








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (10): 97-105.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1607
Previous Articles Next Articles
ZHANG Xiao-ni( ), WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long(
), WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long( )
)
Received:2021-12-30
Online:2022-10-26
Published:2022-11-11
Contact:
ZHANG Yun-long
E-mail:xnn18838917069@163.com;zhyl@dhu.edu.cn
ZHANG Xiao-ni, WENG Yi-chun, FAN Yi-hao, WANG Xiao-juan, ZHAO Jia-yu, ZHANG Yun-long. Mito-OS-Timer:A Targeted Fluorescent Stopwatch for Monitoring Mitochondrial Oxidative Stress[J]. Biotechnology Bulletin, 2022, 38(10): 97-105.
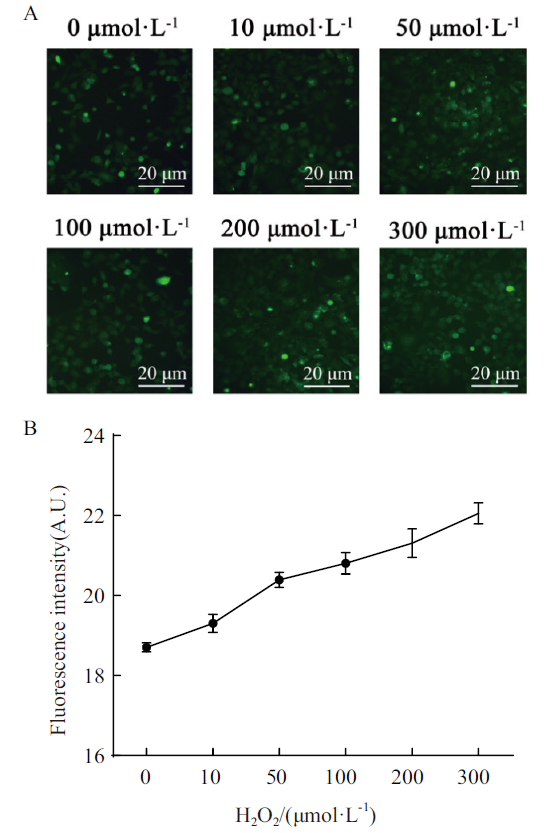
Fig. 1 Evaluation of H2O2-induced oxidative stress cell model A:Oxidative stress state of HEK293T cells treated with different concentrations of H2O2 was detected by DCFH-DA. B:Image J software for analyzing correlation between mean fluorescence intensity and H2O2 concentration in Fig. A
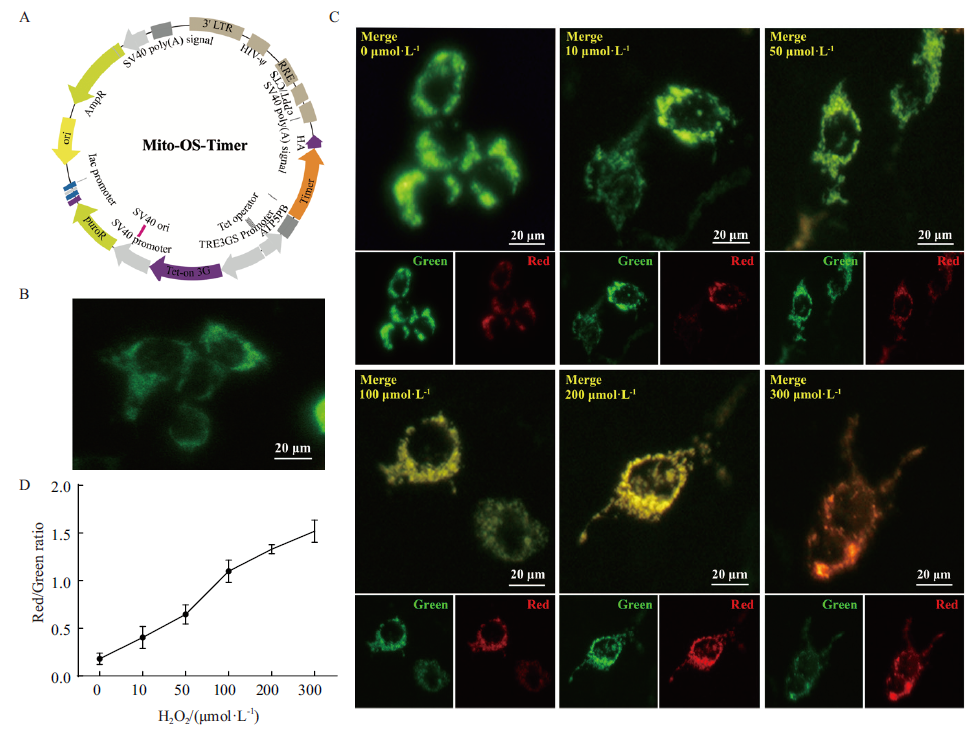
Fig. 2 Mito-OS-Timer targets to monitor the dynamic changes of mitochondrial oxidative stress induced by H2O2 in cell models A:Mito-OS-Timer,namely pLVX-Mito-OS-Timer recombinant expression plasmid map. B:Evaluate the expression of HEK293T cells transfected with Mito-OS-Timer plasmid. The fluorescence protein expression was detected by inverted fluorescence microscope after induction by Doxycyclin. Bar:20 μm. C:Mito-OS-Timer targets to monitor the changes of mitochondrial oxidative stress induced by H2O2 in cell models. The concentrations of H2O2 in the 6 groups were 0,10,50,100,200 and 300 μmol/L,respectively. Light avoidance treatment for 24 h(Green:Green fluorescence. Red:Red fluorescence. Merge:Red-green fluorescence superposition. Bar:20 μm). D:Image J software for analyzing correlation between H2O2-induced concentration and changes in intracellular red-green fluorescence ratio in Fig. C
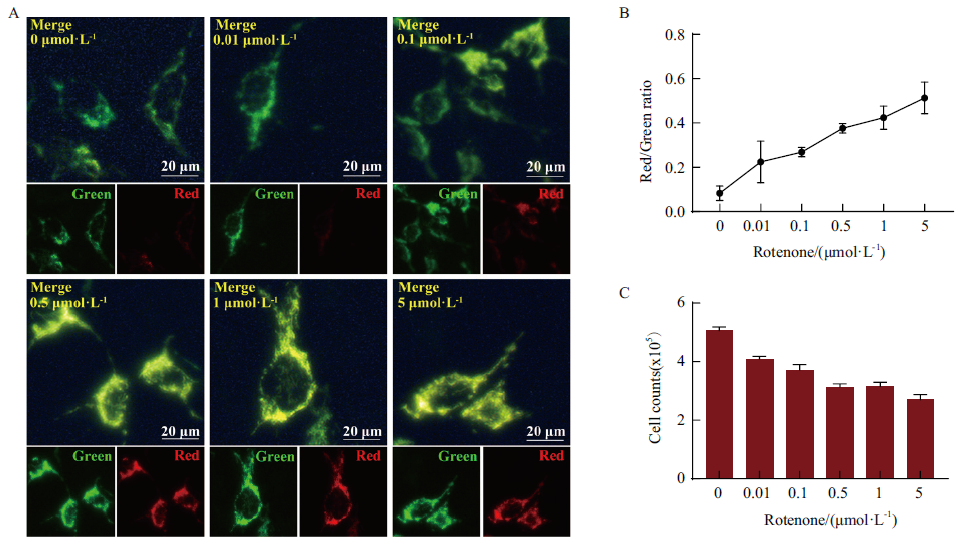
Fig. 3 Evaluation of Mito-OS-Timer system in rotenone-induced mitochondrial oxidation cell model A:Evaluation of targeted monitoring effect of Mito-OS-Timer based on rotenone-induced oxidative stress cell model Doxycyclin-induced HEK293T cells were treated with rotenone at different concentrations of 0,0.01,0.1,0.5,1 and 5 μmol/L for 24 h(Green:green fluorescence;Red:red fluorescence;Merge:red-green fluorescence superposition;Bar:20 μm);B:Image J software analysis of correlation between rotenone induced concentration and intracellular red-green fluorescence ratio in Fig. A;C:Statistical results of cell count of HEK293T cells treated with different concentrations of rotenone

Fig. 4 Flow cytometry results of rotenone-induced mitochondrial oxidation in rotenone-induced cell models monitored by Mito-OS-Timer A:Fluorescence expression of rotenone treated cells was detected by flow cytometry. HEK293T cells were treated with 0,0.01,0.1,0.5,1 and 5 μmol/L rotenone for 24 h,and the fluorescence intensity was detected by flow cytometry;B:Image J software analysis the correlation between rotenone induced concentration in Fig. A and changes in intracellular red-green fluorescence ratio

Fig. 5 Relationship between FLCN gene expression and mitochondrial oxidative stress dynamic changes detected by Mito-OS-Timer A:pLVX-shFLCN recombinant plasmid map;B:1% agarose gel electrophoresis detection map(M:DNA Marker;1:FLCN gene fragment digested by EcoR I /Xho I,1 740 bp;2:pLVX-shRNA vector fragment digested by EcoR I /Xho I,7 881 bp;3:pLVX-shFLCN plasmid DNA,9621 bp);C:FLCN gene expression in transfected cells was detected by western blot(WT:Wide type,FLCN gene expression in normal HEK293T cells;OE:overexpression,FLCN overexpression in HEK293T cells transfected with pcDNA-3HA-FLCN);D:Mito-OS-Timer was used to detect dynamic changes of mitochondrial oxidative stress in OE,FLCN silencing(RNAi)and 0.1 μmol/L rotenone-induced(OS)cells(Green:green fluorescence;Red:red fluorescence;Merge:red-green fluorescence superposition;Bar:20 μm);E:Flow cytometry was used to detect Mito-OS-Timer to monitor the cells;F:Quantitative analysis of flow cytometry results for the changes of red-green fluorescence ratio in various cells
| [1] |
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species(ROS)and ROS-induced ROS release[J]. Physiol Rev, 2014, 94(3):909-950.
doi: 10.1152/physrev.00026.2013 URL |
| [2] | Brillo V, Chieregato L, Leanza L, et al. Mitochondrial dynamics, ROS, and cell signaling:a blended overview[J]. Life(Basel), 2021, 11(4):332-351. |
| [3] |
Saotome M, Katoh H, Yaguchi Y, et al. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury[J]. Am J Physiol Heart Circ Physiol, 2009, 296(4):H1125-H1132.
doi: 10.1152/ajpheart.00436.2008 URL |
| [4] |
Tatsuta T, Langer T. Quality control of mitochondria:protection against neurodegeneration and ageing[J]. EMBO J, 2008, 27(2):306-314.
doi: 10.1038/sj.emboj.7601972 URL |
| [5] |
Pereira CV, Lebiedzinska M, Wieckowski MR, et al. Regulation and protection of mitochondrial physiology by sirtuins[J]. Mitochondrion, 2012, 12(1):66-76.
doi: 10.1016/j.mito.2011.07.003 pmid: 21787885 |
| [6] |
Gao H, Liu YH, Zheng M, et al. Characterization of murine mammary stem/progenitor cells in a D-galactose-induced aging model[J]. Aging, 2021, 13(8):11762-11773.
doi: 10.18632/aging.202870 URL |
| [7] |
McDonald S, Rubin P, Chang AY, et al. Pulmonary changes induced by combined mouse beta-interferon(rMuIFN-beta)and irradiation in normal mice—toxic versus protective effects[J]. Radiother Oncol J Eur Soc Ther Radiol Oncol, 1993, 26(3):212-218.
doi: 10.1016/0167-8140(93)90262-7 URL |
| [8] |
Choudhary I, Vo T, Paudel K, et al. Vesicular and extravesicular protein analyses from the airspaces of ozone-exposed mice revealed signatures associated with mucoinflammatory lung disease[J]. Sci Rep, 2021, 11(1):23203-23225.
doi: 10.1038/s41598-021-02256-5 pmid: 34853335 |
| [9] |
Miura T, Shimizu M, Uehara S, et al. Different hepatic concentrations of bromobenzene, 1, 2-dibromobenzene, and 1, 4-dibromobenzene in humanized-liver mice predicted using simplified physiologically based pharmacokinetic models as putative markers of toxicological potential[J]. Chem Res Toxicol, 2020, 33(12):3048-3053.
doi: 10.1021/acs.chemrestox.0c00387 URL |
| [10] |
Mu HM, Liu HW, Zhang JY, et al. Ursolic acid prevents doxorubicin-induced cardiac toxicity in mice through ENOS activation and inhibition of ENOS uncoupling[J]. J Cell Mol Med, 2019, 23(3):2174-2183.
doi: 10.1111/jcmm.14130 URL |
| [11] |
Matsuura Y, Yamashita A, Zhao Y, et al. Altered glucose metabolism and hypoxic response in alloxan-induced diabetic atherosclerosis in rabbits[J]. PLoS One, 2017, 12(4):e0175976.
doi: 10.1371/journal.pone.0175976 URL |
| [12] |
Malińska D, Więckowski MR, Michalska B, et al. Mitochondria as a possible target for nicotine action[J]. J Bioenerg Biomembr, 2019, 51(4):259-276.
doi: 10.1007/s10863-019-09800-z pmid: 31197632 |
| [13] |
MacDougall G, Anderton RS, Mastaglia FL, et al. Proteomic analysis of cortical neuronal cultures treated with poly-arginine peptide-18(R18)and exposed to glutamic acid excitotoxicity[J]. Mol Brain, 2019, 12(1):66-81.
doi: 10.1186/s13041-019-0486-8 pmid: 31315638 |
| [14] | Sánchez-Rodríguez MA, Mendoza-Núñez VM. Oxidative stress indexes for diagnosis of health or disease in humans[J]. Oxidative Med Cell Longev, 2019, 2019:4128152. |
| [15] |
Terskikh A, Fradkov A, Ermakova G, et al. “Fluorescent timer”:protein that changes color with time[J]. Science, 2000, 290(5496):1585-1588.
doi: 10.1126/science.290.5496.1585 pmid: 11090358 |
| [16] |
Wilson RJ, Drake JC, Cui D, et al. Conditional MitoTimer reporter mice for assessment of mitochondrial structure, oxidative stress, and mitophagy[J]. Mitochondrion, 2019, 44:20-26.
doi: S1567-7249(17)30196-4 pmid: 29274400 |
| [17] |
Hernandez G, Thornton C, Stotland A, et al. MitoTimer:a novel tool for monitoring mitochondrial turnover[J]. Autophagy, 2013, 9(11):1852-1861.
doi: 10.4161/auto.26501 pmid: 24128932 |
| [18] |
Schmidt LS, Warren MB, Nickerson ML, et al. Birt-Hogg-Dubé syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11. 2[J]. Am J Hum Genet, 2001, 69(4):876-882.
pmid: 11533913 |
| [19] | Sies H, Jones D. Oxidative stress[M]// Fink G. Encyclopedia of Stress. Amsterdam:Elsevier, 2007:45-48. |
| [20] |
Zhang BB, Li M, Zou Y, et al. NFκB/Orai1 facilitates endoplasmic Reticulum stress by oxidative stress in the pathogenesis of non-alcoholic fatty liver disease[J]. Front Cell Dev Biol, 2019, 7:202-214.
doi: 10.3389/fcell.2019.00202 URL |
| [21] |
Margolin W. Green fluorescent protein as a reporter for macromolecular localization in bacterial cells[J]. Methods, 2000, 20(1):62-72.
pmid: 10610805 |
| [22] |
Cunningham JT, Rodgers JT, Arlow DH, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex[J]. Nature, 2007, 450(7170):736-740.
doi: 10.1038/nature06322 URL |
| [23] |
Baba M, Hong SB, Sharma N, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling[J]. Proc Natl Acad Sci USA, 2006, 103(42):15552-15557.
doi: 10.1073/pnas.0603781103 URL |
| [24] |
Hasumi H, Baba M, Hasumi Y, et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN[J]. J Natl Cancer Inst, 2012, 104(22):1750-1764.
doi: 10.1093/jnci/djs418 pmid: 23150719 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||