








生物技术通报 ›› 2021, Vol. 37 ›› Issue (6): 286-294.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1587
潘志文( ), 陈伟庭, 高洁儿, 周峰, 姚涓, 王声斌, 姜大刚(
), 陈伟庭, 高洁儿, 周峰, 姚涓, 王声斌, 姜大刚( )
)
收稿日期:2020-12-30
出版日期:2021-06-26
发布日期:2021-07-08
作者简介:潘志文,男,硕士,研究方向:转基因生物安全检测技术;E-mail: 基金资助:
PAN Zhi-wen( ), CHEN Wei-ting, GAO Jie-er, ZHOU Feng, YAO Juan, WANG Sheng-bin, JIANG Da-gang(
), CHEN Wei-ting, GAO Jie-er, ZHOU Feng, YAO Juan, WANG Sheng-bin, JIANG Da-gang( )
)
Received:2020-12-30
Published:2021-06-26
Online:2021-07-08
摘要:
转基因番木瓜检测中,模板DNA的制备是关键一步。为了提高检测效率,建立快速制备番木瓜样品基因组DNA和PCR检测方法十分重要。建立了番木瓜基因组DNA的快速制备方法,包括样品准备、制备缓冲液匀浆和稀释上清。在完成不同番木瓜样品的基因组DNA快速制备后,对获得的DNA进行PCR检测验证。普通PCR验证结果显示,本方法制备的叶片和果肉基因组DNA溶液稀释5倍时,PCR扩增条带清晰,更高稀释倍数时,叶片基因组DNA扩增条带强于果肉基因组DNA;实时荧光PCR验证结果显示,叶片基因组DNA的PCR扩增效率位于合理区间。灵敏度测试结果表明,含量低至0.1%的叶片和果肉样品,内标准基因和外源基因特异性片段普通PCR和实时荧光PCR均能得到预期扩增,且重复性较好。本研究建立的番木瓜基因组DNA快速制备与PCR方法效果良好,体系稳定。
潘志文, 陈伟庭, 高洁儿, 周峰, 姚涓, 王声斌, 姜大刚. 转基因番木瓜基因组DNA的快速制备和PCR检测方法[J]. 生物技术通报, 2021, 37(6): 286-294.
PAN Zhi-wen, CHEN Wei-ting, GAO Jie-er, ZHOU Feng, YAO Juan, WANG Sheng-bin, JIANG Da-gang. Genomic DNA Rapid Preparation and PCR Detection Methods for Genetically Modified Papaya[J]. Biotechnology Bulletin, 2021, 37(6): 286-294.
| 检测对象 Detection targets | 引物/探针 Primers/Probes | 引物/探针序列 Primers/probes sequence(5'-3') | 引物出处 Reference |
|---|---|---|---|
| Papain | Papain-QF | TGCTTGACTGCGACAGAC | [12] |
| Papain-QR | CTTTCTCCCTTGAGCGAC | ||
| Papain-QP | FAM-AGCTACGGGTGCAATGGAGGTTACC-TAMRA | ||
| NPT Ⅱ | NPT-QF | AGGATCTCGTCGTGACCCAT | [13] |
| NPT-QR | GCACGAGGAAGCGGTCA | ||
| NPT-QP | FAM-CACCCAGCCGGCCACAGTCGAT-TAMRA | ||
| CaMV35S | P35S-F | GCTCCTACAAATGCCATCA | [14] |
| P35S-R | GATAGTGGGATTGTGCGTCA | ||
| P35S-QF | CGACAGTGGTCCCAAAGA | ||
| P35S-QR | AAGACGTGGTTGGAACGTCTTC | ||
| P35S-QP | FAM-TGGACCCCCACCCACGAGGAGCATC-TAMRA | ||
| NOS | TNOS-F | GAATCCTGTTGCCGGTCTTG | [14] |
| TNOS-R | TTATCCTAGTTTGCGCGCTA | ||
| TNOS-QF | ATCGTTCAAACATTTGGCA | ||
| TNOS-QR | ATTGCGGGACTCTAATCATA | ||
| TNOS-QP | FAM-CATCGCAAGACCGGCAACAGG-TAMRA |
表1 转基因木瓜筛选检测实时荧光PCR所用引物及探针序列信息
Table 1 Primers and probes sequence information used in transgenic papaya real-time PCR detection
| 检测对象 Detection targets | 引物/探针 Primers/Probes | 引物/探针序列 Primers/probes sequence(5'-3') | 引物出处 Reference |
|---|---|---|---|
| Papain | Papain-QF | TGCTTGACTGCGACAGAC | [12] |
| Papain-QR | CTTTCTCCCTTGAGCGAC | ||
| Papain-QP | FAM-AGCTACGGGTGCAATGGAGGTTACC-TAMRA | ||
| NPT Ⅱ | NPT-QF | AGGATCTCGTCGTGACCCAT | [13] |
| NPT-QR | GCACGAGGAAGCGGTCA | ||
| NPT-QP | FAM-CACCCAGCCGGCCACAGTCGAT-TAMRA | ||
| CaMV35S | P35S-F | GCTCCTACAAATGCCATCA | [14] |
| P35S-R | GATAGTGGGATTGTGCGTCA | ||
| P35S-QF | CGACAGTGGTCCCAAAGA | ||
| P35S-QR | AAGACGTGGTTGGAACGTCTTC | ||
| P35S-QP | FAM-TGGACCCCCACCCACGAGGAGCATC-TAMRA | ||
| NOS | TNOS-F | GAATCCTGTTGCCGGTCTTG | [14] |
| TNOS-R | TTATCCTAGTTTGCGCGCTA | ||
| TNOS-QF | ATCGTTCAAACATTTGGCA | ||
| TNOS-QR | ATTGCGGGACTCTAATCATA | ||
| TNOS-QP | FAM-CATCGCAAGACCGGCAACAGG-TAMRA |
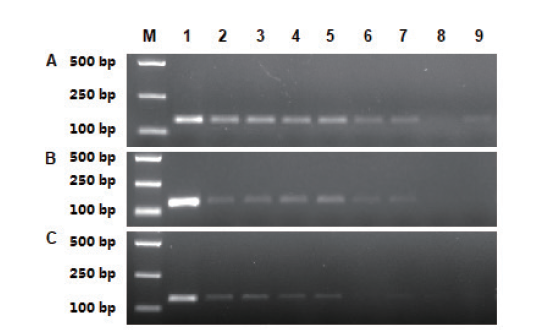
图2 内标准基因Papain普通PCR扩增 A:鲜叶片样品,B:干叶片样品;C:果肉样品. M:分子量标记DL2000,1:阳性对照,2、3:原液,4、5:5倍稀释,6、7:25倍稀释,8、9:125倍稀释
Fig. 2 PCR amplification of endogenous gene Papain A: Fresh leaf. B: Wilted leaf. C: Fruit. M: DL2000 marker. 1: Positive control. 2 and 3: Undiluted solution. 4 and 5: 5 times dilution. 6 and 7: 25 times dilution. 8 and 9: 125 times dilution
| 样品名称 Sample name | DNA浓度/稀释倍数 DNA concentration/ Dilution time | 平行1 Repeat 1 | 平行2 Repeat 2 | 平行3 Repeat 3 | Mean Ct | SD | RSD/% | 扩增效率 Amplification efficiency/% |
|---|---|---|---|---|---|---|---|---|
| DNA-Std1 | 25 ng/μL | 21.41 | 21.35 | 21.34 | 21.37 | 0.038 | 0.177 | 90.73 |
| DNA-Std2 | 5 ng/μL | 24.47 | 24.48 | 24.38 | 24.44 | 0.055 | 0.225 | |
| DNA-Std3 | 1 ng/μL | 26.72 | 26.70 | 26.72 | 26.71 | 0.012 | 0.043 | |
| DNA-Std4 | 0.2 ng/μL | 29.29 | 29.34 | 29.29 | 29.31 | 0.029 | 0.099 | |
| DNA-Std5 | 0.04 ng/μL | 31.70 | 31.63 | 31.57 | 31.63 | 0.065 | 0.206 | |
| DNA-Std6 | 0.008 ng/μL | 34.04 | 34.18 | 34.38 | 34.20 | 0.171 | 0.500 | |
| DNA-Std7 | 0.0016 ng/μL | 36.68 | 36.14 | 36.64 | 36.49 | 0.301 | 0.825 | |
| 鲜叶片样品 Fresh leaf sample | 原液 | 29.42 | 29.26 | 29.41 | 29.36 | 0.090 | 0.305 | 95.83 |
| 5倍稀释 | 31.98 | 31.75 | 31.72 | 31.82 | 0.142 | 0.447 | ||
| 25倍稀释 | 33.77 | 33.59 | 33.66 | 33.67 | 0.091 | 0.270 | ||
| 125倍稀释 | 36.63 | 37.35 | 36.20 | 36.73 | 0.581 | 1.582 | ||
| 干叶片样品 Wilted leaf sample | 原液 | 27.43 | 27.39 | 27.48 | 27.43 | 0.045 | 0.164 | 102.09 |
| 5倍稀释 | 29.49 | 29.75 | 29.62 | 29.62 | 0.130 | 0.439 | ||
| 25倍稀释 | 31.67 | 31.97 | 31.99 | 31.88 | 0.179 | 0.562 | ||
| 125倍稀释 | 34.21 | 34.60 | 34.11 | 34.31 | 0.259 | 0.755 | ||
| 果肉样品 Fruit sample | 原液 | 33.01 | 32.86 | 33.00 | 32.96 | 0.084 | 0.255 | 145.32 |
| 5倍稀释 | 34.15 | 34.24 | 34.03 | 34.14 | 0.105 | 0.309 | ||
| 25倍稀释 | 35.99 | 36.68 | 36.23 | 36.30 | 0.350 | 0.965 | ||
| 125倍稀释 | 37.22 | 39.21 | N/A | 38.22 | 1.407 | 3.682 |
表2 番木瓜Papain基因实时荧光定量PCR
Table 2 Real-time fluorescence PCR of papaya endogenous Papain gene
| 样品名称 Sample name | DNA浓度/稀释倍数 DNA concentration/ Dilution time | 平行1 Repeat 1 | 平行2 Repeat 2 | 平行3 Repeat 3 | Mean Ct | SD | RSD/% | 扩增效率 Amplification efficiency/% |
|---|---|---|---|---|---|---|---|---|
| DNA-Std1 | 25 ng/μL | 21.41 | 21.35 | 21.34 | 21.37 | 0.038 | 0.177 | 90.73 |
| DNA-Std2 | 5 ng/μL | 24.47 | 24.48 | 24.38 | 24.44 | 0.055 | 0.225 | |
| DNA-Std3 | 1 ng/μL | 26.72 | 26.70 | 26.72 | 26.71 | 0.012 | 0.043 | |
| DNA-Std4 | 0.2 ng/μL | 29.29 | 29.34 | 29.29 | 29.31 | 0.029 | 0.099 | |
| DNA-Std5 | 0.04 ng/μL | 31.70 | 31.63 | 31.57 | 31.63 | 0.065 | 0.206 | |
| DNA-Std6 | 0.008 ng/μL | 34.04 | 34.18 | 34.38 | 34.20 | 0.171 | 0.500 | |
| DNA-Std7 | 0.0016 ng/μL | 36.68 | 36.14 | 36.64 | 36.49 | 0.301 | 0.825 | |
| 鲜叶片样品 Fresh leaf sample | 原液 | 29.42 | 29.26 | 29.41 | 29.36 | 0.090 | 0.305 | 95.83 |
| 5倍稀释 | 31.98 | 31.75 | 31.72 | 31.82 | 0.142 | 0.447 | ||
| 25倍稀释 | 33.77 | 33.59 | 33.66 | 33.67 | 0.091 | 0.270 | ||
| 125倍稀释 | 36.63 | 37.35 | 36.20 | 36.73 | 0.581 | 1.582 | ||
| 干叶片样品 Wilted leaf sample | 原液 | 27.43 | 27.39 | 27.48 | 27.43 | 0.045 | 0.164 | 102.09 |
| 5倍稀释 | 29.49 | 29.75 | 29.62 | 29.62 | 0.130 | 0.439 | ||
| 25倍稀释 | 31.67 | 31.97 | 31.99 | 31.88 | 0.179 | 0.562 | ||
| 125倍稀释 | 34.21 | 34.60 | 34.11 | 34.31 | 0.259 | 0.755 | ||
| 果肉样品 Fruit sample | 原液 | 33.01 | 32.86 | 33.00 | 32.96 | 0.084 | 0.255 | 145.32 |
| 5倍稀释 | 34.15 | 34.24 | 34.03 | 34.14 | 0.105 | 0.309 | ||
| 25倍稀释 | 35.99 | 36.68 | 36.23 | 36.30 | 0.350 | 0.965 | ||
| 125倍稀释 | 37.22 | 39.21 | N/A | 38.22 | 1.407 | 3.682 |
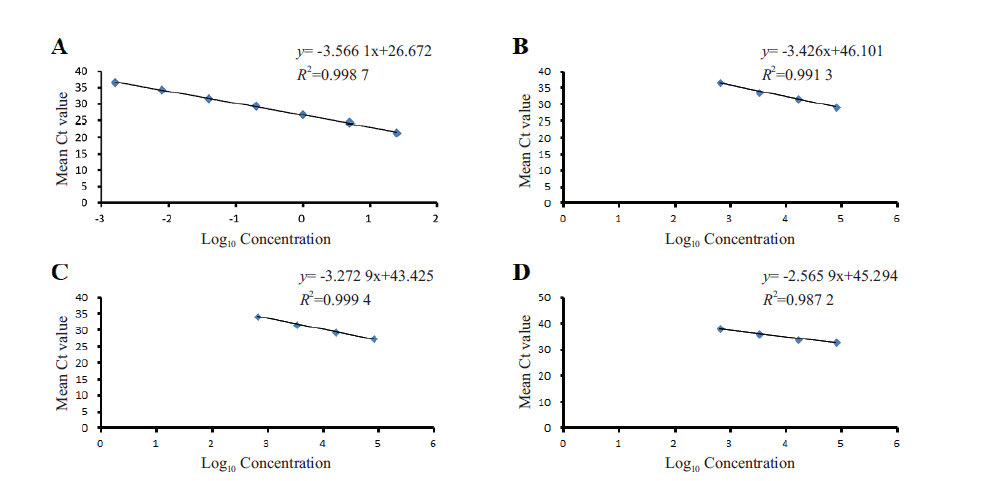
图3 番木瓜Papain基因实时荧光PCR标准曲线 A:纯化的番木瓜基因组DNA;B:鲜叶片样品;C:干叶片样品;D:果肉样品
Fig. 3 Real-time fluorescence PCR standard curve of Papain gene A: Purified papaya genomic DNA. B: Fresh leaf sample. C: Wilted leaf sample. D: Fruit sample
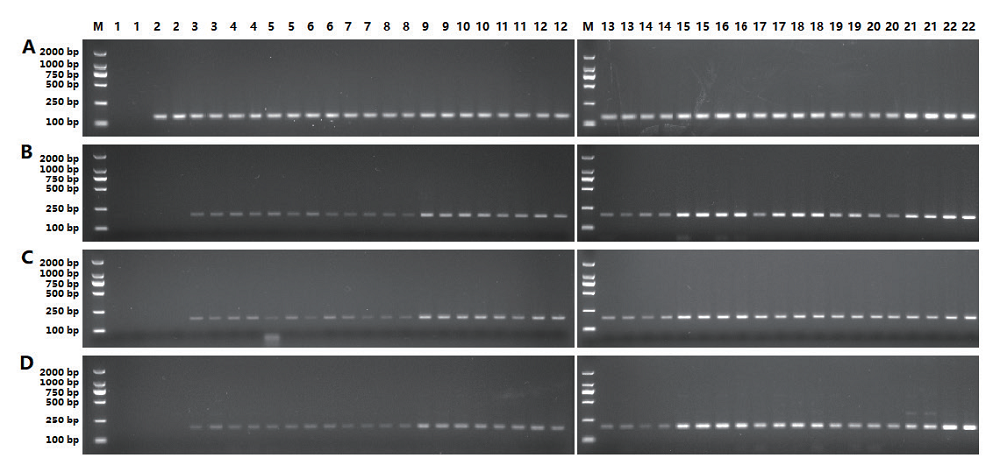
图4 转基因番木瓜普通PCR电泳结果 A:Papain;B:CaMV 35S启动子;C:NOS终止子;D为NPT Ⅱ基因;M:分子量标记marker DL2000;1:空白对照;2:阴性对照;3,4:0.1%鲜叶片样品;5,6:0.1%干叶片样品;7,8:0.1%果肉样品;9,10:1%鲜叶片样品;11,12:1%干叶片样品;13,14:1%果肉样品;15,16:5%鲜叶片样品;17,18:5%干叶片样品;19,20:5%果肉样品;21:0.1%阳性对照;22:1%阳性对照
Fig. 4 PCR electrophoresis results of transgenic papaya A: Papain gene. B: CaMV35S promoter. C: NOS terminator. D: NPT II gene. M: DL2000 marker, 1: blank, 2: negative control, 3 and 4: 0.1% fresh leaf, 5 and 6: 0.1% wilted leaf, 7 and 8: 0.1% fruit, 9 and 10: 1% fresh leaf, 11 and 12: 1% wilted leaf, 13 and 14: 1% fruit, 15 and 16: 5% fresh leaf, 17 and 18: 5% wilted leaf, 19 and 20: 5% fruit, 21: 0.1% positive control, 22: 1% positive control
| 样品名称 Sample name | 检测参数 Detection item | 平行1 Repeat 1 | 平行2 Repeat 2 | 平行3 Repeat 3 | Mean Ct | SD | RSD |
|---|---|---|---|---|---|---|---|
| 阴性对照 Negative control | Papain | 22.94 | 22.75 | 22.80 | 22.83 | 0.099 | 0.431% |
| CaMV35S启动子 | N/A | N/A | N/A | N/A | N/A | N/A | |
| NOS终止子 | N/A | N/A | N/A | N/A | N/A | N/A | |
| NPT Ⅱ | N/A | N/A | N/A | N/A | N/A | N/A | |
| 1%阳性对照 1% positive control | Papain | 23.26 | 23.15 | 23.20 | 23.20 | 0.055 | 0.237% |
| CaMV35S启动子 | 26.43 | 26.49 | 26.55 | 26.49 | 0.060 | 0.227% | |
| NOS终止子 | 27.22 | 27.17 | 27.18 | 27.19 | 0.027 | 0.097% | |
| NPT Ⅱ | 26.25 | 26.39 | 26.28 | 26.31 | 0.074 | 0.280% | |
| 5%鲜叶片样品 5% fresh leaf sample | Papain | 27.40 | 27.47 | 27.46 | 27.44 | 0.038 | 0.138% |
| CaMV35S启动子 | 29.05 | 29.07 | 28.80 | 28.97 | 0.150 | 0.519% | |
| NOS终止子 | 29.85 | 29.82 | 29.99 | 29.89 | 0.091 | 0.304% | |
| NPT Ⅱ | 29.17 | 29.30 | 29.33 | 29.27 | 0.085 | 0.291% | |
| 5%干叶片样品 5% wilted leaf sample | Papain | 27.96 | 28.07 | 28.02 | 28.02 | 0.055 | 0.197% |
| CaMV35S启动子 | 30.33 | 30.14 | 30.22 | 30.23 | 0.095 | 0.316% | |
| NOS终止子 | 30.55 | 30.57 | 30.31 | 30.48 | 0.145 | 0.475% | |
| NPT Ⅱ | 29.91 | 29.90 | 29.85 | 29.89 | 0.032 | 0.108% | |
| 5%果肉样品 5% fruit sample | Papain | 29.27 | 29.29 | 29.28 | 29.28 | 0.010 | 0.034% |
| CaMV35S启动子 | 30.45 | 30.49 | 30.59 | 30.51 | 0.072 | 0.236% | |
| NOS终止子 | 31.35 | 31.45 | 31.53 | 31.44 | 0.090 | 0.287% | |
| NPT Ⅱ | 30.62 | 30.45 | 30.47 | 30.51 | 0.093 | 0.305% | |
| 1%鲜叶片样品 1% fresh leaf sample | Papain | 27.09 | 27.12 | 27.16 | 27.12 | 0.035 | 0.130% |
| CaMV35S启动子 | 30.68 | 30.86 | 30.57 | 30.70 | 0.146 | 0.477% | |
| NOS终止子 | 31.96 | 32.04 | 32.01 | 32.00 | 0.040 | 0.126% | |
| NPT Ⅱ | 31.23 | 31.15 | 31.11 | 31.16 | 0.061 | 0.196% | |
| 1%干叶片样品 1% wilted leaf sample | Papain | 28.28 | 28.27 | 28.26 | 28.27 | 0.010 | 0.035% |
| CaMV35S启动子 | 31.54 | 31.73 | 32.31 | 31.86 | 0.401 | 1.259% | |
| NOS终止子 | 32.49 | 32.52 | 32.10 | 32.37 | 0.234 | 0.724% | |
| NPT Ⅱ | 31.36 | 32.12 | 31.75 | 31.74 | 0.380 | 1.197% | |
| 1%果肉样品 1% fruit sample | Papain | 29.30 | 29.27 | 29.33 | 29.30 | 0.030 | 0.102% |
| CaMV35S启动子 | 33.72 | 33.39 | 32.73 | 33.28 | 0.504 | 1.515% | |
| NOS终止子 | 33.55 | 33.73 | 33.30 | 33.53 | 0.216 | 0.644% | |
| NPT Ⅱ | 32.71 | 32.52 | 32.46 | 32.56 | 0.131 | 0.401% | |
| 0.1%鲜叶片样品 0.1% fresh leaf sample | Papain | 27.38 | 27.48 | 27.29 | 27.38 | 0.095 | 0.347% |
| CaMV35S启动子 | 33.76 | 33.91 | 33.90 | 33.86 | 0.084 | 0.248% | |
| NOS终止子 | 34.82 | 33.88 | 34.59 | 34.43 | 0.490 | 1.423% | |
| NPT Ⅱ | 33.79 | 33.61 | 34.04 | 33.81 | 0.216 | 0.639% | |
| 0.1%干叶片样品 0.1% wilted leaf sample | Papain | 27.74 | 27.72 | 27.67 | 27.71 | 0.036 | 0.130% |
| CaMV35S启动子 | 34.05 | 33.97 | 33.51 | 33.84 | 0.291 | 0.861% | |
| NOS终止子 | 34.83 | 35.79 | 34.84 | 35.15 | 0.551 | 1.569% | |
| NPT Ⅱ | 34.39 | 34.27 | 34.56 | 34.41 | 0.146 | 0.424% | |
| 0.1%果肉样品 0.1% fruit sample | Papain | 29.38 | 29.22 | 29.06 | 29.22 | 0.160 | 0.548% |
| CaMV35S启动子 | 36.35 | 36.36 | 39.23 | 37.31 | 1.660 | 4.449% | |
| NOS终止子 | 36.50 | 36.11 | 37.35 | 36.65 | 0.634 | 1.730% | |
| NPT Ⅱ | 35.46 | 34.58 | 37.35 | 35.80 | 1.415 | 3.954% |
表3 转基因番木瓜实时荧光定量PCR数据与分析
Table 3 Data and analysis of real-time fluorescence PCR of transgenic papaya
| 样品名称 Sample name | 检测参数 Detection item | 平行1 Repeat 1 | 平行2 Repeat 2 | 平行3 Repeat 3 | Mean Ct | SD | RSD |
|---|---|---|---|---|---|---|---|
| 阴性对照 Negative control | Papain | 22.94 | 22.75 | 22.80 | 22.83 | 0.099 | 0.431% |
| CaMV35S启动子 | N/A | N/A | N/A | N/A | N/A | N/A | |
| NOS终止子 | N/A | N/A | N/A | N/A | N/A | N/A | |
| NPT Ⅱ | N/A | N/A | N/A | N/A | N/A | N/A | |
| 1%阳性对照 1% positive control | Papain | 23.26 | 23.15 | 23.20 | 23.20 | 0.055 | 0.237% |
| CaMV35S启动子 | 26.43 | 26.49 | 26.55 | 26.49 | 0.060 | 0.227% | |
| NOS终止子 | 27.22 | 27.17 | 27.18 | 27.19 | 0.027 | 0.097% | |
| NPT Ⅱ | 26.25 | 26.39 | 26.28 | 26.31 | 0.074 | 0.280% | |
| 5%鲜叶片样品 5% fresh leaf sample | Papain | 27.40 | 27.47 | 27.46 | 27.44 | 0.038 | 0.138% |
| CaMV35S启动子 | 29.05 | 29.07 | 28.80 | 28.97 | 0.150 | 0.519% | |
| NOS终止子 | 29.85 | 29.82 | 29.99 | 29.89 | 0.091 | 0.304% | |
| NPT Ⅱ | 29.17 | 29.30 | 29.33 | 29.27 | 0.085 | 0.291% | |
| 5%干叶片样品 5% wilted leaf sample | Papain | 27.96 | 28.07 | 28.02 | 28.02 | 0.055 | 0.197% |
| CaMV35S启动子 | 30.33 | 30.14 | 30.22 | 30.23 | 0.095 | 0.316% | |
| NOS终止子 | 30.55 | 30.57 | 30.31 | 30.48 | 0.145 | 0.475% | |
| NPT Ⅱ | 29.91 | 29.90 | 29.85 | 29.89 | 0.032 | 0.108% | |
| 5%果肉样品 5% fruit sample | Papain | 29.27 | 29.29 | 29.28 | 29.28 | 0.010 | 0.034% |
| CaMV35S启动子 | 30.45 | 30.49 | 30.59 | 30.51 | 0.072 | 0.236% | |
| NOS终止子 | 31.35 | 31.45 | 31.53 | 31.44 | 0.090 | 0.287% | |
| NPT Ⅱ | 30.62 | 30.45 | 30.47 | 30.51 | 0.093 | 0.305% | |
| 1%鲜叶片样品 1% fresh leaf sample | Papain | 27.09 | 27.12 | 27.16 | 27.12 | 0.035 | 0.130% |
| CaMV35S启动子 | 30.68 | 30.86 | 30.57 | 30.70 | 0.146 | 0.477% | |
| NOS终止子 | 31.96 | 32.04 | 32.01 | 32.00 | 0.040 | 0.126% | |
| NPT Ⅱ | 31.23 | 31.15 | 31.11 | 31.16 | 0.061 | 0.196% | |
| 1%干叶片样品 1% wilted leaf sample | Papain | 28.28 | 28.27 | 28.26 | 28.27 | 0.010 | 0.035% |
| CaMV35S启动子 | 31.54 | 31.73 | 32.31 | 31.86 | 0.401 | 1.259% | |
| NOS终止子 | 32.49 | 32.52 | 32.10 | 32.37 | 0.234 | 0.724% | |
| NPT Ⅱ | 31.36 | 32.12 | 31.75 | 31.74 | 0.380 | 1.197% | |
| 1%果肉样品 1% fruit sample | Papain | 29.30 | 29.27 | 29.33 | 29.30 | 0.030 | 0.102% |
| CaMV35S启动子 | 33.72 | 33.39 | 32.73 | 33.28 | 0.504 | 1.515% | |
| NOS终止子 | 33.55 | 33.73 | 33.30 | 33.53 | 0.216 | 0.644% | |
| NPT Ⅱ | 32.71 | 32.52 | 32.46 | 32.56 | 0.131 | 0.401% | |
| 0.1%鲜叶片样品 0.1% fresh leaf sample | Papain | 27.38 | 27.48 | 27.29 | 27.38 | 0.095 | 0.347% |
| CaMV35S启动子 | 33.76 | 33.91 | 33.90 | 33.86 | 0.084 | 0.248% | |
| NOS终止子 | 34.82 | 33.88 | 34.59 | 34.43 | 0.490 | 1.423% | |
| NPT Ⅱ | 33.79 | 33.61 | 34.04 | 33.81 | 0.216 | 0.639% | |
| 0.1%干叶片样品 0.1% wilted leaf sample | Papain | 27.74 | 27.72 | 27.67 | 27.71 | 0.036 | 0.130% |
| CaMV35S启动子 | 34.05 | 33.97 | 33.51 | 33.84 | 0.291 | 0.861% | |
| NOS终止子 | 34.83 | 35.79 | 34.84 | 35.15 | 0.551 | 1.569% | |
| NPT Ⅱ | 34.39 | 34.27 | 34.56 | 34.41 | 0.146 | 0.424% | |
| 0.1%果肉样品 0.1% fruit sample | Papain | 29.38 | 29.22 | 29.06 | 29.22 | 0.160 | 0.548% |
| CaMV35S启动子 | 36.35 | 36.36 | 39.23 | 37.31 | 1.660 | 4.449% | |
| NOS终止子 | 36.50 | 36.11 | 37.35 | 36.65 | 0.634 | 1.730% | |
| NPT Ⅱ | 35.46 | 34.58 | 37.35 | 35.80 | 1.415 | 3.954% |
| [1] | 国际农业生物技术应用服务组织. 2018年全球生物技术/转基因作物商业化发展态势[J]. 中国生物工程杂志, 2019, 39(8):1-6. |
| International service for the acquisition of agri-biotech applications. The global status of commercialized biotech/GM crops in 2018[J]. China Biotechnology, 2019, 39(8):1-6. | |
| [2] |
Fitch MMM, Manshardt RM, Gonsalves D, et al. Virus resistant papaya plants derived from tissues bombarded with the coat protein gene of papaya ringspot virus[J]. Nature Biotechnology, 1992, 10(11):1466-1472.
doi: 10.1038/nbt1192-1466 URL |
| [3] | 阮小蕾, 李华平, 周国辉. 转PRSV复制酶基因T2代番木瓜植株的抗病性测定[J]. 华南农业大学学报, 2004, 25(4):12-15. |
| Ruan XL, Li HP, Zhou GH. Evaluation of PRSV resistance of T2 transgenic papaya with replicase gene [J]. Journal of South China Agricultural University, 2004, 25(4):12-15. | |
| [4] |
Cheng YH, Yang JS, Yeh SD. Efficient transformation of papaya by coat protein gene of papaya ringspot virus mediated by Agrobacterium following liquid-phase wounding of embryogenic tissues with caborundum[J]. Plant Cell Reports, 1996, 16:127-132.
doi: 10.1007/BF01890852 URL |
| [5] |
Thion L, Vossen C, Couderc B, et al. Detection of genetically modified organisms in food by DNA extraction and PCR amplification[J]. Biochemistry and Molecular Biology Education, 2002, 30(1):51-55.
doi: 10.1002/(ISSN)1539-3429 URL |
| [6] | 农业部科技发展中心. 转基因植物检测[M]. 北京: 中国农业出版社, 2009. |
| Science and Technology Development Center of ministry of Agriculture. Detection of genetically modified plants[M]. Beijing: China Agriculture Press, 2009. | |
| [7] | 汪秀峰, 杨剑波, 向太和, 等. 一种叶片直接用作PCR扩增的新方法及其应用[J]. 中国水稻科学, 2002, 16(1):67-70. |
| Wang XF, Yang JB, Xiang TH, et al. A new method for PCR reaction from alkali-treated rice leaf tissues[J]. Chinese Journal of Rice Science, 2006, 16(1):67-70. | |
| [8] | 赵红霞, 谢攀, 黄志坚, 等. 一种改良的水稻总DNA提取方法[J]. 湖北大学学报:自然科学版, 2006, 28(4):389-392. |
| Zhao HX, Xie P, Huang ZJ, et al. An improved method of extracting rice genomic DNA[J]. Journal of Hubei University:Natural Science, 2006, 28(4):389-392. | |
| [9] | 王兰, 龙云铭, 刘耀光. 一种用于PCR的植物基因组DNA快速制备方法[J]. 分子植物育种, 2009, 7(2):425-428. |
| Wang L, Long YM, Liu YG. A method for rapid preparation of plant genomic DNA for PCR analysis[J]. Molecular Plant Breeding, 2009, 7(2):425-428. | |
| [10] | 孙川, 陈刚, 饶玉春, 等. 水稻基因组DNA简易制备方法[J]. 中国水稻科学, 2010, 24(6):677-680. |
| Sun C, Chen G, Rao YC, et al. A simple method for rapid preparation of rice genomic DNA[J]. Chinese Journal of Rice Science, 2010, 24(6):677-680. | |
| [11] |
Zou YP, Michael GM, Wang YL, et al. Nucleic acid purification from plants, animals and microbes in under 30 seconds[J]. PLoS Biology, 2017, 15(11):e2003916.
doi: 10.1371/journal.pbio.2003916 URL |
| [12] | 姜大刚, 李夏莹, 潘志文, 等. 转基因植物及其产品成分检测 番木瓜内标准基因定性PCR方法[S]. 农业农村部公告第323号-1-2020. 北京: 中国农业出版社, 2020. |
| Jiang DG, Li XY, Pan ZW, et al. Detection of genetically modified plants and derived products- target-taxon-specific qualitative PCR method for Papaya[S]. Announcement of the Ministry of Agriculture and Rural Affairs No. 323-1-2020. Beijing: China Agriculture Press, 2020. | |
| [13] | 曹际娟, 徐君怡, 赵昕, 等. 转基因成分检测 玉米检测方法[S]. SN/T 1196-2012. 北京: 中国标准出版社, 2013. |
| Cao JJ, Xu JY, Zhao X, et al. Detection of genetically modified components-Maize test methods[S]. SN/T 1196-2012. Beijing: Standards Press of China, 2013. | |
| [14] | 谢家建, 沈平, 彭于发, 等. 转基因植物及其产品成分检测 调控元件CaMV 35S启动子、FMV 35S启动子、NOS启动子、NOS终止子和CaMV 35S终止子定性PCR方法[S]. 农业部1782号公告-3-2012. 北京: 中国农业出版社, 2012. |
| Xie JJ, Shen P, Peng YF, et al. Qualitative PCR method for the regulatory elements CaMV 35S promoter, FMV 35S promoter, NOS promoter, NOS terminator and CaMV 35S terminator[S]. Announcement of the Ministry of Agriculture No. 1782-3-2012. Beijing: China Agriculture Press, 2012. |
| [1] | 余慧, 王静, 梁昕昕, 辛亚平, 周军, 赵会君. 宁夏枸杞铁镉响应基因的筛选及其功能验证[J]. 生物技术通报, 2023, 39(7): 195-205. |
| [2] | 姚姿婷, 曹雪颖, 肖雪, 李瑞芳, 韦小妹, 邹承武, 朱桂宁. 火龙果溃疡病菌实时荧光定量PCR内参基因的筛选[J]. 生物技术通报, 2023, 39(5): 92-102. |
| [3] | 郭三保, 宋美玲, 李灵心, 尧子钊, 桂明明, 黄胜和. 斑地锦查尔酮合酶基因及启动子的克隆与分析[J]. 生物技术通报, 2023, 39(4): 148-156. |
| [4] | 宋海娜, 吴心桐, 杨鲁豫, 耿喜宁, 张华敏, 宋小龙. 葱鳞葡萄孢菌诱导下韭菜RT-qPCR内参基因的筛选和验证[J]. 生物技术通报, 2023, 39(3): 101-115. |
| [5] | 李天顺, 李宸葳, 王佳, 朱龙佼, 许文涛. 功能核酸筛选过程中次级文库的有效制备[J]. 生物技术通报, 2023, 39(3): 116-122. |
| [6] | 余世洲, 曹领改, 王世泽, 刘勇, 边文杰, 任学良. 烟草种质基因分型核心SNP标记的开发[J]. 生物技术通报, 2023, 39(3): 89-100. |
| [7] | 穆德添, 万凌云, 章瑶, 韦树根, 陆英, 付金娥, 田艺, 潘丽梅, 唐其. 钩藤管家基因筛选及生物碱合成相关基因的表达分析[J]. 生物技术通报, 2023, 39(2): 126-138. |
| [8] | 李会杰, 董莲华, 陈桂芳, 刘思渊, 杨佳怡, 杨靖亚. 食品中椰毒假单胞菌微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2023, 39(1): 127-136. |
| [9] | 胡雪莹, 张越, 郭雅杰, 仇天雷, 高敏, 孙兴滨, 王旭明. 不同施肥处理农田土壤中噬菌体与细菌携带抗生素抗性基因的比较[J]. 生物技术通报, 2022, 38(9): 116-126. |
| [10] | 程深伟, 张克强, 梁军锋, 刘福元, 郜兴亮, 杜连柱. 畜禽养殖粪污中典型致病菌的三重微滴式数字PCR定量检测方法的建立[J]. 生物技术通报, 2022, 38(9): 271-280. |
| [11] | 刘娜, 焦京琳, 饶正华. 短链脂肪酸在动物样本中的检测方法研究进展[J]. 生物技术通报, 2022, 38(8): 84-91. |
| [12] | 曹英芳, 赵新, 刘双, 李瑞环, 刘娜, 徐石勇, 高芳瑞, 马卉, 兰青阔, 檀建新, 王永. 抗除草剂大豆GE-J12实时荧光定量PCR检测方法的建立[J]. 生物技术通报, 2022, 38(7): 146-152. |
| [13] | 申恒, 刘思慧, 李跃, 李敬涛, 梁文星. 一种用于PCR的番茄DNA快速粗提方法[J]. 生物技术通报, 2022, 38(6): 74-80. |
| [14] | 易芳, 来鹏程, 郑希鳌, 胡帅, 高燕丽. Kod DNA聚合酶的制备及纯化研究[J]. 生物技术通报, 2022, 38(5): 183-190. |
| [15] | 孙宝箴, 全龙萍, 康慧, 姚玉新, 沈甜, 陈卫平, 杜远鹏, 高振. 基于跨反向剪接位点引物特异性检测circRNA的PCR方法[J]. 生物技术通报, 2022, 38(5): 279-285. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||