








Biotechnology Bulletin ›› 2025, Vol. 41 ›› Issue (7): 226-236.doi: 10.13560/j.cnki.biotech.bull.1985.2024-1056
Previous Articles Next Articles
HUANG Xu-sheng( ), ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun(
), ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun( ), LI Run-zhi(
), LI Run-zhi( )
)
Received:2024-10-28
Online:2025-07-26
Published:2025-07-22
Contact:
JIA Xiao-yun, LI Run-zhi
E-mail:hxss214809@163.com;jiaxiaoyun@sxau.edu.cn;rli2001@126.com
HUANG Xu-sheng, ZHOU Ya-li, CHAI Xu-dong, WEN Jing, WANG Ji-ping, JIA Xiao-yun, LI Run-zhi. Cloning of Plastidial PfLPAT1B Gene from Perilla frutescens and Its Functional Analysis in Oil Biosynthesis[J]. Biotechnology Bulletin, 2025, 41(7): 226-236.
引物名称 Primer name | 引物序列 Primer sequence (5′‒3′) | 酶切位点 Restriction site | 用途 Purpose |
|---|---|---|---|
| PfActin-q-F | AGACCTTCAATGTGCCAGCCA | 内参基因 | |
| PfActin-q-R | CACGACCAGCAAGATCCAACC | ||
| PfLPAT1B-q-F | TTAGACAGCAGAAGCCAAT | 实时荧光定量PCR | |
| PfLPAT1B-q-R | CCAGTTCCAACCAGAGTAA | ||
| PfLPAT1B-1300-F | ATACACCAAATCGAC | Xba Ⅰ、 Spe Ⅰ | 亚细胞定位表达载体构建 |
| PfLPAT1B-1300-R | CATGGTACCGGATCC | ||
| PfLPAT1B-pET28a-F | GGATAACAATTCCCC | Xba Ⅰ、 BamH Ⅰ | 大肠杆菌表达载体构建 |
| PfLPAT1B-pET28a-R | ACGGAGCTCGAATTC | ||
| PfLPAT1B-pYES2.0-F | ATTAAGCTTGGTACC | Sac Ⅰ、 Xba Ⅰ | 酵母表达载体构建 |
| PfLPAT1B-pYES2.0-R | TACATGATGCGGCCC | ||
| PfLPAT1B-1303-F | AACCTGCAGGTCGAC | Xba Ⅰ、 Sma Ⅰ | 植物过表达载体构建 |
| PfLPAT1B-1303-R | TAAACGAGCTCGGTA |
Table 1 Primer sequence used in this study
引物名称 Primer name | 引物序列 Primer sequence (5′‒3′) | 酶切位点 Restriction site | 用途 Purpose |
|---|---|---|---|
| PfActin-q-F | AGACCTTCAATGTGCCAGCCA | 内参基因 | |
| PfActin-q-R | CACGACCAGCAAGATCCAACC | ||
| PfLPAT1B-q-F | TTAGACAGCAGAAGCCAAT | 实时荧光定量PCR | |
| PfLPAT1B-q-R | CCAGTTCCAACCAGAGTAA | ||
| PfLPAT1B-1300-F | ATACACCAAATCGAC | Xba Ⅰ、 Spe Ⅰ | 亚细胞定位表达载体构建 |
| PfLPAT1B-1300-R | CATGGTACCGGATCC | ||
| PfLPAT1B-pET28a-F | GGATAACAATTCCCC | Xba Ⅰ、 BamH Ⅰ | 大肠杆菌表达载体构建 |
| PfLPAT1B-pET28a-R | ACGGAGCTCGAATTC | ||
| PfLPAT1B-pYES2.0-F | ATTAAGCTTGGTACC | Sac Ⅰ、 Xba Ⅰ | 酵母表达载体构建 |
| PfLPAT1B-pYES2.0-R | TACATGATGCGGCCC | ||
| PfLPAT1B-1303-F | AACCTGCAGGTCGAC | Xba Ⅰ、 Sma Ⅰ | 植物过表达载体构建 |
| PfLPAT1B-1303-R | TAAACGAGCTCGGTA |
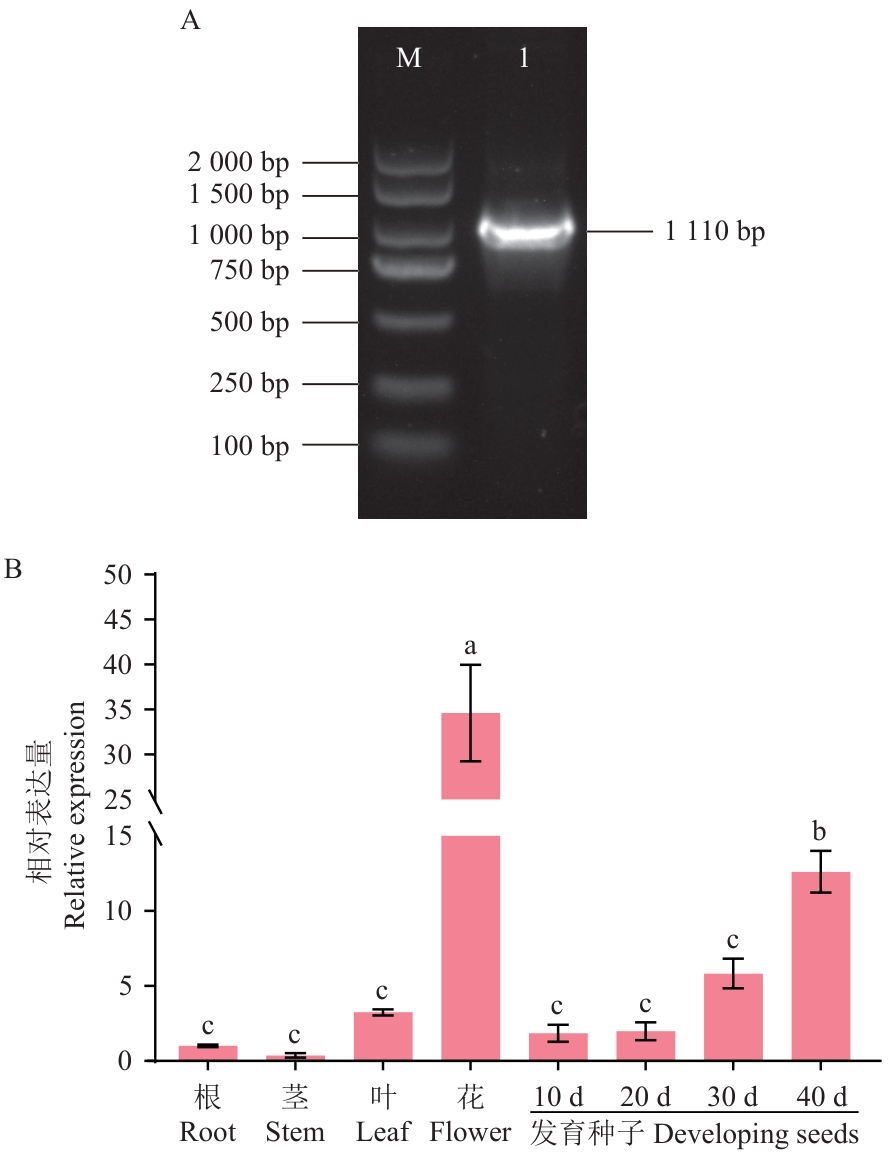
Fig. 2 Cloning of PfLPAT1B gene (A) and its expression patterns in different tissues of perilla (B)M: DNA marker DL 2 000; 1: PCR detection of PfLPAT1B gene. Different lower letters indicate significant differences at P<0.05 level. The same below
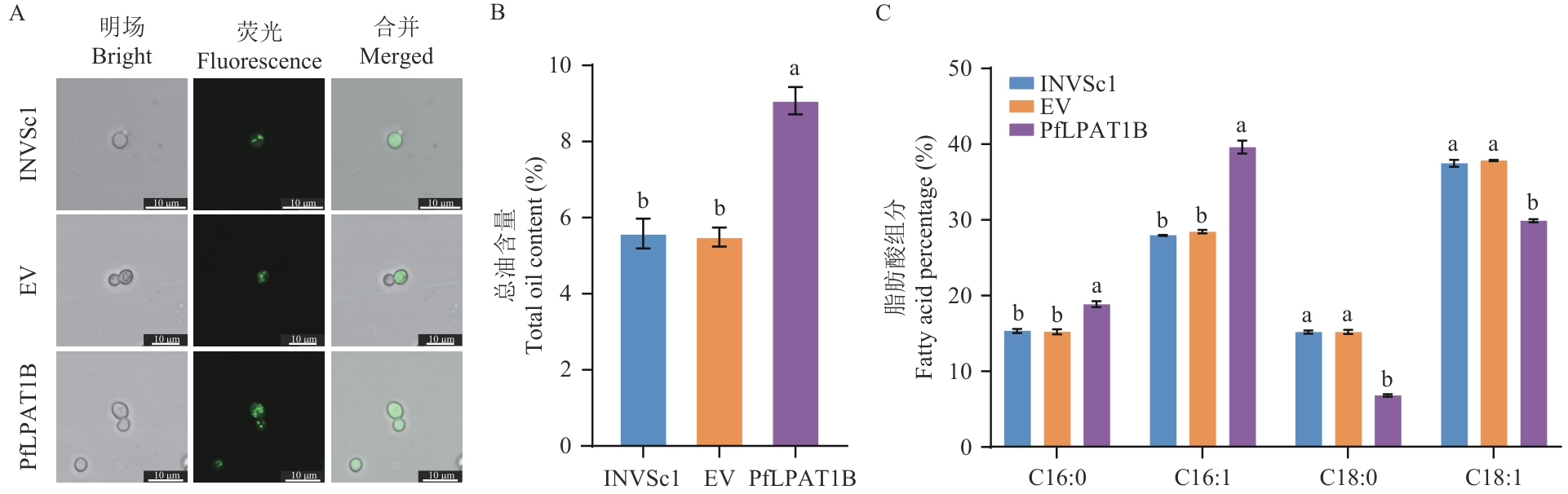
Fig. 6 Lipid droplets (A), total oil (B), and FA profiles (C) of the PfLPAT1B-transgenic yeastINVSc1: The wild-type yeast strain of S. cerevisiae. EV: Yeast strains carrying pYES2.0 empty vector. PfLPAT1B: Yeast strains expressing PfLPAT1B gene
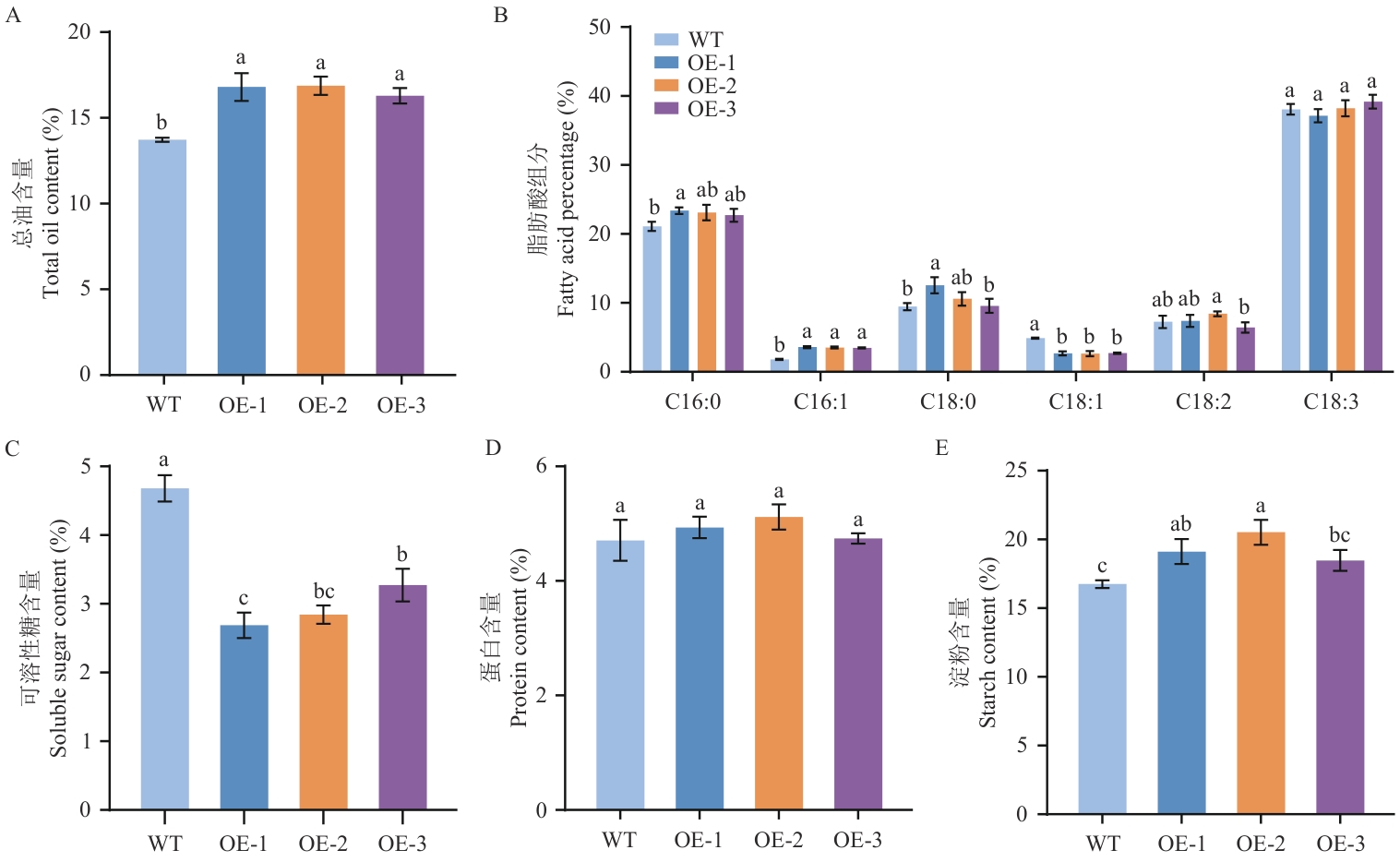
Fig. 7 Contents of oil and other metabolites in PfLPAT1B-transgenic tobaccoWT: Wild type tobacco plant lines. OE-1/-2/-3: Transgenic tobacco plant lines overexpressing PfLPAT1B gene
| [1] | Kim HU, Lee KR, Shim D, et al. Transcriptome analysis and identification of genes associated with ω-3 fatty acid biosynthesis in Perilla frutescens (L.) var. frutescens [J]. BMC Genomics, 2016, 17: 474. |
| [2] | Baker EJ, Miles EA, Burdge GC, et al. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans [J]. Prog Lipid Res, 2016, 64: 30-56. |
| [3] | Wu D, Yang SM, Shang ZW, et al. Genome-wide analysis of the fatty acid desaturase gene family reveals the key role of PfFAD3 in α-linolenic acid biosynthesis in Perilla seeds [J]. Front Genet, 2021, 12: 735862. |
| [4] | Zheng JR, Yang JF, Yang XY, et al. Transcriptome and miRNA sequencing analyses reveal the regulatory mechanism of α-linolenic acid biosynthesis in Paeonia rockii [J]. Food Res Int, 2022, 155: 111094. |
| [5] | Warude D, Joshi K, Harsulkar A. Polyunsaturated fatty acids: biotechnology [J]. Crit Rev Biotechnol, 2006, 26(2): 83-93. |
| [6] | Ohlrogge J, Browse J. Lipid biosynthesis [J]. Plant Cell, 1995, 7(7): 957-970. |
| [7] | Li-Beisson Y, Shorrosh B, Beisson F, et al. Acyl-lipid metabolism [J]. Arabidopsis Book, 2013, 11: e0161. |
| [8] | Fan JL, Andre C, Xu CC. A chloroplast pathway for the de novo biosynthesis of triacylglycerol in Chlamydomonas reinhardtii [J]. FEBS Lett, 2011, 585(12): 1985-1991. |
| [9] | Goodson C, Roth R, Wang ZT, et al. Structural correlates of cytoplasmic and chloroplast lipid body synthesis in Chlamydomonas reinhardtii and stimulation of lipid body production with acetate boost [J]. Eukaryot Cell, 2011, 10(12): 1592-1606. |
| [10] | Kim HU, Huang AHC. Plastid lysophosphatidyl acyltransferase is essential for embryo development in Arabidopsis [J]. Plant Physiol, 2004, 134(3): 1206-1216. |
| [11] | Yu B, Wakao S, Fan JL, et al. Loss of plastidic lysophosphatidic acid acyltransferase causes embryo-lethality in Arabidopsis [J]. Plant Cell Physiol, 2004, 45(5): 503-510. |
| [12] | Misra N, Panda PK, Parida BK. Genome-wide identification and evolutionary analysis of algal LPAT genes involved in TAG biosynthesis using bioinformatic approaches [J]. Mol Biol Rep, 2014, 41(12): 8319-8332. |
| [13] | Kim HU, Li YB, Huang AHC. Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis [J]. Plant Cell, 2005, 17(4): 1073-1089. |
| [14] | Yamaoka Y, Achard D, Jang S, et al. Identification of a Chlamydomonas plastidial 2-lysophosphatidic acid acyltransferase and its use to engineer microalgae with increased oil content [J]. Plant Biotechnol J, 2016, 14(11): 2158-2167. |
| [15] | 郝月茹, 周雅莉, 王志龙, 等. 紫苏油脂合成相关基因家族鉴定及表达分析 [J]. 西北植物学报, 2020, 40(10): 1663-1671. |
| Hao YR, Zhou YL, Wang ZL, et al. Identification and expression analysis of gene family related to Perilla lipid synthesis [J]. Acta Bot Boreali Occidentalia Sin, 2020, 40(10): 1663-1671. | |
| [16] | 肖旦望, 刘聪, 胡学芳, 等. 甘蓝型油菜LPAT4基因的克隆与表达 [J]. 作物学报, 2014, 40(10): 1748-1755. |
| Xiao DW, Liu C, Hu XF, et al. Cloning and expression of LPAT4 genes from Brassica napus [J]. Acta Agron Sin, 2014, 40(10): 1748-1755. | |
| [17] | 尹永泰. 甘蓝型油菜溶血磷脂酰基转移酶家族基因的克隆与表达 [D]. 武汉: 华中科技大学, 2016. |
| Yin YT. Cloning and expression of lysophosphatidis acyltransferase family genes in Brassica napus [D]. Wuhan: Huazhong University of Science and Technology, 2016. | |
| [18] | Chen SL, Lei Y, Xu X, et al. The peanut (Arachis hypogaea L.) gene AhLPAT2 increases the lipid content of transgenic Arabidopsis seeds [J]. PLoS One, 2015, 10(8): e0136170. |
| [19] | Chen GQ, van Erp H, Martin-Moreno J, et al. Expression of Castor LPAT2 enhances ricinoleic acid content at the sn-2 position of triacylglycerols in Lesquerella seed [J]. Int J Mol Sci, 2016, 17(4): 507. |
| [20] | 张凯. 溶血磷脂酸酰基转移酶2和5调控甘蓝型油菜种子油脂合成的功能研究 [D]. 武汉: 华中科技大学, 2021. |
| Zhang K. Study on the function of lysophosphatidic acid acyltransferase 2 and 5 in regulating oil synthesis of Brassica napus seeds [D]. Wuhan: Huazhong University of Science and Technology, 2021. | |
| [21] | 徐华祥, 鲁庚, 郭曦, 等. 紫苏溶血磷脂酰转移酶基因PfLPAAT的克隆及功能研究 [J]. 作物学报, 2022, 48(10): 2494-2504. |
| Xu HX, Lu G, Guo X, et al. Cloning and functional study of lysophosphatidic acid acyltransferase gene in Perilla frutescens [J]. Acta Agron Sin, 2022, 48(10): 2494-2504. | |
| [22] | 周雅莉, 黄旭升, 郝月茹, 等. 紫苏溶血磷脂酸酰基转移酶基因的克隆与功能分析 [J]. 生物工程学报, 2022, 38(8): 3014-3028. |
| Zhou YL, Huang XS, Hao YR, et al. Cloning and functional characterization of a lysophosphatidic acid acyltransferase gene from Perilla frutescens [J]. Chin J Biotechnol, 2022, 38(8): 3014-3028. | |
| [23] | Huang XS, Zhou YL, Shi XF, et al. PfbZIP85 transcription factor mediates ω-3 fatty acid-enriched oil biosynthesis by down-regulating PfLPAT1B gene expression in plant tissues [J]. Int J Mol Sci, 2024, 25(8): 4375. |
| [24] | Zhou YL, Huang XS, Hu T, et al. Genome-wide analysis of glycerol-3-phosphate acyltransferase (GPAT) family in Perilla frutescens and functional characterization of PfGPAT9 crucial for biosynthesis of storage oils rich in high-value lipids [J]. Int J Mol Sci, 2023, 24(20): 15106. |
| [25] | 刘萌萌. 转录因子GhAHL4负调控GhSDP1参与棉籽萌发过程油脂降解 [D]. 太谷: 山西农业大学, 2022. |
| Liu MM. Transcription factor GhAHL4 negatively regulates GhSDP1 to participate in oil degradation during cottonseed germination [D]. Taigu: Shanxi Agricultural University, 2022. | |
| [26] | Barroga NAM, Nguyen VC, Nakamura Y. The role of lysophosphatidic acid acyltransferase 1 in reproductive growth of Arabidopsis thaliana [J]. J Exp Bot, 2024, 75(22): 7190-7201. |
| [27] | Zhang K, He JJ, Yin YT, et al. Lysophosphatidic acid acyltransferase 2 and 5 commonly, but differently, promote seed oil accumulation in Brassica napus [J]. Biotechnol Biofuels Bioprod, 2022, 15(1): 83. |
| [28] | Zhang QY, Niu LX, Yu R, et al. Cloning, characterization, and expression analysis of a gene encoding a putative lysophosphatidic acid acyltransferase from seeds of Paeonia rockii [J]. Appl Biochem Biotechnol, 2017, 182(2): 721-741. |
| [29] | Yin YT, Raboanatahiry N, Chen K, et al. Class A lysophosphatidic acid acyltransferase 2 from Camelina sativa promotes very long-chain fatty acids accumulation in phospholipid and triacylglycerol [J]. Plant J, 2022, 112(5): 1141-1158. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||