








生物技术通报 ›› 2024, Vol. 40 ›› Issue (10): 139-148.doi: 10.13560/j.cnki.biotech.bull.1985.2024-0484
耿若涵1,2,3,4( ), 王炳贺1,2,3,4, 徐昌文1,2,3,4, 钱虹萍1,2,3,4, 林金星1,2,3,4, 崔亚宁1,2,3,4(
), 王炳贺1,2,3,4, 徐昌文1,2,3,4, 钱虹萍1,2,3,4, 林金星1,2,3,4, 崔亚宁1,2,3,4( )
)
收稿日期:2024-05-24
出版日期:2024-10-26
发布日期:2024-11-20
通讯作者:
崔亚宁,女,副教授,研究方向:植物分子细胞生物学;E-mail: cuiyaning@bjfu.edu.cn作者简介:耿若涵,女,研究方向:植物分子细胞生物学;E-mail: GengRH123@bjfu.edu.cn
基金资助:
GENG Ruo-han1,2,3,4( ), WANG Bing-he1,2,3,4, XU Chang-wen1,2,3,4, QIAN Hong-ping1,2,3,4, LIN Jin-xing1,2,3,4, CUI Ya-ning1,2,3,4(
), WANG Bing-he1,2,3,4, XU Chang-wen1,2,3,4, QIAN Hong-ping1,2,3,4, LIN Jin-xing1,2,3,4, CUI Ya-ning1,2,3,4( )
)
Received:2024-05-24
Published:2024-10-26
Online:2024-11-20
摘要:
蛋白质翻译后修饰是蛋白质合成后经历的一系列化学修饰过程,这些修饰能够显著影响蛋白质的结构、定位、稳定性和功能。在细胞生物学中,蛋白质翻译后修饰在几乎所有的细胞信号通路和调控网络中扮演着至关重要的角色,尤其是在囊泡转运这一细胞内物质运输的关键过程中,蛋白质翻译后修饰对于蛋白质的运输调控和信号传导具有决定性的影响。囊泡转运是指细胞内物质通过囊泡从一处运输到另一处的过程,这一过程涉及多个步骤,包括囊泡的形成、运输、融合等。在这个过程中,蛋白质的正确修饰和定位对于囊泡的形成和运输方向至关重要。本文首先介绍了几种重要的蛋白翻译后修饰的作用及分类,随后,系统地总结了蛋白质翻译后修饰在囊泡转运中的研究进展,为揭示细胞内囊泡转运的分子机制、深入研究膜蛋白生物学功能提供重要参考。未来,应进一步探索蛋白质翻译后修饰在植物囊泡转运中的具体机制,以及这些机制如何影响植物的生长发育和环境适应性。这一研究将为植物遗传改良和抗逆境育种提供新的策略和方法,有助于提高作物的产量和品质,增强植物对环境变化的适应能力。总之,通过深入研究这一领域,不仅有助于我们揭示植物细胞中特定蛋白质修饰的基本原理,还可能为改良作物性状和提高植物抗逆境能力提供新的策略。
耿若涵, 王炳贺, 徐昌文, 钱虹萍, 林金星, 崔亚宁. 蛋白的翻译后修饰调控植物囊泡转运的研究进展[J]. 生物技术通报, 2024, 40(10): 139-148.
GENG Ruo-han, WANG Bing-he, XU Chang-wen, QIAN Hong-ping, LIN Jin-xing, CUI Ya-ning. Research Progress in the Regulation of Protein Post-translational Modification in Plant Vesicle Transport[J]. Biotechnology Bulletin, 2024, 40(10): 139-148.

图1 磷酸化修饰对EXO70C2胞吞作用的影响 磷酸化修饰能够诱导EXO70C2在细胞膜与细胞内特定区域之间进行迁移,并触发其与ROH1的相互作用。该相互作用对ROH1的活性产生显著影响,进而促进转运囊泡的生成及物质运输
Fig. 1 Impact of phosphorylation modification on EXO70C2 endocytic function Phosphorylation modification induces the migration of EXO70C2 between the cell membrane and specific intracellular regions and triggers its interaction with ROH1. This interaction significantly impacts the activity of ROH1, subsequently promoting the formation of transport vesicles and material transport
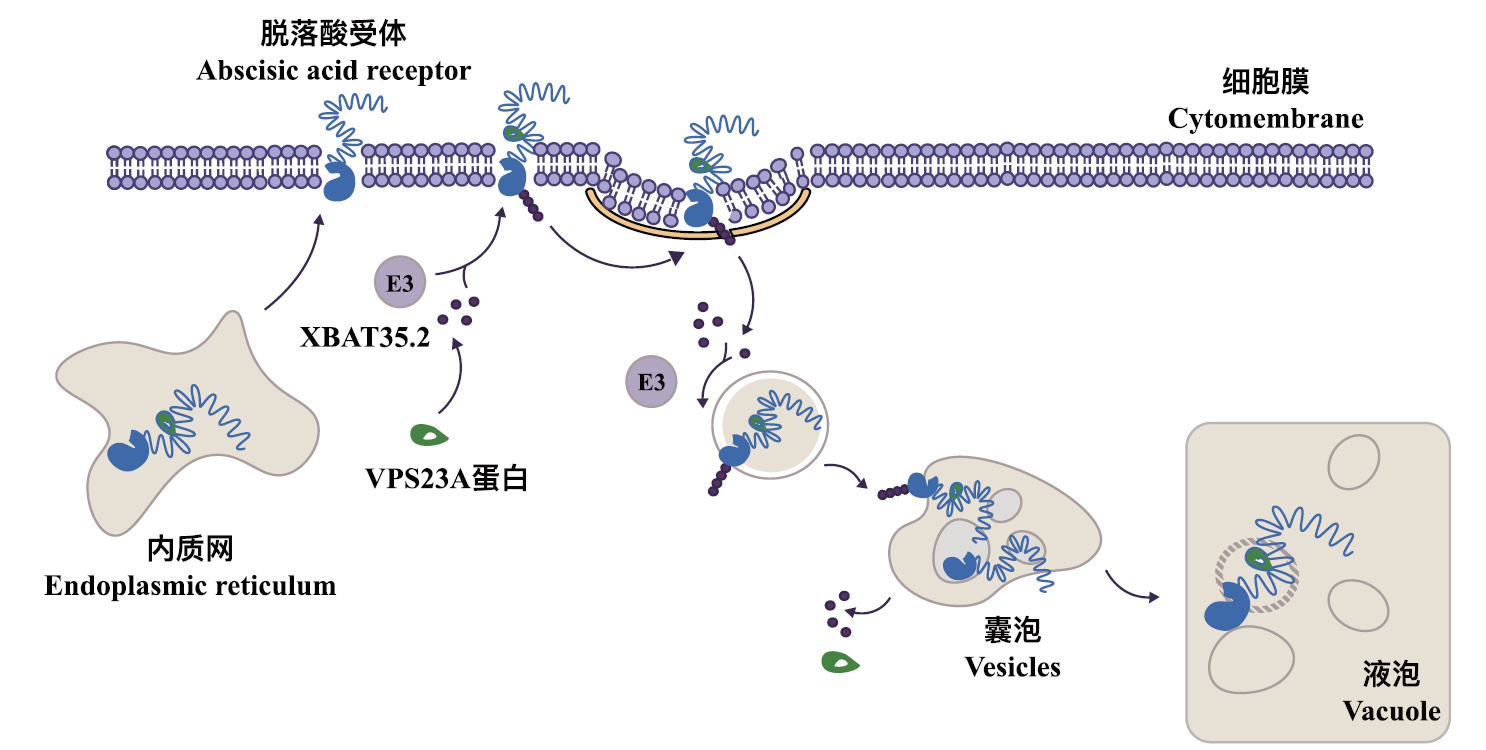
图2 VPS23A介导的泛素化修饰促进ABA受体的液泡降解机制 ESCRT复合体中的关键蛋白VPS23A,通过特异性识别非泛素化的ABA受体及其K63位连接的泛素链,促进ABA受体进入膜运输途径。这一泛素化修饰过程将ABA受体引导至细胞内,形成囊泡,随后被转运至内质网,最终在液泡中实现降解
Fig. 2 Ubiquitination modification mediated by VPS23A promotes the vacuolar degradation mechanism of ABA receptors The key protein VPS23A in the ESCRT complex specifically recognizes non-ubiquitinated ABA receptors and their K63-linked ubiquitin chains, facilitating the entry of ABA receptors into the membrane trafficking pathway. This ubiquitination process guides ABA receptors into the cell, forming vesicles that are subsequently transported to the endoplasmic reticulum and ultimately degraded in the vacuole
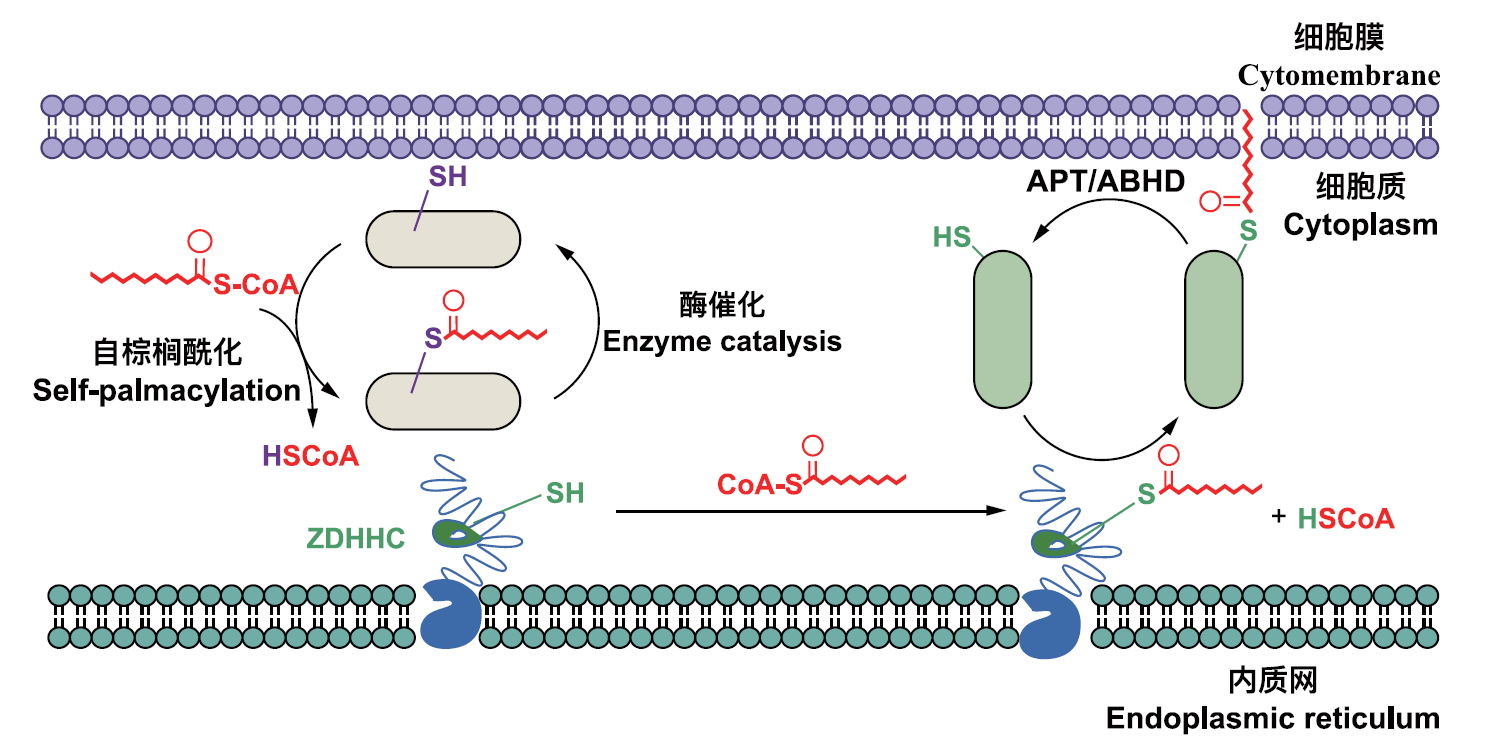
图3 膜蛋白棕榈酰化修饰及去棕榈酰化修饰的机制 细胞内质网膜蛋白的棕榈酰化修饰,是由ZDHHC酶催化,通过棕榈酰基辅酶A(S-CoA)中的棕榈酰基与蛋白质分子上的巯基(SH)发生反应而实现。去棕榈酰化过程则由ABHDS酶介导,通过解离蛋白质上的棕榈酸链,使其恢复至初始状态
Fig. 3 Mechanism of membrane protein palmitoylation and depalmitoylation The palmitoylation modification of intracellular endoplasmic reticulum membrane proteins is catalyzed by ZDHHC enzymes, achieved through the reaction of the palmitoyl group in palmitoyl-coenzyme A(S-CoA)with the sulfhydryl(SH)group on the protein molecule. The depalmitoylation process, on the other hand, is mediated by ABHDS enzymes, which dissociate the palmitate chain from the protein, restoring it to its initial state
| [1] | 谭锬, 柯柏怡, 梁前进. 蛋白质磷酸化修饰及其在细胞周期调控中的作用研究进展[J]. 北京师范大学学报:自然科学版, 2024, 60(1): 38-45. |
| Tan Y, Ke BY, Liang QJ. Research progress on protein phosphorylation modification and its role in cell cycle regulation[J]. Journal of Beijing Normal University Natural Science Edition, 2024, 60(1): 38-45. | |
| [2] | 张莹华. ITSN1蛋白磷酸化的研究进展[J]. 天津医科大学学报, 2019, 25(6): 666-669. |
| Zhang YH. Research progress on phosphorylation of ITSN1 protein[J]. J Tianjin Med Univ, 2019, 25(6): 666-669. | |
| [3] | 李构思, 张雅玲, 马坤, 等. 蛋白修饰调控植物育性与生殖发育的研究进展[J]. 华南农业大学学报, 2022, 43(6): 36-47. |
| Li GS, Zhang YL, Ma K, et al. Advances in protein modifications for fertility regulation and reproductive development in plants[J]. J South China Agric Univ, 2022, 43(6): 36-47. | |
| [4] | Lin CH, Shen YR, Wang HY, et al. Regulation of septin phosphorylation: SEPT12 phosphorylation in sperm septin assembly[J]. Cytoskeleton, 2019, 76(1): 137-142. |
| [5] | Longoni FP, Goldschmidt-Clermont M. Thylakoid protein phosphorylation in chloroplasts[J]. Plant Cell Physiol, 2021, 62(7): 1094-1107. |
| [6] | Saccomanno A, Potocký M, Pejchar P, et al. Regulation of exocyst function in pollen tube growth by phosphorylation of exocyst subunit EXO70C2[J]. Front Plant Sci, 2021, 11: 609600. |
| [7] |
Schink KO, Tan KW, Stenmark H. Phosphoinositides in control of membrane dynamics[J]. Annu Rev Cell Dev Biol, 2016, 32: 143-171.
pmid: 27576122 |
| [8] |
Xing JJ, Zhang L, Duan ZK, et al. Coordination of phospholipid-based signaling and membrane trafficking in plant immunity[J]. Trends Plant Sci, 2021, 26(4): 407-420.
doi: 10.1016/j.tplants.2020.11.010 pmid: 33309101 |
| [9] | Kamal MM, Ishikawa S, Takahashi F, et al. Large-scale phosphoproteomic study of Arabidopsis membrane proteins reveals early signaling events in response to cold[J]. Int J Mol Sci, 2020, 21(22): 8631. |
| [10] | Delom F, Fessart D. Role of phosphorylation in the control of clathrin-mediated internalization of GPCR[J]. Int J Cell Biol, 2011, 2011: 246954. |
| [11] |
Kalachova T, Škrabálková E, Pateyron S, et al. DIACYLGLYCEROL KINASE 5 participates in flagellin-induced signaling in Arabidopsis[J]. Plant Physiol, 2022, 190(3): 1978-1996.
doi: 10.1093/plphys/kiac354 pmid: 35900211 |
| [12] | Posor Y, Jang W, Haucke V. Phosphoinositides as membrane organizers[J]. Nat Rev Mol Cell Biol, 2022, 23(12): 797-816. |
| [13] |
Giannini JL, Gildensoph LH, Reynolds-Niesman I, et al. Calcium transport in sealed vesicles from red beet(Beta vulgaris L.) storage tissue: I. characterization of a Ca-pumping ATPase associated with the endoplasmic reticulum[J]. Plant Physiol, 1987, 85(4): 1129-1136.
doi: 10.1104/pp.85.4.1129 pmid: 16665816 |
| [14] | Ueda H, Yokota E, Kuwata K, et al. Phosphorylation of the C terminus of RHD3 has a critical role in homotypic ER membrane fusion in Arabidopsis[J]. Plant Physiol, 2016, 170(2): 867-880. |
| [15] |
Malmersjö S, Di Palma S, Diao JJ, et al. Phosphorylation of residues inside the SNARE complex suppresses secretory vesicle fusion[J]. EMBO J, 2016, 35(16): 1810-1821.
doi: 10.15252/embj.201694071 pmid: 27402227 |
| [16] |
Isono E, Kalinowska K. ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants[J]. Curr Opin Plant Biol, 2017, 40: 49-55.
doi: S1369-5266(17)30087-0 pmid: 28753460 |
| [17] | Coego A, Julian J, Lozano-Juste J, et al. Ubiquitylation of ABA receptors and protein phosphatase 2C coreceptors to modulate ABA signaling and stress response[J]. Int J Mol Sci, 2021, 22(13): 7103. |
| [18] |
Feng J, Shen WH. Dynamic regulation and function of histone monoubiquitination in plants[J]. Front Plant Sci, 2014, 5: 83.
doi: 10.3389/fpls.2014.00083 pmid: 24659991 |
| [19] | Ling JJ, Li J, Zhu DM, et al. Noncanonical role of Arabidopsis COP1/SPA complex in repressing BIN2-mediated PIF3 phosphorylation and degradation in darkness[J]. Proc Natl Acad Sci U S A, 2017, 114(13): 3539-3544. |
| [20] |
Hua ZH. Deciphering the protein ubiquitylation system in plants[J]. J Exp Bot, 2023, 74(21): 6487-6504.
doi: 10.1093/jxb/erad354 pmid: 37688404 |
| [21] | Liu GC, Liang JX, Lou LJ, et al. The deubiquitinases UBP12 and UBP13 integrate with the E3 ubiquitin ligase XBAT35.2 to modulate VPS23A stability in ABA signaling[J]. Sci Adv, 2022, 8(14): eabl5765. |
| [22] |
Chen Q, Yu FF, Xie Q. Insights into endoplasmic reticulum-associated degradation in plants[J]. New Phytol, 2020, 226(2): 345-350.
doi: 10.1111/nph.16369 pmid: 31838748 |
| [23] |
Liu BY, Zhang M, Chu HL, et al. The ubiquitin E3 ligase TRIM31 promotes aggregation and activation of the signaling adaptor MAVS through Lys63-linked polyubiquitination[J]. Nat Immunol, 2017, 18(2): 214-224.
doi: 10.1038/ni.3641 pmid: 27992402 |
| [24] | Wang X, Zhang XY, Song CP, et al. PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis[J]. Plant Cell, 2023, 35(9): 3585-3603. |
| [25] |
Foot N, Henshall T, Kumar S. Ubiquitination and the regulation of membrane proteins[J]. Physiol Rev, 2017, 97(1): 253-281.
pmid: 27932395 |
| [26] | Migliano SM, Teis D. ESCRT and membrane protein ubiquitination[M]// Endocytosis and Signaling. Cham: Springer, 2018: 107-135. |
| [27] |
Morvan J, Froissard M, Haguenauer-Tsapis R, et al. The ubiquitin ligase Rsp5p is required for modification and sorting of membrane proteins into multivesicular bodies[J]. Traffic, 2004, 5(5): 383-392.
pmid: 15086787 |
| [28] |
Amodio G, Margarucci L, Moltedo O, et al. Identification of cysteine ubiquitylation sites on the Sec23A protein of the COPII complex required for vesicle formation from the ER[J]. Open Biochem J, 2017, 11: 36-46.
doi: 10.2174/1874091X01711010036 pmid: 28553408 |
| [29] | Zhao R, Cao YY, Ge YR, et al. Single-molecule and vesicle trafficking analysis of ubiquitination involved in the activity of ammonium transporter AMT1;3 in Arbidopsis under high ammonium stress[J]. Cells, 2022, 11(22): 3651. |
| [30] | Gu XY, Brennan A, Wei WB, et al. Vesicle transport in plants: a revised phylogeny of SNARE proteins[J]. Evol Bioinform Online, 2020, 16: 1176934320956575. |
| [31] |
Yu FF, Lou LJ, Tian MM, et al. ESCRT-I component VPS23A affects ABA signaling by recognizing ABA receptors for endosomal degradation[J]. Mol Plant, 2016, 9(12): 1570-1582.
doi: S1674-2052(16)30271-4 pmid: 27856401 |
| [32] |
Lunghi G, Fazzari M, Ciampa MG, et al. Regulation of signal transduction by gangliosides in lipid rafts: focus on GM3-IR and GM1-TrkA interactions[J]. FEBS Lett, 2022, 596(24): 3124-3132.
doi: 10.1002/1873-3468.14532 pmid: 36331354 |
| [33] | Sun TJ, Zhang YL. MAP kinase cascades in plant development and immune signaling[J]. EMBO Rep, 2022, 23(2): e53817. |
| [34] |
Zhang L, Luo P, Bai J, et al. Function of histone H2B monoubiquitination in transcriptional regulation of auxin biosynthesis in Arabidopsis[J]. Commun Biol, 2021, 4(1): 206.
doi: 10.1038/s42003-021-01733-x pmid: 33589721 |
| [35] | 于菲菲, 谢旗. 泛素化修饰调控脱落酸介导的信号途径[J]. 遗传, 2017, 39(8): 692-706. |
| Yu FF, Xie Q. Ubiquitination modification precisely modulates the ABA signaling pathway in plants[J]. Hereditas(Beijing), 2017, 39(8): 692-706. | |
| [36] | Liu C, Yu HS, Li LG. SUMO modification of LBD30 by SIZ1 regulates secondary cell wall formation in Arabidopsis thaliana[J]. PLoS Genet, 2019, 15(1): e1007928. |
| [37] | 许凤浩. 蛋白质的脂化修饰[J]. 生物化学与生物物理进展, 1989, 16(2): 82-86. |
| Xu FH. Lipidation modification of proteins[J]. Progress in Biochemistry and Biophysics, 1989, 16(2): 82-86. | |
| [38] | Lai LY, Ruan JT, Xiao CW, et al. The putative myristoylome of Physcomitrium patens reveals conserved features of myristoylation in basal land plants[J]. Plant Cell Rep, 2023, 42(6): 1107-1124. |
| [39] | Utsumi T, Ohta H, Kayano Y, et al. The N-terminus of B96Bom, a Bombyx mori G-protein-coupled receptor, is N-myristoylated and translocated across the membrane[J]. FEBS J, 2005, 272(2): 472-481. |
| [40] | Carluccio AV, Prigigallo MI, Rosas-Diaz T, et al. S-acylation mediates Mungbean yellow mosaic virus AC4 localization to the plasma membrane and in turns gene silencing suppression[J]. PLoS Pathog, 2018, 14(8): e1007207. |
| [41] |
Running MP. The role of lipid post-translational modification in plant developmental processes[J]. Front Plant Sci, 2014, 5: 50.
doi: 10.3389/fpls.2014.00050 pmid: 24600462 |
| [42] | Zaballa ME, van der Goot FG. The molecular era of protein S-acylation: spotlight on structure, mechanisms, and dynamics[J]. Crit Rev Biochem Mol Biol, 2018, 53(4): 420-451. |
| [43] |
Fu S, Xu Y, Li CY, et al. Rice stripe virus interferes with S-acylation of remorin and induces its autophagic degradation to facilitate virus infection[J]. Mol Plant, 2018, 11(2): 269-287.
doi: S1674-2052(17)30366-0 pmid: 29229567 |
| [44] |
Xie X, Li XM, Qin FF, et al. Genetically encoded photoaffinity histone marks[J]. J Am Chem Soc, 2017, 139(19): 6522-6525.
doi: 10.1021/jacs.7b01431 pmid: 28459554 |
| [45] |
Su BD, Wang AQ, Shan XY. The role of N-myristoylation in homeostasis of brassinosteroid signaling kinase 1[J]. Planta, 2022, 255(4): 73.
doi: 10.1007/s00425-022-03861-y pmid: 35220507 |
| [46] | Zhang HY, Bussmann J, Huhnke FH, et al. Together is better: mRNA co-encapsulation in lipoplexes is required to obtain ratiometric co-delivery and protein expression on the single cell level[J]. Adv Sci, 2022, 9(4): e2102072. |
| [47] |
Su BD, Zhang X, Li L, et al. Dynamic spatial reorganization of BSK1 complexes in the plasma membrane underpins signal-specific activation for growth and immunity[J]. Mol Plant, 2021, 14(4): 588-603.
doi: 10.1016/j.molp.2021.01.019 pmid: 33524551 |
| [48] |
Chen BE, Sun Y, Niu JX, et al. Protein lipidation in cell signaling and diseases: function, regulation, and therapeutic opportunities[J]. Cell Chem Biol, 2018, 25(7): 817-831.
doi: S2451-9456(18)30150-8 pmid: 29861273 |
| [49] | Murphy J, Kolandaivelu S. Palmitoylation of progressive rod-cone degeneration(PRCD)regulates protein stability and localization[J]. J Biol Chem, 2016, 291(44): 23036-23046. |
| [50] |
Hemsley PA, Grierson CS. Multiple roles for protein palmitoylation in plants[J]. Trends Plant Sci, 2008, 13(6): 295-302.
doi: 10.1016/j.tplants.2008.04.006 pmid: 18501662 |
| [51] |
Qu MY, Liu XY, Wang XT, et al. Palmitoylation of vacuole membrane protein 1 promotes small extracellular vesicle secretion via interaction with ALIX and influences intercellular communication[J]. Cell Commun Signal, 2024, 22(1): 150.
doi: 10.1186/s12964-024-01529-6 pmid: 38403678 |
| [52] |
Bachmann SJ, Kotar J, Parolini L, et al. Melting transition in lipid vesicles functionalised by mobile DNA linkers[J]. Soft Matter, 2016, 12(37): 7804-7817.
pmid: 27722701 |
| [53] | Whitley JA, Kim S, Lou L, et al. Encapsulating Cas9 into extracellular vesicles by protein myristoylation[J]. J Extracell Vesicles, 2022, 11(4): e12196. |
| [54] |
Turnbull D, Hemsley PA. Fats and function: protein lipid modifications in plant cell signalling[J]. Curr Opin Plant Biol, 2017, 40: 63-70.
doi: S1369-5266(17)30050-X pmid: 28772175 |
| [55] | Kwiatkowska K, Matveichuk OV, Fronk J, et al. Flotillins: At the intersection of protein S- palmitoylation and lipid-mediated signaling[J]. Int J Mol Sci, 2020, 21(7): 2283. |
| [1] | 周恒, 谢彦杰. 植物氧化胁迫信号应答的研究进展[J]. 生物技术通报, 2023, 39(11): 36-43. |
| [2] | 贾海红, 李冰清. 超氧化物歧化酶翻译后修饰的研究进展[J]. 生物技术通报, 2022, 38(2): 237-244. |
| [3] | 郭会灿;. 蛋白质翻译后修饰研究进展[J]. , 2011, 0(07): 18-21. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||