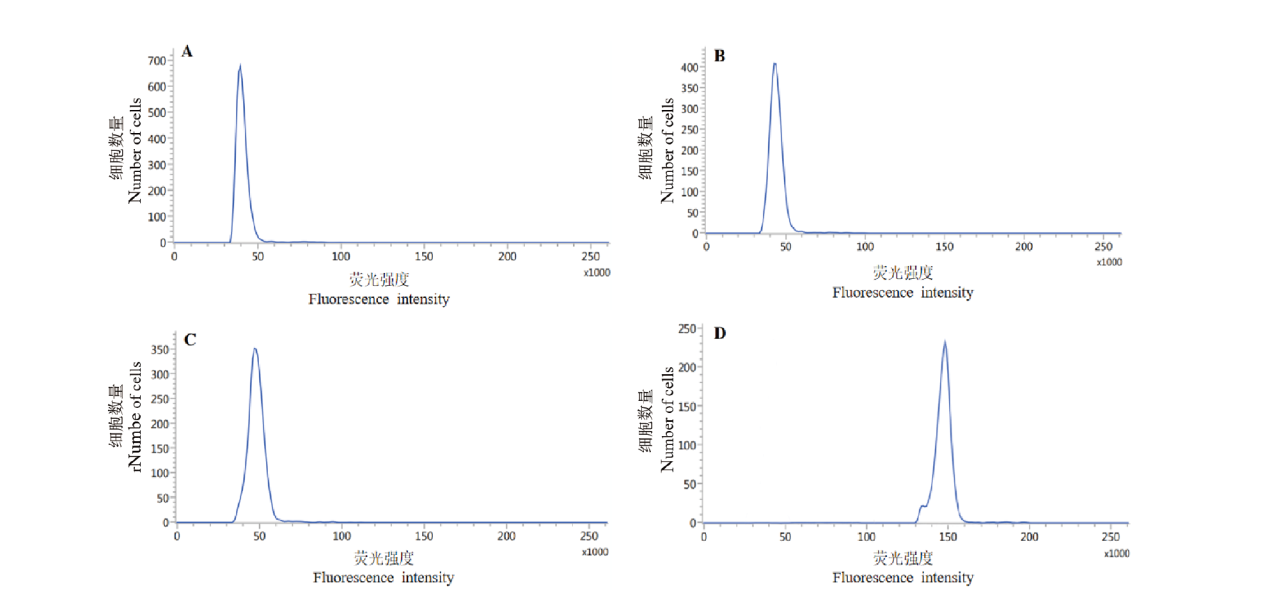
Fig. 4 Relative DNA contents of P. guajava petals with different ploidy levels A: ‘Zhenzhu’(diploid); B: ‘Ziguo’(diploid); C: ‘Jindouxiang’(diploid); D: ‘Strawberry’(hexaploid)
| 品种编号Sample No. | 名称Name | 倍性系数Coefficient of ploidy | 倍性估算值Ploidy estimated value | 倍性Ploidy |
|---|---|---|---|---|
| 1 | 紫果Ziguo | 0.49 | 1.96 | 2n |
| 2 | 泰国2号Thailand 2 | 0.45 | 1.80 | 2n |
| 3 | 泰国3号Thailand 3 | 0.48 | 1.92 | 2n |
| 4 | 软香Ruanxiang | 0.43 | 1.72 | 2n |
| 5 | 木瓜Mugua | 0.53 | 2.12 | 2n |
| 6 | 红肉实生Hongrou shisheng | 0.59 | 2.36 | 2n |
| 7 | 红肉翁拔Hongrouwengba | 0.51 | 2.04 | 2n |
| 8 | 帝王Diwang | 0.48 | 1.92 | 2n |
| 9 | 草莓Strawberry | 0.52 | 2.01 | 6n |
| 10 | 西瓜红Xiguahong | 0.44 | 1.76 | 2n |
| 11 | 东莞红肉Dongguanhongrou | 0.45 | 1.80 | 2n |
| 12 | 佳硕Jiashuo | 0.46 | 1.84 | 2n |
| 13 | 水蜜Shuimi | 0.49 | 1.96 | 2n |
| 14 | 细叶小果Xiyexiaoguo | 0.44 | 1.76 | 2n |
| 15 | 细叶大果Xiyedaguo | 0.43 | 1.72 | 2n |
| 16 | 金斗香Jin douxiang | 0.45 | 1.80 | 2n |
| 17 | 白肉翁拔Bairouwengba | 0.52 | 2.08 | 2n |
| 18 | 翡翠Feicui | 0.51 | 2.04 | 2n |
| 19 | 青皮红肉Qingpihongrou | 0.53 | 2.12 | 2n |
| 20 | 金石宫Jinshigong | 0.47 | 1.88 | 2n |
| 21 | 珍珠Zhenzhu | 0.50 | 2.00 | 2n |
| 22 | 红心软果Hongxinruanguo | 0.42 | 1.68 | 2n |
| 23 | 粉红蜜Fenhongmi | 0.51 | 2.04 | 2n |
| 24 | 卵红Luanhong | 0.50 | 2.00 | 2n |
| 25 | 宫粉红Gongfenhong | 0.58 | 2.32 | 2n |
| 26 | 黄皮红肉Huangpihongrou | 0.53 | 2.12 | 2n |
| 27 | B1 | 0.51 | 2.04 | 2n |
| 28 | B2 | 0.56 | 2.24 | 2n |
| 29 | B3 | 0.51 | 2.04 | 2n |
| 30 | B4 | 0.54 | 2.16 | 2n |
| 31 | B5 | 0.53 | 2.12 | 2n |
| 32 | B6 | 0.53 | 2.12 | 2n |
| 33 | 穗红1号Suihong 1 | 0.49 | 1.96 | 2n |
Table 1 FCM identification of 33 P. guajava germplasms with ‘Zhenzhu’as reference
| 品种编号Sample No. | 名称Name | 倍性系数Coefficient of ploidy | 倍性估算值Ploidy estimated value | 倍性Ploidy |
|---|---|---|---|---|
| 1 | 紫果Ziguo | 0.49 | 1.96 | 2n |
| 2 | 泰国2号Thailand 2 | 0.45 | 1.80 | 2n |
| 3 | 泰国3号Thailand 3 | 0.48 | 1.92 | 2n |
| 4 | 软香Ruanxiang | 0.43 | 1.72 | 2n |
| 5 | 木瓜Mugua | 0.53 | 2.12 | 2n |
| 6 | 红肉实生Hongrou shisheng | 0.59 | 2.36 | 2n |
| 7 | 红肉翁拔Hongrouwengba | 0.51 | 2.04 | 2n |
| 8 | 帝王Diwang | 0.48 | 1.92 | 2n |
| 9 | 草莓Strawberry | 0.52 | 2.01 | 6n |
| 10 | 西瓜红Xiguahong | 0.44 | 1.76 | 2n |
| 11 | 东莞红肉Dongguanhongrou | 0.45 | 1.80 | 2n |
| 12 | 佳硕Jiashuo | 0.46 | 1.84 | 2n |
| 13 | 水蜜Shuimi | 0.49 | 1.96 | 2n |
| 14 | 细叶小果Xiyexiaoguo | 0.44 | 1.76 | 2n |
| 15 | 细叶大果Xiyedaguo | 0.43 | 1.72 | 2n |
| 16 | 金斗香Jin douxiang | 0.45 | 1.80 | 2n |
| 17 | 白肉翁拔Bairouwengba | 0.52 | 2.08 | 2n |
| 18 | 翡翠Feicui | 0.51 | 2.04 | 2n |
| 19 | 青皮红肉Qingpihongrou | 0.53 | 2.12 | 2n |
| 20 | 金石宫Jinshigong | 0.47 | 1.88 | 2n |
| 21 | 珍珠Zhenzhu | 0.50 | 2.00 | 2n |
| 22 | 红心软果Hongxinruanguo | 0.42 | 1.68 | 2n |
| 23 | 粉红蜜Fenhongmi | 0.51 | 2.04 | 2n |
| 24 | 卵红Luanhong | 0.50 | 2.00 | 2n |
| 25 | 宫粉红Gongfenhong | 0.58 | 2.32 | 2n |
| 26 | 黄皮红肉Huangpihongrou | 0.53 | 2.12 | 2n |
| 27 | B1 | 0.51 | 2.04 | 2n |
| 28 | B2 | 0.56 | 2.24 | 2n |
| 29 | B3 | 0.51 | 2.04 | 2n |
| 30 | B4 | 0.54 | 2.16 | 2n |
| 31 | B5 | 0.53 | 2.12 | 2n |
| 32 | B6 | 0.53 | 2.12 | 2n |
| 33 | 穗红1号Suihong 1 | 0.49 | 1.96 | 2n |
| [1] |
Kumar M, Tomar M, Amarowicz R, et al. Guava(Psidium guaja-va L.)leaves: nutritional composition, phytochemical profile, and health-promoting bioactivities[J]. Foods, 2021, 10(4): 752.
doi: 10.3390/foods10040752 URL |
| [2] |
Zou XF, Liu HY. A review of meroterpenoids and of their bioactivity from guava(Psidium guajava L.)[J]. J Future Foods, 2023, 3(2): 142-154.
doi: 10.1016/j.jfutfo.2022.12.005 URL |
| [3] |
Jaiarj P, Khoohaswan P, Wongkrajang Y, et al. Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract[J]. J Ethnopharmacol, 1999, 67(2): 203-212.
pmid: 10619385 |
| [4] |
Gutiérrez RMP, Mitchell S, Solis RV. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology[J]. J Ethnopharmacol, 2008, 117(1): 1-27.
doi: 10.1016/j.jep.2008.01.025 pmid: 18353572 |
| [5] |
Morais-Braga MFB, Carneiro JNP, Machado AJT, et al. Psidium guajava L., from ethnobiology to scientific evaluation: elucidating bioactivity against pathogenic microorganisms[J]. J Ethnopharmacol, 2016, 194: 1140-1152.
doi: S0378-8741(16)31814-1 pmid: 27845266 |
| [6] |
Naseer S, Hussain S, Naeem N, et al. The phytochemistry and medicinal value of Psidium guajava(guava)[J]. Clin Phytosci, 2018, 4(1): 32.
doi: 10.1186/s40816-018-0093-8 |
| [7] |
Amadike Ugbogu E, Emmanuel O, Ebubechi Uche M, et al. The ethnobotanical, phytochemistry and pharmacological activities of Psidium guajava L[J]. Arab J Chem, 2022, 15(5): 103759.
doi: 10.1016/j.arabjc.2022.103759 URL |
| [8] |
Liu HX, Wei SS, Shi LL, et al. Preparation, structural characterization, and bioactivities of polysaccharides from Psidium guajava: a review[J]. Food Chem, 2023, 411: 135423.
doi: 10.1016/j.foodchem.2023.135423 URL |
| [9] |
Kumar M, Kapoor S, Dhumal S, et al. Guava(Psidium guajava L.)seed: a low-volume, high-value byproduct for human health and the food industry[J]. Food Chem, 2022, 386: 132694.
doi: 10.1016/j.foodchem.2022.132694 URL |
| [10] |
邵雪花, 赖多, 肖维强, 等. 番石榴遗传多样性的ISSR分析及指纹图谱构建[J]. 中国农学通报, 2023, 39(21): 39-47.
doi: 10.11924/j.issn.1000-6850.casb2022-0420 |
| Shao XH, Lai D, Xiao WQ, et al. Psidium guajava L.: genetic diversity analysis based on ISSR markers and construction of fingerprints[J]. Chin Agric Sci Bull, 2023, 39(21): 39-47. | |
| [11] | 陈宜木, 周群, 钟颖颖, 等. 利用流式细胞术鉴定68份三角梅基因组大小与染色体倍性[J]. 亚热带植物科学, 2022, 51(6): 417-423. |
| Chen YM, Zhou Q, Zhong YY, et al. Genome size estimation and ploidy identification of 68 servings Bougainvillea by flow cytometry[J]. Subtrop Plant Sci, 2022, 51(6): 417-423. | |
| [12] |
Brown M, Wittwer C. Flow cytometry: principles and clinical applications in hematology[J]. Clin Chem, 2000, 46(8 Pt 2):1221-1229.
pmid: 10926916 |
| [13] |
郑英转, 吕燕, 杨东旭, 等. 基于液氮研磨法的流式细胞术检测马铃薯倍性的研究[J]. 生物技术通报, 2021, 37(1): 282-288.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0478 |
| Zheng YZ, Lü Y, Yang DX, et al. Study on the identification of potato ploidy using flow cytometry based on liquid nitrogen grinding method[J]. Biotechnol Bull, 2021, 37(1): 282-288. | |
| [14] |
陈林, 潘贞志, 戴毅, 等. 适合流式细胞仪分析的大豆细胞核解离液的筛选与应用[J]. 生物技术通报, 2020, 36(11): 230-237.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0426 |
|
Chen L, Pan ZZ, Dai Y, et al. Screening and application of the nuclear dissociation solutions of soybeans suitable for flow cytometry analysis[J]. Biotechnol Bull, 2020, 36(11): 230-237.
doi: 10.13560/j.cnki.biotech.bull.1985.2020-0426 |
|
| [15] | 赵孟良, 任延靖, 田闵玉, 等. 基于流式细胞仪鉴定菊芋倍性方法的建立及应用[J]. 西北农林科技大学学报: 自然科学版, 2021, 49(3): 138-146. |
| Zhao ML, Ren YJ, Tian MY, et al. Establishment and application of aploidy method for identification of Helianthus tuberosus L. by flow cytometry[J]. J Northwest A F Univ Nat Sci Ed, 2021, 49(3): 138-146. | |
| [16] |
王娅丽, 周利利, 王娜, 等. 利用流式细胞仪快速鉴定棉花倍性的方法比较[J]. 生物技术通报, 2022, 38(12): 144-148.
doi: 10.13560/j.cnki.biotech.bull.1985.2022-0783 |
| Wang YL, Zhou LL, Wang N, et al. Comparison of methods for rapid determination of cotton ploidy by flow cytometry[J]. Biotechnol Bull, 2022, 38(12): 144-148. | |
| [17] | 吕顺, 任毅, 王芳, 等. 利用流式细胞术快速鉴定169份香蕉种质资源的染色体倍性[J]. 果树学报, 2018, 35(6): 668-684. |
| Lü S, Ren Y, Wang F, et al. Ploidy identification of 169 Musa germplasms by flow cytometry[J]. J Fruit Sci, 2018, 35(6): 668-684. | |
| [18] | 田新民, 周香艳, 弓娜. 流式细胞术在植物学研究中的应用——检测植物核DNA含量和倍性水平[J]. 中国农学通报, 2011, 27(9): 21-27. |
| Tian XM, Zhou XY, Gong N. Applications of flow cytometry in plant research—analysis of nuclear DNA content and ploidy level in plant cells[J]. Chin Agric Sci Bull, 2011, 27(9): 21-27. | |
| [19] |
熊文艳, 普冉, 刘云礼, 等. 基于流式细胞术对27种石斛的倍性鉴定和基因组大小分析[J]. 热带作物学报, 2022, 43(11): 2249-2257.
doi: 10.3969/j.issn.1000-2561.2022.11.009 |
| Xiong WY, Pu R, Liu YL, et al. Estimation ploidy and genome size of 27 Dendrobium species by flow cytometry[J]. Chin J Trop Crops, 2022, 43(11): 2249-2257. | |
| [20] | 张桂芳, 王艳, 闫小巧, 等. 流式细胞仪检测铁皮石斛核DNA初探[J]. 现代中药研究与实践, 2017, 31(1): 16-19. |
| Zhang GF, Wang Y, Yan XQ, et al. Study on flow cytometer for detecting nuclear DNA contents in Dendrobium officinal[J]. Res Pract Chin Med, 2017, 31(1): 16-19. | |
| [21] | 杨静, 宋勤霞, 宁军权, 等. 利用流式细胞术鉴定桑树染色体倍性的方法[J]. 蚕业科学, 2017, 43(1): 8-17. |
| Yang J, Song QX, Ning JQ, et al. Establishment of Morus L. chromosome ploidy identification method using flow cytometry[J]. Sci Seric, 2017, 43(1): 8-17. | |
| [22] | 王利虎, 吕晔, 罗智, 等. 流式细胞术估测枣染色体倍性和基因组大小方法的建立及应用[J]. 农业生物技术学报, 2018, 26(3): 511-520. |
| Wang LH, Lv Y, Luo Z, et al. Establishment and application of a method for chromosome ploidy identification and genome size estimation using flow cytometry in Ziziphus jujuba[J]. J Agric Biotechnol, 2018, 26(3): 511-520. | |
| [23] |
Georgiev V, Weber J, Bley T, et al. Improved procedure for nucleus extraction for DNA measurements by flow cytometry of red beet(Beta vulgaris L.)hairy roots[J]. Journal of Bioscience and Bioengineering, 2009, 107(4): 439-441.
doi: 10.1016/j.jbiosc.2008.12.023 pmid: 19332305 |
| [1] | GAO Kai-yue, GUO Yu-ting, DU Yi-mou, ZHENG Xiao-mei, MA Xin-rong, ZHAO Wei, ZHENG Ping, SUN Ji-bin. A Quantitative Detection Approach for Glucose Uptake in Aspergillus niger: A Case Study of Glucose Transporter MstC [J]. Biotechnology Bulletin, 2023, 39(12): 71-80. |
| [2] | LIU Jin-sheng, CHEN Zhen-ya, HUO Yi-xin, GUO Shu-yuan. Application of FACS Technology in the Directed Evolution of Enzyme [J]. Biotechnology Bulletin, 2023, 39(10): 93-106. |
| [3] | WANG Ya-li, WANG Na, CHENG Hong-mei. Comparison of Methods for Rapid Determination of Cotton Ploidy by Flow Cytometry [J]. Biotechnology Bulletin, 2022, 38(12): 144-148. |
| [4] | ZHENG Ying-zhuan, LÜ Yan, YANG Dong-xu, LI Guo-wei, WANG Hong-yang, LI Can-hui. Study on the Identification of Potato Ploidy Using Flow Cytometry Based on Liquid Nitrogen Grinding Method [J]. Biotechnology Bulletin, 2021, 37(1): 282-288. |
| [5] | MI Liang-bo, ZENG Wei-zhu, HUANG Ke-xue, WANG De-ming, DU Guo-cheng, ZHOU Jing-wen, CHEN Jian. High-throughput Screening High-yield Bacitracin Strain from Bacillus licheniformis DW2 [J]. Biotechnology Bulletin, 2020, 36(7): 90-96. |
| [6] | CHEN Lin, PAN Zhen-zhi, DAI Yi, SONG Li. Screening and Application of the Nuclear Dissociation Solutions of Soybeans Suitable for Flow Cytometry Analysis [J]. Biotechnology Bulletin, 2020, 36(11): 230-237. |
| [7] | WANG Ya-nan, WEN Hai-ruo, WANG Xue. Establishment and Preliminary Exploration of in vitro Pig-a Gene Mutation Assay Based on L5178Y Cells [J]. Biotechnology Bulletin, 2020, 36(1): 220-228. |
| [8] | LI Yan-wei, SONG Xing-hui, WANG Jia-jia, LIU Li, HUANG Ying-ying, GUO Chun. Establishment of the Real-time and Label-free Screening System for Tumor Cell Apoptosis [J]. Biotechnology Bulletin, 2019, 35(10): 220-226. |
| [9] | HE Shuo-kang, LUO Ze-wei. Development and Phenotypic Analysis of Tetraploid Arabidopsis thaliana with QUARTET Mutation [J]. Biotechnology Bulletin, 2018, 34(7): 119-125. |
| [10] | LU Jia, DENG Qiu-ping, REN Wen-hua. Mechanism of Antimicrobial Peptide Scolopin 2-NH2 Isolated from Scolopendra subspinipes mutilans [J]. Biotechnology Bulletin, 2018, 34(11): 179-190. |
| [11] | ZHANG Chao, LIU Yu-cheng, WANG Yi-guang, FU Jian-xin, ZHAO Hong-bo. Cloning and Expression Analysis of Carotenoid Isomerase Gene in Osmanthus fragrans [J]. Biotechnology Bulletin, 2017, 33(6): 89-96. |
| [12] | SUN Wen, ZHENG Feng. Molecular Cloning of Gene for Enolase in Highly Virulent Strains from Streptococcus suis serotype 2 and Its Protein Biological Function [J]. Biotechnology Bulletin, 2017, 33(4): 222-230. |
| [13] | Han Yawei, Wang Xihua, Chen Liping, Shi Guiqin, Sun Liping, Zhou Wenshan. Toxic Effects of NNK on NCTC 1469 Cells [J]. Biotechnology Bulletin, 2015, 31(9): 218-223. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||