








生物技术通报 ›› 2021, Vol. 37 ›› Issue (6): 295-304.doi: 10.13560/j.cnki.biotech.bull.1985.2020-1502
收稿日期:2020-12-11
出版日期:2021-06-26
发布日期:2021-07-08
作者简介:章英才,男,硕士,教授,研究方向:药用植物结构和有效成分关系;E-mail: 基金资助:
ZHANG Ying-cai( ), HUANG Yue, HAI Yuan, ZHANG Yuan, HU Ya-jie, ZHAO Meng-yi
), HUANG Yue, HAI Yuan, ZHANG Yuan, HU Ya-jie, ZHAO Meng-yi
Received:2020-12-11
Published:2021-06-26
Online:2021-07-08
摘要:
本研究以不同发育时期的“灵武长枣”果实为实验材料,采用石蜡切片技术明确不同发育时期果实维管束结构特征和变化规律,在此基础上,通过枣果实维管组织中荧光示踪剂引入方法的应用,采用荧光染料活细胞示踪技术,探讨枣果实维管束韧皮部同化物卸载路径的研究方法。结果表明:(1)膨大前期果实的维管束较多,尤其是内果皮附近的维管束数量较多、分布集中,此时有单个维管束进行发育,也有两个维管束背靠背进行发育。快速膨大期果实中果皮内薄壁细胞发育迅速,由外向内体积逐渐增大,排列松散,有些甚至排列成网状结构,围成一个个较大的空腔,相同视野内维管束数量下降,维管束多为两两背靠背式、3个或4个聚在一起发育。着色期中果皮的细胞空腔进一步增大、数量增多,相比前两个时期相同视野内的维管束数量更少,由多个维管束形成了多分支结构。与着色期相比,完熟期果实薄壁细胞形成的空腔空间继续增大,维管束数量无明显变化,多维管束分支结构略有差异。(2)采用荧光染料活细胞示踪技术,在不同发育时期枣果实维管束韧皮部中引入了荧光示踪剂羧基荧光素酯CFDA、质膜通透剂洋地黄皂苷,在维管束木质部中引入了荧光示踪剂Texas-Red,通过激光共聚焦显微镜CLSM观察荧光特征,获得了枣果实中韧皮部同化物卸载路径的研究方法,为研究枣果实同化物积累和品质调控奠定了基础。
章英才, 黄月, 海源, 张媛, 扈亚杰, 赵梦怡. 枣果实维管组织中荧光示踪剂引入方法的应用[J]. 生物技术通报, 2021, 37(6): 295-304.
ZHANG Ying-cai, HUANG Yue, HAI Yuan, ZHANG Yuan, HU Ya-jie, ZHAO Meng-yi. Method Application of Leading Fluorescent Tracers into the Vascular Tissues of Ziziphus jujuba Mill cv. Lingwuchangzao Fruits[J]. Biotechnology Bulletin, 2021, 37(6): 295-304.

图1 CFDA的引入方法1图示 A:用一细针将棉线从上向下穿过短枝韧皮部;B:用细铁丝将Eppendorf管固定并保持竖直;C:将棉线缠绕于短枝四周进行固定,并用微量注射器向Eppendorf管中注入CFDA稀释液;D:Eppendorf管盖好并用锡箔纸包好
Fig.1 Photos showing the introduction method 1 of CFDA A:A cotton thread passes through the short branch phloem from up to down with a fine needle. B:Fix the Eppendorf tube with fine iron wire and keep it upright. C:Fix the cotton thread around the short branch and inject CFDA diluent into the Eppendorf tube with microinjector. D:The Eppendorf tube is closed with the lid and wrapped in silver paper

图2 CFDA的引入方法2图示 A:砂布磨擦后将棉花团缠绕在靠近果柄的短枝;B:用封口膜将棉花团部分密封后微量注射器注入CFDA溶液;C:用封口膜将棉花团完全密封,两端涂抹凡士林;D:用锡箔纸将密封的棉花团完全覆盖
Fig.2 Photos showing the introduction method 2 of CFDA A:Intertwine the cotton dough around short branch near fruit stalk after rubbing with sandcloth. B:Inject CFDA into the cotton dough with a microinjector after partly sealing up the cotton dough with sealing film. C:Completely seal up the cotton dough with sealing film and applying vaseline on both ends. D:Completely wrap the sealed cotton dough with silver paper

图3 CFDA和洋地黄皂苷同时引入的方法图示 A:棉线穿过短枝韧皮部,在针穿进和穿出的部位滴加适量EDTA;B:用细铁丝将Eppendorf管固定并保持竖直,将棉线缠绕于短枝四周进行固定,在Eppendorf管附近短枝砂布磨擦后用棉花团缠绕;C:将棉花团用封口膜部分密封后用微量注射器注入洋地黄皂苷,然后全部密封;D:在Eppendorf管中用微量注射器注入CFDA稀释液;E:Eppendorf管中注入CFDA稀释液后迅速盖好盖子;F:用锡箔纸将密封的棉花团和Eppendorf管完全包好
Fig.3 Photos showing the simultaneous introduction meth-ods of CFDA and digitonin A:A cotton thread is passed through the phloem of short branch and the appropriate amount of EDTA into and out of the needle is dripped. B:Fix the Eppendorf tube with a fine iron wire and keep it upright,fix the cotton thread around the short branch,intertwine the cotton dough around the short branch near the Eppendorf tube after rubbing with sandcloth. C:Inject digitonin into the cotton dough with a microinjector after partly sealing up the cotton dough with sealing film,then completely sealing up. D:Inject CFDA diluent into the Eppendorf tube with a microinjector. E:The Eppendorf tube is closed with a lid rapidly after injecting CFDA diluent. F:Seal the cotton dough and completely wrap the Eppendorf tube with silver paper
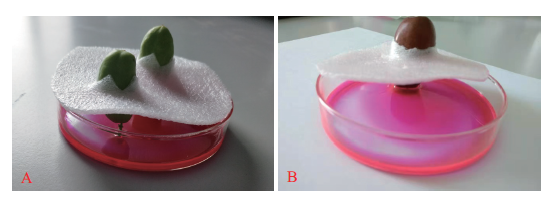
图4 Texas-Red标记的方法图示 A:快速膨大期果实果柄向下引入Texas-Red图示;B:完熟期果实果柄向下引入Texas-Red图示
Fig.4 Photos showing the introduction method of Texas-Red A:Photo showing the introduction of fruit stalk downward Texas-Red during the rapid enlargement period. B:Photo showing the introduction of fruit stalk downward Texas-Red during the maturation period
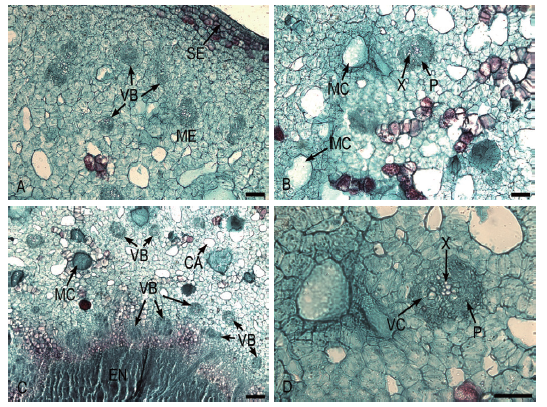
图5 膨大前期果实维管束特征 A:中果皮中的维管束;B:中果皮中的维管束及其他结构;C:内果皮及外部的维管束;D:果实中维管束的结构 X:木质部;P:韧皮部;VC:维管形成层;VB:维管束;MC:黏液腔;CA:空腔;ME:中果皮;EN:内果皮;SE:内表皮。Bar=50 μm。下同
Fig.5 Vascular bundles characteristic of fruit during the early bulking period A:Vascular bundles of mesocarp. B:Vascular bundles and other structures of mesocarp. C:Endocarp and external vascular bundles. D:Vascular bundle structures of fruit X:Xylem. P:Phloem. VC:Vascular bundle cambium. VB:Vascular bundle. MC:Mucous cavity. CA:Cavity. ME:Mesocarp. EN:Endocarp. SE:Inner epidermis. Bar=50 μm. The same below

图6 快速膨大期果实维管束特征 A:中果皮中的维管束及其他结构;B:果实中维管束的结构;C-D:果实中维管束的结构及其分支
Fig.6 Vascular bundles characteristic of fruit during the rapid enlargement period A:Vascular bundles and other structures of mesocarp. B:Vascular bundle structures of fruit. C-D:Vascular bundle structures and branches of fruit
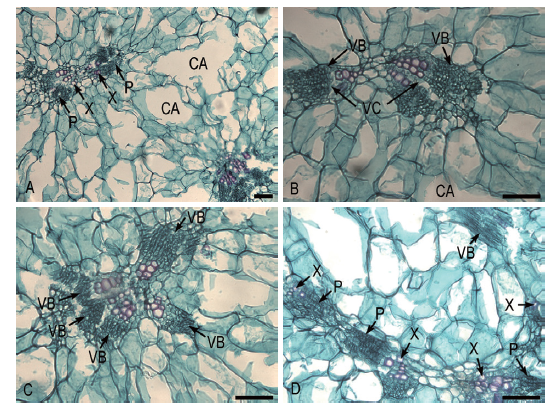
图7 着色期果实维管束特征 A:果实中果皮结构及其维管束的结构;B:中果皮2个维管束的分支结构;C:中果皮多维管束的分支结构;D:多个维管束彼此相连的结构
Fig.7 Vascular bundles characteristic of fruit during the coloring period A:Structures of mesocarp and vascular bundles in fruit. B:Branch structures of two vascular bundles of mesocarp. C:Branch structures of multiple vascular bundles of mesocarp. D:Structures connected by multiple vascular bundles

图8 完熟期果实维管束特征 A:果实中果皮结构及其维管束的结构;B:中果皮中维管束的结构;C:多维管束的分支结构;D:维管束的分支及同时出现的纵横切结构
Fig.8 Vascular bundles characteristic of fruit during the maturation period A:Structures of mesocarp and vascular bundles in fruit. B:Vascular bundles structures of mesocarp. C:Branch structures of multiple vascular bundles. D:Branches of vascular bundles and length cutting and cross section structures

图9 枣果实维管束荧光示踪图 A1、A2、A3分别为膨大前期果实引入CFDA 48 h后维管束横切荧光图、对应的透射图、对应的荧光图和明视野的叠加图;B1、B2分别为快速膨大期果实只引入CFDA、同时引入CFDA和洋地黄皂苷48 h后维管束纵切荧光图;C1、C2分别为着色期果实只引入CFDA、同时引入CFDA和洋地黄皂苷48 h后维管束纵切荧光图;D1、D2分别为完熟期果实只引入CFDA、同时引入CFDA和Texas-Red后荧光在维管束中的分布(韧皮部为CF标记的绿色荧光,木质部为Texas-Red标记的红色荧光)。X:木质部;P:韧皮部。A1-A3,Bar=100 μm;B1、B2、C1、C2、D1、D2,Bar=250 μm
Fig.9 Fluorescence tracing map of vascular bundles in jujube fruit A1,A2,and A3 shows vascular bundles fluorography,the corresponding bright field,the overlaid pictures from fluorescence and bright field,respectively after CFDA was introduced into the phloem of the early bulking period fruit for 48 h. B1 andB2 shows vascular bundles fluorography when only CFDA and CFDA and digitonin introduced simultaneously into the phloem of the rapid enlargement period fruit after treatment with 48 h.C1 and C2 shows vascular bundles fluorography when only CFDA and CFDA and digitonin introduced simultaneously into the phloem of the coloring period fruit after treatment with 48 h. D1 and D2 shows vascular bundles fluorography when only CFDA and CFDA and Texas-Red were introduced simultaneously into the maturation period fruit(CF green fluorescence shows the phloem and Texas-Red red fluorescence shows the xylem). X:Xylem. P:Phloem. A1-A3,Bar=100 μm. B1,B2,C1,C2,D1,D2,Bar=250 μm
| [1] | 李进伟, 丁绍东, 李苹苹, 等. 五种枣成分及功能研究[J]. 食品工业科技, 2009, 30(7):294-296. |
| Li JW, Ding SD, Li PP, et al. Study on composition and function of five cultivars of Chinese jujube[J]. Sci Technol Food Ind, 2009, 30(7):294-296. | |
| [2] | 刘鑫, 杨翠花, 雍鹏, 等. 枣成熟期果皮解剖结构特性研究[J]. 山西林业科技, 2013, 42(3):12-14. |
| Liu X, Yang CH, Yong P, et al. Study on pericarp anatomical structure of jujuba in mature stage[J]. Shanxi For Sci Technol, 2013, 42(3):12-14. | |
| [3] | 边媛, 狄龙, 李定红, 等. 枣果实维管束解剖结构研究[J]. 果树学报, 2015, 32(3):448-452, 523. |
| Bian Y, Di L, Li DH, et al. Vascular anatomy of Chinese jujube fruit[J]. J Fruit Sci, 2015, 32(3):448-452, 523. | |
| [4] | 刘世鹏, 刘申, 文欣. 枣果肉解剖结构及其裂果性研究[J]. 北方园艺, 2017(14):32-38. |
| Liu SP, Liu S, Wen X. Relations between anatomical structure and fruit cracking of jujube fruit[J]. North Hortic, 2017(14):32-38. | |
| [5] | 李彦玲, 杨爱珍, 孟泽, 等. 枣果皮组织结构与裂果关系研究[J]. 北京农学院学报, 2016, 31(2):34-41. |
| Li YL, Yang AZ, Meng Z, et al. Study on the relationship between jujube peel histological structure and fruit cracking[J]. J Beijing Univ Agric, 2016, 31(2):34-41. | |
| [6] | 丁改秀, 王保明, 王小原, 等. 壶瓶枣不同发育期果皮显微结构与裂果的关系[J]. 山西农业科学, 2014, 42(9):948-951. |
| Ding GX, Wang BM, Wang XY, et al. Study on the relationship between histological structure and fruit cracking during development in Huping jujube[J]. J Shanxi Agric Sci, 2014, 42(9):948-951. | |
| [7] | 黑淑梅, 冯晓东, 常海飞. 枣果实组织结构影响裂果发生的研究进展[J]. 山西农业科学, 2015, 43(7):916-918. |
| Hei SM, Feng XD, Chang HF. Research progress in jujube fruit cracking affected by anatomical structure of pericarp[J]. J Shanxi Agric Sci, 2015, 43(7):916-918. | |
| [8] | 寇晓虹, 王文生, 吴彩娥, 等. 鲜枣果实解剖结构与其耐藏性关系的研究[J]. 食品科技, 2001, 26(5):67-68. |
| Kou XH, Wang WS, Wu CE, et al. Study on the relationship between anatomical structure and storage life of fresh jujube[J]. Food Sci Technol, 2001, 26(5):67-68. | |
| [9] |
Braun DM, Wang L, Ruan YL. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security[J]. J Exp Bot, 2014, 65(7):1713-1735.
doi: 10.1093/jxb/ert416 URL |
| [10] |
Viola R, Roberts AG, Haupt S, et al. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading[J]. Plant Cell, 2001, 13(2):385-398.
pmid: 11226192 |
| [11] |
Oparka KJ, Duckett CM, Prior DAM, et al. Real-time imaging of phloem unloading in the root tip of Arabidopsis[J]. Plant J, 1994, 6(5):759-766.
doi: 10.1046/j.1365-313X.1994.6050759.x URL |
| [12] |
Liesche J, Martens HJ, Schulz A. Symplasmic transport and phloem loading in gymnosperm leaves[J]. Protoplasma, 2011, 248(1):181-190.
doi: 10.1007/s00709-010-0239-0 URL |
| [13] | Zanon L, Falchi R, Santi S, et al. Sucrose transport and phloem unloading in peach fruit:potential role of two transporters localized in different cell types[J]. Physiol Plant, 2015, 154(2):179-193. |
| [14] |
Nie PX, Wang XY, Hu LP, et al. The predominance of the apoplasmic phloem-unloading pathway is interrupted by a symplasmic pathway during Chinese jujube fruit development[J]. Plant Cell Physiol, 2010, 51(6):1007-1018.
doi: 10.1093/pcp/pcq054 URL |
| [15] | 侯思皓, 边媛, 牛辉陵, 等. 枣和酸枣果实韧皮部糖分卸载途径及其积累研究[J]. 果树学报, 2017, 34(12):1580-1589. |
| Hou SH, Bian Y, Niu HL, et al. Phloem unloading and sugar accumulation in jujube fruits[J]. J Fruit Sci, 2017, 34(12):1580-1589. | |
| [16] | 李春丽, 侯柄竹, 张晓燕, 等. 无花果果实韧皮部卸载路径由共质体向质外体途径转变[J]. 科学通报, 2016, 61(8):835-843. |
|
Li CL, Hou BZ, Zhang XY, et al. A shift of phloem unloading from symplasmic to apoplasmic pathway during fig fruit development[J]. Chin Sci Bull, 2016, 61(8):835-843.
doi: 10.1360/N972015-01056 URL |
|
| [17] | 张鹤华, 李艳芳, 聂佩显, 等. 蓝莓果实同化物韧皮部卸载路径与糖代谢酶活性[J]. 林业科学, 2017, 53(3):40-48. |
| Zhang HH, Li YF, Nie PX, et al. Phloem unloading pathway of photosynthates and sucrose-metabolizing enzymes activities in Vaccinium corymbosum fruit[J]. Sci Silvae Sin, 2017, 53(3):40-48. | |
| [18] | 章英才, 柴雅红, 曹金霞. 灵武长枣果实多糖中单糖组成分析[J]. 干旱地区农业研究, 2018, 36(2):144-152. |
| Zhang YC, Chai YH, Cao JX. Monosaccharide composition of polysaccharides in Ziziphus jujuba Mill cv. lingwuchangzao fruit[J]. Agric Res Arid Areas, 2018, 36(2):144-152. | |
| [19] |
Clearwater MJ, Luo Z, Ong SE, et al. Vascular functioning and the water balance of ripening kiwifruit(Actinidia chinensis)berries[J]. J Exp Bot, 2012, 63(5):1835-1847.
doi: 10.1093/jxb/err352 pmid: 22155631 |
| [20] | 景红霞, 章英才. 灵武长枣果实发育结构特征研究[J]. 广西植物, 2014, 34(4):565-569, 556. |
| Jing HX, Zhang YC. Structure characteristic of developmental fruit of Ziziphus jujuba cv. Lingwuchangzao[J]. Guihaia, 2014, 34(4):565-569, 556. | |
| [21] | 杨淑娟, 章英才, 郑国琦, 等. 灵武长枣正常果与裂果解剖结构的比较研究[J]. 北方园艺, 2010(22):15-18. |
| Yang SJ, Zhang YC, Zheng GQ, et al. Comparative study on dissected structures of normal fruits and cracking ones of Lingwu long-jujube[J]. North Hortic, 2010(22):15-18. | |
| [22] |
Chatelet DS, Rost TL, Matthews MA, et al. The peripheral xylem of grapevine(Vitis vinifera)berries. 2. Anatomy and development[J]. J Exp Bot, 2008, 59(8):1997-2007.
doi: 10.1093/jxb/ern061 URL pmid: 18440930 |
| [1] | 陈强, 邹明康, 宋家敏, 张冲, 吴隆坤. 甜瓜LBD基因家族的鉴定和果实发育进程中的表达分析[J]. 生物技术通报, 2023, 39(3): 176-183. |
| [2] | 孙燕, 王金刚, 臧丹丹, 赵恒田, 刘淑华. 不同发育时期蓝果忍冬果实的转录组分析[J]. 生物技术通报, 2022, 38(12): 204-213. |
| [3] | 王露露, 耿兴敏, 许世达. 乙烯受体在果实成熟及花衰老中的研究进展[J]. 生物技术通报, 2021, 37(3): 144-152. |
| [4] | 李玲, 杨丽霞, 郭梅. CNR转录因子在番茄果实成熟过程中的功能[J]. 生物技术通报, 2021, 37(2): 51-62. |
| [5] | 张圆圆, 邵冬南, 崔百明. 基于CRISPR/Cas9加工番茄α-Man突变体的构建[J]. 生物技术通报, 2019, 35(6): 9-16. |
| [6] | 赵阳阳, 郭雨潇, 张凌云. 文冠果果实转录组测序及分析[J]. 生物技术通报, 2019, 35(6): 24-31. |
| [7] | 莫显兰, 史列琴, 陆秋利, 王小敏, 任振新. Sl-miR482在番茄果实中的表达分析及STTM沉默载体的构建[J]. 生物技术通报, 2019, 35(12): 50-56. |
| [8] | 章英才, 苏伟东, 柴雅红. 宁夏中宁地区灵武长枣果实多糖的单糖成分GC-MS分析[J]. 生物技术通报, 2019, 35(11): 22-29. |
| [9] | 李和平, 姚运法, 练冬梅, 赖正锋, 洪建基. 黄秋葵果实转录组测序及分析[J]. 生物技术通报, 2018, 34(3): 121-127. |
| [10] | 胡晓, 侯旭, 袁雪, 管丹, 刘悦萍,. ARF和Aux/IAA调控果实发育成熟机制研究进展[J]. 生物技术通报, 2017, 33(12): 37-44. |
| [11] | 彭勇, 陈尚武, 马会勤. 黑果枸杞果实成熟发育过程表达谱差异分析[J]. 生物技术通报, 2016, 32(11): 144-151. |
| [12] | 陈宇杰,陈明,乌兰巴特尔,郝金凤,高峰,哈斯阿古拉. 甜瓜乙烯受体基因Cm-ETR1 cDNA的克隆及表达特性分析[J]. 生物技术通报, 2013, 0(7): 54-59. |
| [13] | 周阿涛;刘迪秋;葛锋;陈朝银;饶健;丁为群;. 果实表达PGIPs的基因克隆及功能研究进展[J]. , 2012, 0(01): 14-18. |
| [14] | 姜娜娜;赵传志;赵光敬;李长生;夏晗;王兴军;. 番茄果实特异性启动子的克隆与遗传转化研究[J]. , 2012, 0(01): 74-78. |
| [15] | 李玲;傅达奇;朱毅;田慧琴;罗云波;朱本忠;. 利用染色质免疫共沉淀技术确定转录因子RIN调控的靶基因[J]. , 2011, 0(12): 166-170. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||