








Biotechnology Bulletin ›› 2022, Vol. 38 ›› Issue (10): 106-114.doi: 10.13560/j.cnki.biotech.bull.1985.2021-1172
Previous Articles Next Articles
LIU Ning-ning1,2( ), WANG Xin-xin3, LAN Xin-yue2, CHU Hua-shuo2, CHEN Xu2, CHANG Shi-min1, LI Teng-fei1(
), WANG Xin-xin3, LAN Xin-yue2, CHU Hua-shuo2, CHEN Xu2, CHANG Shi-min1, LI Teng-fei1( ), XU Wen-tao2(
), XU Wen-tao2( )
)
Received:2021-09-13
Online:2022-10-26
Published:2022-11-11
Contact:
LI Teng-fei,XU Wen-tao
E-mail:774602273@qq.com;litengfeibeyond@126.com;xuwentao@cau.edu.cn
LIU Ning-ning, WANG Xin-xin, LAN Xin-yue, CHU Hua-shuo, CHEN Xu, CHANG Shi-min, LI Teng-fei, XU Wen-tao. G-Triplex Visualization Nucleic Acid Sensor for the Detection of Tetracycline[J]. Biotechnology Bulletin, 2022, 38(10): 106-114.
| 名称 Name | 序列Sequence | 碱基数 Number of bases/nt |
|---|---|---|
| G01 | 5'-GGGCACCACCAGGGTTAGGG-3' | 20 |
| G02 | 5'-GGGCACCACAGGGTTAGGG-3' | 19 |
| G03 | 5'-GGGCACCAAGGGTTAGGG-3' | 18 |
| G04 | 5'-GGGCACCAGGGTTAGGG-3' | 17 |
| G05 | 5'-GGGCACAGGGTTAGGG-3' | 16 |
| G06 | 5'-GGGCAAGGGTTAGGG-3' | 15 |
| G07 | 5'-GGGCAGGGTTAGGG-3' | 14 |
| Apt1 | 5'-CGGTGGTGCCC-3' | 11 |
Table 1 Sequence list of aptamers and G-triplex
| 名称 Name | 序列Sequence | 碱基数 Number of bases/nt |
|---|---|---|
| G01 | 5'-GGGCACCACCAGGGTTAGGG-3' | 20 |
| G02 | 5'-GGGCACCACAGGGTTAGGG-3' | 19 |
| G03 | 5'-GGGCACCAAGGGTTAGGG-3' | 18 |
| G04 | 5'-GGGCACCAGGGTTAGGG-3' | 17 |
| G05 | 5'-GGGCACAGGGTTAGGG-3' | 16 |
| G06 | 5'-GGGCAAGGGTTAGGG-3' | 15 |
| G07 | 5'-GGGCAGGGTTAGGG-3' | 14 |
| Apt1 | 5'-CGGTGGTGCCC-3' | 11 |

Fig. 2 Aptamer and sequence optimization diagram A:Color diagram of colloidal gold under different conditions,1 is negative control,2 is positive control,and 3-5 refers to adding tetracycline 1,10,and 100 μmol/L respectively. B:ThT fluorescence intensities before and after adding target while G01-G07 sequences bound with aptamers
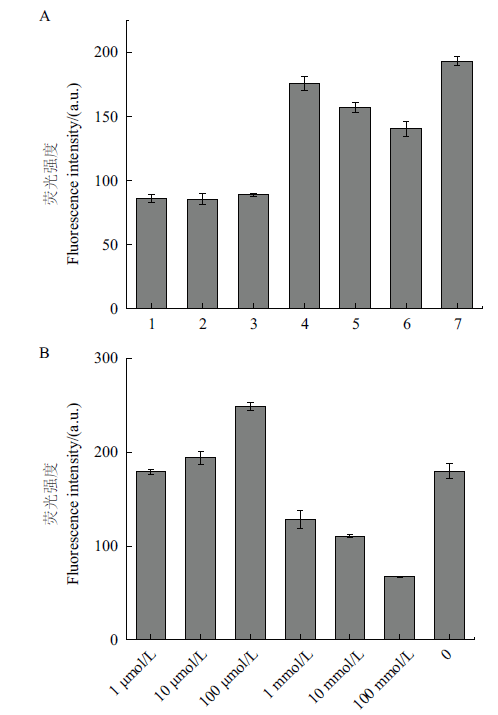
Fig. 3 ThT fluorescence intensities under different conditions A: ThT fluorescence intensity in the presence of different ions(1: Tris-HCl+CaCl2,2: Tris-HCl+MgCl2,3: Tris-HCl+KCl,4: H2O,5: CaCl2,6: MgCl2,and 7: KCl;PH=7.4);B: effect of KCl solution with different concentration on the ThT fluorescence intensity

Fig. 4 Time optimization A: the change of ThT fluorescence intensity during 0-30 min incubation between target and aptamer;B: changes of ThT fluorescence intensity within 0-30 min
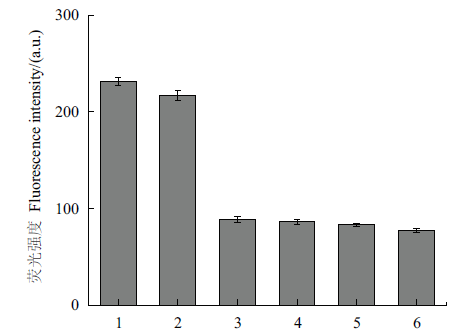
Fig. 6 Specificity verification chart 1:Tetracycline. 2:Tetracycline hydrochloride. 3:Oxytetracycline. 4:Chloramphenicol. 5:Streptavidin. 6:Lincomycin
| 添加浓度Adding concentration/(nmol·L-1) | 荧光强度 Fluorescence intensity/(a.u.) | 回收浓度 Recovery concentration/(nmol·L-1) | 平均浓度 Mean concentration/(nmol·L-1) | 回收率 Recovery rate/% | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 115.2 | 114.1 | 115.1 | 0.01 | 0 | 0.03 | 0.01 | 101.33 |
| 1 | 17.0 | 173.2 | 172.5 | 1.08 | 1.10 | 1.05 | 1.08 | 107.67 |
| 10 | 210 | 208 | 209 | 11.09 | 9.78 | 10.42 | 10.43 | 104.3 |
| 100 | 245 | 245.6 | 246 | 99.89 | 104.41 | 106.35 | 103.55 | 103.55 |
| 1 000 | 282 | 281.1 | 281.5 | 1 019.66 | 1 120.36 | 988.14 | 1 042.72 | 104.27 |
Table 2 Addition and recovery table of tetracycline in milk samples
| 添加浓度Adding concentration/(nmol·L-1) | 荧光强度 Fluorescence intensity/(a.u.) | 回收浓度 Recovery concentration/(nmol·L-1) | 平均浓度 Mean concentration/(nmol·L-1) | 回收率 Recovery rate/% | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 115.2 | 114.1 | 115.1 | 0.01 | 0 | 0.03 | 0.01 | 101.33 |
| 1 | 17.0 | 173.2 | 172.5 | 1.08 | 1.10 | 1.05 | 1.08 | 107.67 |
| 10 | 210 | 208 | 209 | 11.09 | 9.78 | 10.42 | 10.43 | 104.3 |
| 100 | 245 | 245.6 | 246 | 99.89 | 104.41 | 106.35 | 103.55 | 103.55 |
| 1 000 | 282 | 281.1 | 281.5 | 1 019.66 | 1 120.36 | 988.14 | 1 042.72 | 104.27 |
| [1] |
Anderson CR, Rupp HS, Wu WH. Complexities in tetracycline analysis-chemistry, matrix extraction, cleanup, and liquid chromatography[J]. J Chromatogr A, 2005, 1075(1/2):23-32.
doi: 10.1016/j.chroma.2005.04.013 URL |
| [2] |
Liu X, Steele JC, Meng XZ. Usage, residue, and human health risk of antibiotics in Chinese aquaculture:a review[J]. Environ Pollut, 2017, 223:161-169.
doi: 10.1016/j.envpol.2017.01.003 URL |
| [3] |
Liu Y, Yang HL, Yang S, et al. High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish and shrimp[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2013, 917/918:11-17.
doi: 10.1016/j.jchromb.2012.12.036 URL |
| [4] |
Visek WJ. The mode of growth promotion by antibiotics[J]. J Animal Sci, 1978, 46(5):1447-1469.
doi: 10.2527/jas1978.4651447x URL |
| [5] |
Pastor-Navarro N, Morais S, Maquieira A, et al. Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues. Application to honey samples[J]. Anal Chim Acta, 2007, 594(2):211-218.
pmid: 17586117 |
| [6] |
Moats WA. Determination of tetracycline antibiotics in beef and pork tissues using ion-paired liquid chromatography[J]. J Agric Food Chem, 2000, 48(6):2244-2248.
doi: 10.1021/jf990649r URL |
| [7] |
Kowalski P. Capillary electrophoretic method for the simultaneous determination of tetracycline residues in fish samples[J]. J Pharm Biomed Anal, 2008, 47(3):487-493.
doi: 10.1016/j.jpba.2008.01.036 pmid: 18325708 |
| [8] |
Fritz JW, Zuo YG. Simultaneous determination of tetracycline, oxytetracycline, and 4-epitetracycline in milk by high-performance liquid chromatography[J]. Food Chem, 2007, 105(3):1297-1301.
doi: 10.1016/j.foodchem.2007.03.047 URL |
| [9] |
Zhang YL, Lu SX, Liu W, et al. Preparation of anti-tetracycline antibodies and development of an indirect heterologous competitive enzyme-linked immunosorbent assay to detect residues of tetracycline in milk[J]. J Agric Food Chem, 2007, 55(2):211-218.
doi: 10.1021/jf062627s URL |
| [10] |
Okerman L, Croubels S, de Baere S, et al. Inhibition tests for detection and presumptive identification of tetracyclines, beta-lactam antibiotics and quinolones in poultry meat[J]. Food Addit Contam, 2001, 18(5):385-393.
pmid: 11358180 |
| [11] |
Aga DS, O’Connor S, Ensley S, et al. Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry[J]. J Agric Food Chem, 2005, 53(18):7165-7171.
doi: 10.1021/jf050415+ URL |
| [12] |
Chua A, Yean CY, Ravichandran M, et al. A rapid DNA biosensor for the molecular diagnosis of infectious disease[J]. Biosens Bioelectron, 2011, 26(9):3825-3831.
doi: 10.1016/j.bios.2011.02.040 pmid: 21458979 |
| [13] |
Kim YT, Jung JH, Choi YK, et al. A packaged paper fluidic-based microdevice for detecting gene expression of influenza A virus[J]. Biosens Bioelectron, 2014, 61:485-490.
doi: 10.1016/j.bios.2014.06.006 pmid: 24949821 |
| [14] |
Jung JH, Oh SJ, Kim YT, et al. Combination of multiplex reverse-transcription loop-mediated isothermal amplification with an immunochromatographic strip for subtyping influenza A virus[J]. Anal Chim Acta, 2015, 853:541-547.
doi: S0003-2670(14)01253-7 pmid: 25467501 |
| [15] |
Darmostuk M, Rimpelova S, Gbelcova H, et al. Current approaches in SELEX:an update to aptamer selection technology[J]. Biotechnol Adv, 2015, 33(<W>6 Pt 2):1141-1161.
doi: 10.1016/j.biotechadv.2015.02.008 pmid: 25708387 |
| [16] |
Kim YS, Raston NHA, Gu MB. Aptamer-based nanobiosensors[J]. Biosens Bioelectron, 2016, 76:2-19.
doi: 10.1016/j.bios.2015.06.040 pmid: 26139320 |
| [17] |
Mascini M, Palchetti I, Tombelli S. Nucleic acid and peptide aptamers:fundamentals and bioanalytical aspects[J]. Angew Chem Int Ed Engl, 2012, 51(6):1316-1332.
doi: 10.1002/anie.201006630 URL |
| [18] |
Liu LL, Shao Y, Peng J, et al. Molecular rotor-based fluorescent probe for selective recognition of hybrid G-quadruplex and as a K+ sensor[J]. Anal Chem, 2014, 86(3):1622-1631.
doi: 10.1021/ac403326m URL |
| [19] |
Mohanty J, Barooah N, Dhamodharan V, et al. Thioflavin T as an ef-ficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA[J]. J Am Chem Soc, 2013, 135(1):367-376.
doi: 10.1021/ja309588h URL |
| [20] |
Li YN, Wang JY, Zhang B, et al. A rapid fluorometric method for determination of aflatoxin B1 in plant-derived food by using a thioflavin T-based aptasensor[J]. Mikrochim Acta, 2019, 186(4):214.
doi: 10.1007/s00604-019-3325-9 URL |
| [21] |
Kudrya VY, Yashchuk VM, Dubey IY, et al. The spectral properties of the telomere fragments[J]. Ukr J Phys, 2016, 61(6):516-518.
doi: 10.15407/ujpe61.06.0516 URL |
| [22] |
Choudhury SD, Mohanty J, Pal H, et al. Cooperative metal ion binding to a cucurbit[7]uril-thioflavin T complex:demonstration of a stimulus-responsive fluorescent supramolecular capsule[J]. J Am Chem Soc, 2010, 132(4):1395-1401.
doi: 10.1021/ja908795y URL |
| [23] | 曲瑶, 张亚旗, 肖光, 等. 基于核酸碱基猝灭荧光团的核酸适配体传感器检测赭曲霉毒素A[J]. 分析化学, 2020, 48(10):1409-1415. |
| Qu Y, Zhang YQ, Xiao G, et al. Aptasensor based on nucleic acid base quenching fluorophore for detection of ochratoxin A[J]. Chin J Anal Chem, 2020, 48(10):1409-1415. | |
| [24] |
Hao LL, Wang W, Shen XQ, et al. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of ochratoxin A[J]. J Agric Food Chem, 2020, 68(1):369-375.
doi: 10.1021/acs.jafc.9b06021 URL |
| [25] |
Liu SG, Zhang D, He Y, et al. A split aptamer sensing platform for highly sensitive detection of theophylline based on dual-color fluorescence colocalization and single molecule photobleaching[J]. Biosens Bioelectron, 2020, 166:112461.
doi: 10.1016/j.bios.2020.112461 URL |
| [26] |
Khaled A, Gionfriddo E, Acquaro V Jr, et al. Development and validation of a fully automated solid phase microextraction high throughput method for quantitative analysis of multiresidue veterinary drugs in chicken tissue[J]. Anal Chim Acta, 2019, 1056:34-46.
doi: S0003-2670(18)31493-4 pmid: 30797459 |
| [27] |
de Faria HD, Rosa MA, Silveira AT, et al. Direct extraction of tetracyclines from bovine milk using restricted access carbon nanotubes in a column switching liquid chromatography system[J]. Food Chem, 2017, 225:98-106.
doi: S0308-8146(17)30004-3 pmid: 28193438 |
| [28] |
Zhao WJ, Zuo HY, Guo Y, et al. Porous covalent triazine-terphenyl polymer as hydrophilic-lipophilic balanced sorbent for solid phase extraction of tetracyclines in animal derived foods[J]. Talanta, 2019, 201:426-432.
doi: S0039-9140(19)30399-6 pmid: 31122445 |
| [29] |
Sun CY, Su RF, Bie JX, et al. Label-free fluorescent sensor based on aptamer and thiazole orange for the detection of tetracycline[J]. Dyes Pigments, 2018, 149:867-875.
doi: 10.1016/j.dyepig.2017.11.031 URL |
| [30] |
Dai YY, Zhang Y, Liao WL, et al. G-quadruplex specific thioflavin T-based label-free fluorescence aptasensor for rapid detection of tetracycline[J]. Spectrochim Acta A Mol Biomol Spectrosc, 2020, 238:118406.
doi: 10.1016/j.saa.2020.118406 URL |
| [1] | LI Dian-dian, SU Yuan, LI Jie, XU Wen-tao, ZHU Long-jiao. Progress in Selection and Application of Antibacterial Aptamers [J]. Biotechnology Bulletin, 2023, 39(6): 126-132. |
| [2] | LI Tian-shun, LI Chen-wei, WANG Jia, ZHU Long-Jiao, XU Wen-tao. Efficient Generation of Secondary Libraries During Functional Nucleic Acids Screening [J]. Biotechnology Bulletin, 2023, 39(3): 116-122. |
| [3] | ZHOU Zi-qi, ZHANG Yang-zi, LAN Xin-yue, LIU Yang-er, ZHU Long-jiao, XU Wen-tao. Selection and Application of Light-up Nucleic Acid Aptamers [J]. Biotechnology Bulletin, 2022, 38(5): 240-247. |
| [4] | LAN Xin-yue, LIU Ning-ning, ZHU Long-jiao, CHEN Xu, CHU Hua-shuo, LI Xiang-yang, DUAN Nuo, XU Wen-tao. Tetracycline Bivalent Aptamer Non-enzyme Label-free Sensor [J]. Biotechnology Bulletin, 2022, 38(3): 276-284. |
| [5] | ZHENG Fang-fang, LIN Jun-sheng. Selection and Specificity of Nucleic Acid Aptamers for a Proliferation Inducing Ligand [J]. Biotechnology Bulletin, 2021, 37(10): 196-202. |
| [6] | PENG Yuan-yuan, XIAO Xing-ning, ZHU Long-jiao, TAO Xiao-qi, XU Wen-tao. The Interaction Law Between Small Molecular Substances and Aptamers [J]. Biotechnology Bulletin, 2020, 36(8): 201-209. |
| [7] | ZHAO Ying, WANG Nan, LU An-xiang, FENG Xiao-yuan, GUO Xiao-jun, LUAN Yun-xia. Application in the Detection of Fungal Toxins by Nucleic Acid Aptamer Lateral Flow Chromatography Analysis Technique [J]. Biotechnology Bulletin, 2020, 36(8): 217-227. |
| [8] | FANG Shun-yan, SONG Dan, LIU Yan-ping, XU Wen-juan, LIU Jia-yao, HAN Xiang-zhi, LONG Feng. Study on Evanescent Wave Fluorescence Aptasensor for Direct and Rapid Detection of Escherichia coli O157∶H7 [J]. Biotechnology Bulletin, 2020, 36(7): 228-234. |
| [9] | YUHAN Jie-yu, ZHU Li-ye, CHEN Xu, HE Xiao-yun, XU Wen-tao. Screening and Evaluation Strategies of Cell-specific Nucleic Acid Aptamers [J]. Biotechnology Bulletin, 2020, 36(7): 235-244. |
| [10] | YANG Min, LI Shu-ting, YANG Wen-ping, LI Xiang-yang, XU Wen-tao. Research Progress on Functional Nucleic Acid Biosensors Mediated by DNA/Silver Nanoclusters [J]. Biotechnology Bulletin, 2020, 36(6): 245-254. |
| [11] | WANG Qi, YAN Chun-lei, GAO Hong-wei, WU Wei, YANG Qing-li. Research Progress of DNA Aptasensors for Foodborne Pathogen Detection [J]. Biotechnology Bulletin, 2020, 36(11): 245-258. |
| [12] | WU Xue-ling, ZHOU Xiang-yu, WU Xiao-yan, LUO Kui, GU Yi-chao, ZHOU Han, LIAO Wan-qing, ZENG Wei-min. Construction of Tetracycline-degrading Bacterial Co-culture System and Community Analysis of Wastewater Remediation [J]. Biotechnology Bulletin, 2020, 36(10): 116-126. |
| [13] | WU Ya, XU Zhi-hui, ZHANG Biao, ZHAO Dong-fang, CAO Wen-xin, ZHANG Xing-ping. Research Progress of Nucleic Acid Aptamer Optical Biosensor in Kanamycin Detection [J]. Biotechnology Bulletin, 2020, 36(1): 193-201. |
| [14] | XIE Yin-xia, WANG Wei-ran, CHENG Nan, XU Wen-tao. Research Progress on Electrical Signal Molecules in Electrochemical Functional Nucleic Acids Biosensors [J]. Biotechnology Bulletin, 2019, 35(5): 157-169. |
| [15] | LI Xue-tong, LIN Ying, ZHANG Yuan, LI Ying, LÜ Shu-xia, XU Wen-tao. Application Progress on Aptasensors in Detection of Food-born Pathogenic Bacteria [J]. Biotechnology Bulletin, 2019, 35(4): 125-138. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||