








生物技术通报 ›› 2020, Vol. 36 ›› Issue (11): 230-237.doi: 10.13560/j.cnki.biotech.bull.1985.2020-0426
收稿日期:2020-04-05
出版日期:2020-11-26
发布日期:2020-11-20
作者简介:陈林,男,硕士研究生,研究方向:大豆遗传育种;E-mail: 基金资助:
CHEN Lin1,2( ), PAN Zhen-zhi1, DAI Yi1, SONG Li1(
), PAN Zhen-zhi1, DAI Yi1, SONG Li1( )
)
Received:2020-04-05
Published:2020-11-26
Online:2020-11-20
摘要:
利用大豆Williams 82等多个品种为材料,比较6种不同细胞核解离液的核分离效果,以期制备适合流式细胞仪分析的大豆不同组织部位的高纯度细胞核悬液,并检测其在大豆细胞周期调控中的应用。将新鲜幼嫩的大豆叶和根组织在不同的细胞核解离液中进行机械切割后,细胞核从组织释放游离到核解离液,过400目滤网,离心富集及DAPI染色,流式细胞仪检测分析细胞核解离液中生物颗粒特性和DNA含量。结果表明:Galbraith’s和LB01 核解离液适用于大豆叶和根中细胞核的解离,其变异系数CV值较低,分别是2.78%和2.96%。其中LB01 核解离液可检测到显著的G2/M 期峰,在细胞周期及遗传倍性的研究中应用相对更好,且核解离液在不同大豆品种上具有一定的适用性,可成功检测PEG模拟的干旱胁迫对不同大豆根尖细胞周期的调控。
陈林, 潘贞志, 戴毅, 宋丽. 适合流式细胞仪分析的大豆细胞核解离液的筛选与应用[J]. 生物技术通报, 2020, 36(11): 230-237.
CHEN Lin, PAN Zhen-zhi, DAI Yi, SONG Li. Screening and Application of the Nuclear Dissociation Solutions of Soybeans Suitable for Flow Cytometry Analysis[J]. Biotechnology Bulletin, 2020, 36(11): 230-237.
| 核解离液 | 配制方法和储存使用 |
|---|---|
| Galbraith’s[ | 45 mmol/L MgCl2,20 mmol/L MOPS,30 mmol/L Sodium citrate,0.1%(V/V)Triton X-100。1 mol/L NaOH调pH至7.0,0.22 μm 过滤并分装贮存于-20℃。 |
| mG[ | 45 mmol/L MgCl2,20 mmol/L MOPS,30 mmol/L Sodium citrate,10 mmol/L Na2EDTA,1% PVP-40,0.2%(V/V)Triton X-100。1 mol/L NaOH调pH至7.0,0.22 μm过滤,按照20 μL/mL加入2-mercaptoethanol 并分装贮存于-20℃。 |
| LB01[ | 15 mmol/L Tris,2 mmol/L Na2EDTA,0.5 mmol/L Spermine tetrahydrochloride,80 mmol/L KCl,20 mmol/L NaCl,0.1%(V/V)Triton X-100。1 mol/L NaOH调pH至7.5,0.22 μm过滤,加入2-mercaptoethanol使得终浓度为15 mmol/L并分装贮存于-20℃。 |
| WPB[ | 200 mmol/L Tris,4 mmol/L MgCl2,2 mmol/L Na2EDTA,86 mmol/L NaCl,10 mmol/L Sodium pyrosulfite,1%(W/V)PVP-10,1%(V/V)Triton X-100。HCl调pH至7.5,0.22 μm过滤并分装贮存于-20℃。 |
| GPB[ | 0.5 mmol/L Spermine tetrahydrochloride,30 mmol/L Sodium citrate,80 mmol/L KCl,20 mmol/L NaCl,20 mmol/L MOPS,0.5%(V/V)Triton X-100。HCl调pH至7.0,0.22 μm并分装贮存于-20℃。 |
| Tris·MgCl2[ | 200 mmol/L Tris,4 mmol/L MgCl2,0.5%(V/V)Triton X-100。HCl调pH至7.5,0.22 μm过滤并分装贮存于-20℃。 |
表1 核解离缓冲液配方
| 核解离液 | 配制方法和储存使用 |
|---|---|
| Galbraith’s[ | 45 mmol/L MgCl2,20 mmol/L MOPS,30 mmol/L Sodium citrate,0.1%(V/V)Triton X-100。1 mol/L NaOH调pH至7.0,0.22 μm 过滤并分装贮存于-20℃。 |
| mG[ | 45 mmol/L MgCl2,20 mmol/L MOPS,30 mmol/L Sodium citrate,10 mmol/L Na2EDTA,1% PVP-40,0.2%(V/V)Triton X-100。1 mol/L NaOH调pH至7.0,0.22 μm过滤,按照20 μL/mL加入2-mercaptoethanol 并分装贮存于-20℃。 |
| LB01[ | 15 mmol/L Tris,2 mmol/L Na2EDTA,0.5 mmol/L Spermine tetrahydrochloride,80 mmol/L KCl,20 mmol/L NaCl,0.1%(V/V)Triton X-100。1 mol/L NaOH调pH至7.5,0.22 μm过滤,加入2-mercaptoethanol使得终浓度为15 mmol/L并分装贮存于-20℃。 |
| WPB[ | 200 mmol/L Tris,4 mmol/L MgCl2,2 mmol/L Na2EDTA,86 mmol/L NaCl,10 mmol/L Sodium pyrosulfite,1%(W/V)PVP-10,1%(V/V)Triton X-100。HCl调pH至7.5,0.22 μm过滤并分装贮存于-20℃。 |
| GPB[ | 0.5 mmol/L Spermine tetrahydrochloride,30 mmol/L Sodium citrate,80 mmol/L KCl,20 mmol/L NaCl,20 mmol/L MOPS,0.5%(V/V)Triton X-100。HCl调pH至7.0,0.22 μm并分装贮存于-20℃。 |
| Tris·MgCl2[ | 200 mmol/L Tris,4 mmol/L MgCl2,0.5%(V/V)Triton X-100。HCl调pH至7.5,0.22 μm过滤并分装贮存于-20℃。 |
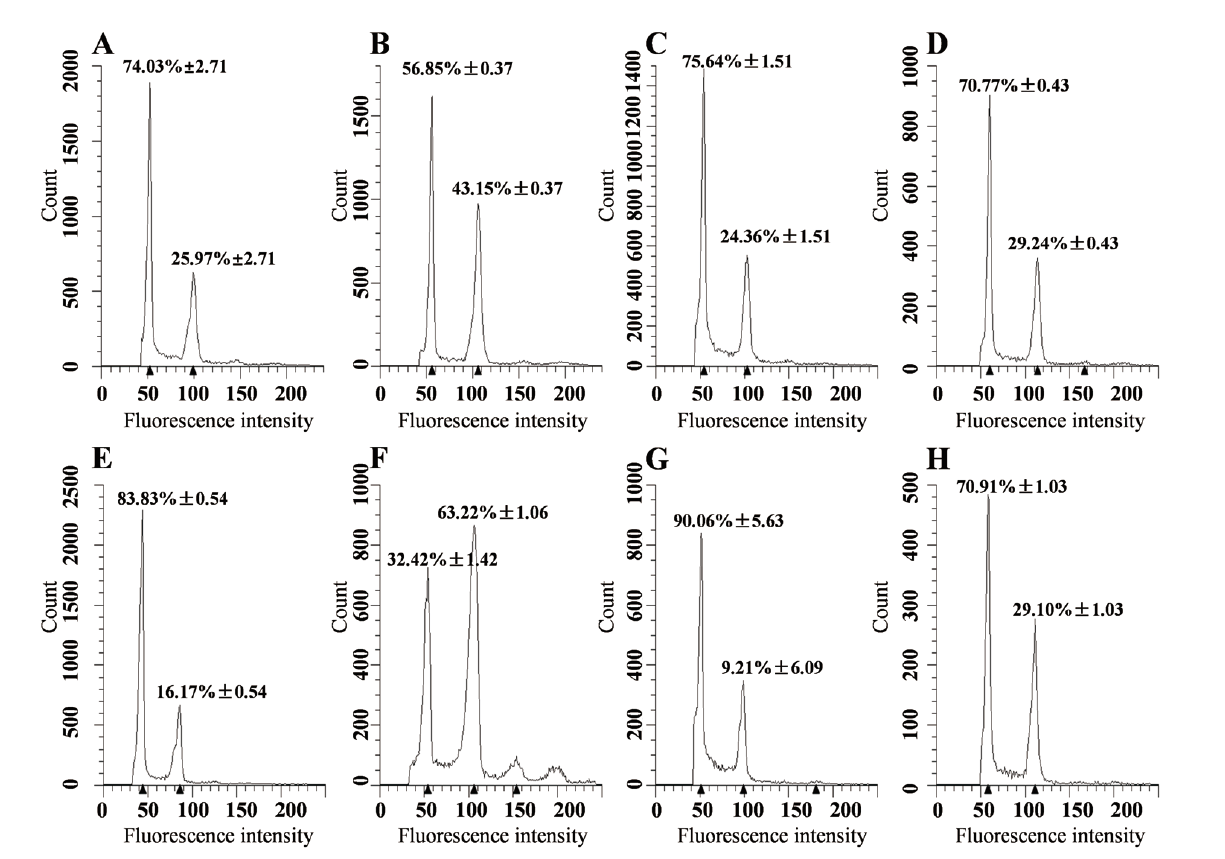
图4 不同大豆品种在PEG模拟干旱胁迫前后细胞周期的分析 A、C、E、G:不同大豆品种在正常生长状态时根尖细胞核的DNA相对含量;B、D、F、H不同大豆品种在20% PEG 6000模拟干旱胁迫处理后根尖细胞核的DNA相对含量。(A和B)Holladay,P<0.001;(C和D)PI 567611,P<0.05;(E和F)PI 567651,P<0.001;(G和H)Fiskeby III,P<0.001
| [1] |
Martin S, Lamb H, Brady C, et al. Inducing apoptosis of cancer cells using small-molecule plant compounds that bind to GRP78[J]. British Journal of Cancer, 2013,109(2):433-443.
doi: 10.1038/bjc.2013.325 URL pmid: 23807168 |
| [2] |
Henry CM, Hollville E, Martin S. Measuring apoptosis by microscopy and flow cytometry[J]. Methods, 2013,61(2):90-97.
doi: 10.1016/j.ymeth.2013.01.008 URL pmid: 23403105 |
| [3] |
Alam H, Sehgal L, Kundu ST, et al. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells[J]. Molecular Biology of the Cell, 2011,22(21):4068-4078.
doi: 10.1091/mbc.E10-08-0703 URL pmid: 21900500 |
| [4] | Basu S, Campbell HM, Dittel BN, et al. Purification of specific cell population by fluorescence activated cell sorting(FACS)[J]. Journal of Visualized Experiments, 2010,41:e1546. |
| [5] | Rewers M, Sliwinska E. Endoreduplication intensity as a marker of seed developmental stage in the Fabaceae[J]. Cytometry Part A. 2012,81(12):1067-1075. |
| [6] | Wang Y, Bigelow CA, Jiang Y. Ploidy level and DNA content of perennial ryegrass germplasm as determined by flow cytometry[J]. HortScience, 2009,44(7):2049-2052. |
| [7] |
Doležel J, Bartoš J. Plant DNA flow cytometry and estimation of nuclear genome size[J]. Ann Bot, 2005,95(1):99-110.
doi: 10.1093/aob/mci005 URL pmid: 15596459 |
| [8] |
Sliwinska E, Pisarczyk I, Pawlik A, et al. Measuring genome size of desert plants using dry seeds[J]. Botany, 2009,87(2):127-135.
doi: 10.1139/B08-120 URL |
| [9] | Vrána J, Cápal P, Číhalíková J, et al. Flow sorting plant chromosomes[M] // Kianian S, Kianian P. Plant Cytogenetics. New York: Humana Press, 2016: 119-134. |
| [10] | Kovářová P, Navrátilová A, Macas J, et al. Chromosome analysis and sorting in Vicia sativa using flow cytometry[J]. Biologia Plantarum, 2007,51(1):43-48. |
| [11] |
Ortiz-Ramírez C, Arevalo ED, Xu X, et al. An efficient cell sorting protocol for maize protoplasts[J]. Current Protocols in Plant Biology, 2018,3(3):e20072.
doi: 10.1002/cppb.20072 URL pmid: 30138552 |
| [12] | Loureiro J, Trávníček P, Rauchová J, et al. The use of flow cytometry in the biosystematics, ecology and population biology of homoploid plants[J]. Preslia, 2010,82(1):3-21. |
| [13] |
Doležel J, Greilhuber J, Suda J. Estimation of nuclear DNA content in plants using flow cytometry[J]. Nature Protocols, 2007,2(9):2233-2244.
doi: 10.1038/nprot.2007.310 URL pmid: 17853881 |
| [14] |
Galbraith DW, Harkins KR, Maddox JM, et al. Rapid flow cytometric analysis of the cell cycle in intact plant tissues[J]. Science, 1983,220(4601):1049-1051.
doi: 10.1126/science.220.4601.1049 URL pmid: 17754551 |
| [15] |
Lee HC, Lin TY. Isolation of plant nuclei suitable for flow cytometry from recalcitrant tissue by use of a filtration column[J]. Plant Molecular Biology Reporter, 2005,23(1):53-58.
doi: 10.1007/BF02772646 URL |
| [16] |
Loureiro J, Rodriguez E, Doležel J, et al. Two new nuclear isolation buffers for plant DNA flow cytometry:a test with 37 species[J]. Annals of Botany, 2007,100(4):875-888.
doi: 10.1093/aob/mcm152 URL pmid: 17684025 |
| [17] | 李思璐. 玉米细胞内多倍化的发生规律研究[D]. 杨凌:西北农林科技大学, 2019. |
| Li SL. Study on the emerging and development of endopolyploidy in maize[D]. Yangling:Northwest A&F University, 2019. | |
| [18] | 张琳琳, 曹博, 白成科. 应用流式细胞术测定药用植物黄芩基因组大小[J]. 中国农学通报, 2013,29(25):130-135. |
| Zhang LL, Cao B, Bai CK. The detection of genome size of medicinal plant scutellaria baicalensis georgi by flow cytometry[J]. Chinese Agricultural Science Bulletin, 2013,29(25):130-135. | |
| [19] | 刘凤霞, 李京一, 王志刚, 等. 适合流式细胞仪分析的梨叶片细胞核提取缓冲液筛选[J]. 农业生物技术学报, 2018,26(6):1034-1042. |
| Liu FX, Li JY, Wang ZG, et al. The screening of the optimum buffer to produce pear(pyrus)leaf nuclear suspensions for flow cytometry analysis[J]. Chinese Journal of Agricultural Biotechnology, 2018,26(6):128-136. | |
| [20] | 郭宾会, 戴毅, 宋丽. 干旱下植物激素影响作物根系发育的研究进展[J]. 生物技术通报, 2018,34(7):48-56. |
| Guo BH, Dai Y, Song L. Research progress on the effects of phytohormones on crop root system development under drought condition[J]. Biotechnology Bulletin, 2018,34(7):48-56. | |
| [21] | 吴娇娇, 张谦, 刘士平, 等. 细胞周期因子与植物根系发育[J]. 植物生理学通讯, 2008,44(4):621-629. |
| Wu JJ, Zhang Q, Liu SP, et al. Cell cycle factors and plant root development[J]. Plant Physiology Journal, 2008(4):621-629. | |
| [22] |
Bramsiepe J, Wester K, Weinl C, et al. Endoreplication controls cell fate maintenance[J]. PLoS Genetics, 2010,6(6):e1000996.
doi: 10.1371/journal.pgen.1000996 URL pmid: 20585618 |
| [23] |
Scholes DR, Paige KN. Plasticity in ploidy:a generalized response to stress[J]. Trends in Plant Science, 2015,20(3):165-175.
doi: 10.1016/j.tplants.2014.11.007 URL pmid: 25534217 |
| [24] |
Bhosale R, Boudolf V, Cuevas F, et al. A spatiotemporal DNA endoploidy map of the Arabidopsis root reveals roles for the endocycle in root development and stress adaptation[J]. Plant Cell, 2018,30(10):2330-2351.
doi: 10.1105/tpc.17.00983 URL pmid: 30115738 |
| [25] | 田新民, 周香艳, 弓娜. 流式细胞术在植物学研究中的应用---检测植物核DNA含量和倍性水平[J]. 中国农学通报, 2011,27(9):21-27. |
| Tian XM, Zhou XY, Gong N, et al. Applications of flow cytometry in plant research—analysis of nuclear DNA content and ploidy level in plant cells[J]. Chinese Agriculture Science Bulletin, 2011,27(9):21-27. | |
| [26] | 杜立颖, 冯仁青. 流式细胞术[M]. 北京: 北京大学出版社, 2008: 16-16. |
| Du LY, Feng RQ. Flow cytometry[M]. Beijing: Peking University Press, 2008: 16-16. | |
| [27] |
Zhao L, Li Y, Xie Q, et al. Loss of CDKC;2 increases both cell division and drought tolerance in Arabidopsis thaliana[J]. Plant Journal, 2017,91(5):816-828.
doi: 10.1111/tpj.13609 URL pmid: 28622431 |
| [28] |
Li F, Wang L, Zhang Z, et al. ZmSMR4, a novel cyclin-dependent kinase inhibitor(CKI)gene in maize(Zea mays L.), functions as a key player in plant growth, development and tolerance to abiotic stress[J]. Plant Science, 2019,280:120-131.
doi: 10.1016/j.plantsci.2018.03.007 URL pmid: 30823990 |
| [1] | 王子颖, 龙晨洁, 范兆宇, 张蕾. 利用酵母双杂交系统筛选水稻中与OsCRK5互作蛋白[J]. 生物技术通报, 2023, 39(9): 117-125. |
| [2] | 刘雯锦, 马瑞, 刘升燕, 杨江伟, 张宁, 司怀军. 马铃薯StCIPK11的克隆及响应干旱胁迫分析[J]. 生物技术通报, 2023, 39(9): 147-155. |
| [3] | 刘玉玲, 王梦瑶, 孙琦, 马利花, 朱新霞. 启动子RD29A对转雪莲SikCDPK1基因烟草抗逆性的影响[J]. 生物技术通报, 2023, 39(9): 168-175. |
| [4] | 王帅, 冯宇梅, 白苗, 杜维俊, 岳爱琴. 大豆GmHMGR基因响应外源激素及非生物胁迫功能研究[J]. 生物技术通报, 2023, 39(7): 131-142. |
| [5] | 李文辰, 刘鑫, 康越, 李伟, 齐泽铮, 于璐, 王芳. TRV病毒诱导大豆基因沉默体系优化及应用[J]. 生物技术通报, 2023, 39(7): 143-150. |
| [6] | 丁凯鑫, 王立春, 田国奎, 王海艳, 李凤云, 潘阳, 庞泽, 单莹. 烯效唑缓解植物干旱损伤的研究进展[J]. 生物技术通报, 2023, 39(6): 1-11. |
| [7] | 王春语, 李政君, 王平, 张丽霞. 高粱表皮蜡质缺失突变体sb1抗旱生理生化分析[J]. 生物技术通报, 2023, 39(5): 160-167. |
| [8] | 翟莹, 李铭杨, 张军, 赵旭, 于海伟, 李珊珊, 赵艳, 张梅娟, 孙天国. 异源表达大豆转录因子GmNF-YA19提高转基因烟草抗旱性[J]. 生物技术通报, 2023, 39(5): 224-232. |
| [9] | 侯筱媛, 车郑郑, 李姮静, 杜崇玉, 胥倩, 王群青. 大豆膜系统cDNA文库的构建及大豆疫霉效应子PsAvr3a互作蛋白的筛选[J]. 生物技术通报, 2023, 39(4): 268-276. |
| [10] | 王海龙, 李雨倩, 王勃, 邢国芳, 张杰伟. 谷子SiMAPK3基因的克隆和表达特性分析[J]. 生物技术通报, 2023, 39(3): 123-132. |
| [11] | 杨春洪, 董璐, 陈林, 宋丽. 大豆VAS1基因家族的鉴定及参与侧根发育的研究[J]. 生物技术通报, 2023, 39(3): 133-142. |
| [12] | 王琪, 胡哲, 富薇, 李光哲, 郝林. 伯克霍尔德氏菌GD17对黄瓜幼苗耐干旱的调节[J]. 生物技术通报, 2023, 39(3): 163-175. |
| [13] | 陈奕博, 杨万明, 岳爱琴, 王利祥, 杜维俊, 王敏. 基于SLAF标记的大豆遗传图谱构建及苗期耐盐性QTL定位[J]. 生物技术通报, 2023, 39(2): 70-79. |
| [14] | 苗淑楠, 高宇, 李昕儒, 蔡桂萍, 张飞, 薛金爱, 季春丽, 李润植. 大豆GmPDAT1参与油脂合成和非生物胁迫应答的功能分析[J]. 生物技术通报, 2023, 39(2): 96-106. |
| [15] | 于波, 秦晓惠, 赵杨. 植物感应干旱信号的机制[J]. 生物技术通报, 2023, 39(11): 6-17. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||