








生物技术通报 ›› 2021, Vol. 37 ›› Issue (9): 77-85.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0717
• 青贮微生物专题(专题主编:杨富裕 教授) • 上一篇 下一篇
王琦( ), 武之绚, 陈钟玲, 吴白乙拉, 胡宗福(
), 武之绚, 陈钟玲, 吴白乙拉, 胡宗福( ), 牛化欣(
), 牛化欣( )
)
收稿日期:2021-06-07
出版日期:2021-09-26
发布日期:2021-10-25
作者简介:王琦,男,硕士研究生,研究方向:动物营养与饲料科学;E-mail: 基金资助:
WANG Qi( ), WU Zhi-xuan, CHEN Zhong-ling, WU Bai-yi-la, HU Zong-fu(
), WU Zhi-xuan, CHEN Zhong-ling, WU Bai-yi-la, HU Zong-fu( ), NIU Hua-xin(
), NIU Hua-xin( )
)
Received:2021-06-07
Published:2021-09-26
Online:2021-10-25
摘要:
采用16S rRNA高通量测序技术,检测青贮苜蓿有氧暴露细菌群落动态变化,旨在为青贮苜蓿有氧暴露细菌多样性变化及其接种乳酸菌防止有氧变质提供依据。将未接种(CON)和接种副干酪乳杆菌(LCP)的苜蓿青贮发酵56 d,分析有氧暴露后0 d、7 d、14 d青贮发酵品质和细菌多样性的动态变化。结果表明,有氧暴露使未接种青贮苜蓿pH上升,乳酸含量下降。相比CON,接种副干酪乳杆菌在有氧暴露期间pH升高幅度更小,乳酸含量更高(P<0.05)。CON主要有乳杆菌属、肠杆菌属和肠球菌属为主,其中肠杆菌属和肠球菌属丰度逐渐下降;LCP主要以乳杆菌属为主,其丰度逐渐下降。此外,有氧暴露14 d时,LCP降低了醋杆菌属的丰度。关联性分析发现,乳杆菌属与pH呈负相关(P<0.05),肠杆菌属、肠球菌属、魏氏菌属、Cedecea、Sporolactobacillus与pH呈正相关(P<0.05),醋杆菌属Acetobacter与乳酸和乙酸负相关(P< 0.05)。综上,本研究发现醋杆菌属在长期有氧暴露的青贮苜蓿中大量存在,降低了乳酸和乙酸的含量。接种副干酪乳杆菌可提高有氧暴露青贮苜蓿乳杆菌属的丰度,降低长期(14 d)有氧暴露醋杆菌属的丰度。因此青贮苜蓿接种副干酪乳杆菌可改善短期有氧暴露青贮品质,提高其有氧稳定性,减缓有氧变质。
王琦, 武之绚, 陈钟玲, 吴白乙拉, 胡宗福, 牛化欣. 副干酪乳杆菌对青贮苜蓿有氧暴露品质和细菌多样性的影响[J]. 生物技术通报, 2021, 37(9): 77-85.
WANG Qi, WU Zhi-xuan, CHEN Zhong-ling, WU Bai-yi-la, HU Zong-fu, NIU Hua-xin. Effects of Lactobacillus paracasei on the Quality and Bacterial Diversity of Silage Alfalfa After Aerobic Exposure[J]. Biotechnology Bulletin, 2021, 37(9): 77-85.
| 组别Group | 鲜样Fresh sample | PO0 | PO7 | PO14 | SEM | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | LCP | CON | LCP | CON | LCP | I | S | I×S | |||
| DM /% | 23.92 | 20.85 | 22.26 | 20.57 | 21.49 | 20.79 | 21.72 | 0.48 | ** | NS | NS |
| pH | 6.27 | 5.46 | 4.55 | 5.44 | 4.74 | 6.43 | 4.83 | 0.01 | ** | ** | ** |
| LAB/(log10 CFU/g FM) | 5.53 | 7.22 | 7.68 | 6.56 | 7.34 | 5.38 | 6.28 | 0.06 | ** | ** | ** |
| Yeasts/(log10 CFU/g FM) | 3.76 | 3.28 | 2.56 | 4.46 | 3.90 | 7.13 | 6.07 | 0.09 | ** | ** | ** |
| Molds/(log10 CFU/g FM) | 3.92 | 2.80 | <2.00 | 3.08 | 3.51 | 6.24 | 4.68 | 0.09 | — | — | — |
| LA/(g·kg-1 DM) | 31.67 | 48.61 | 11.56 | 26.85 | 4.73 | 15.49 | 1.04 | ** | ** | ** | |
| AA/(g·kg-1 DM) | 18.32 | 15.88 | 11.55 | 12.52 | 7.43 | 10.41 | 0.63 | ** | ** | ** | |
| PA/(g·kg-1 DM) | 3.75 | 2.64 | 1.56 | 1.24 | 0.82 | 0.68 | 0.28 | * | ** | NS | |
| BA/(g·kg-1 DM) | 1.43 | 0.45 | 2.01 | 1.48 | 3.21 | 2.61 | 0.04 | ** | ** | ** | |
表1 有氧暴露苜蓿青贮品质
Table 1 Fermentation quality of alfalfa silage during aerobic exposure from 0 to 14 d
| 组别Group | 鲜样Fresh sample | PO0 | PO7 | PO14 | SEM | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | LCP | CON | LCP | CON | LCP | I | S | I×S | |||
| DM /% | 23.92 | 20.85 | 22.26 | 20.57 | 21.49 | 20.79 | 21.72 | 0.48 | ** | NS | NS |
| pH | 6.27 | 5.46 | 4.55 | 5.44 | 4.74 | 6.43 | 4.83 | 0.01 | ** | ** | ** |
| LAB/(log10 CFU/g FM) | 5.53 | 7.22 | 7.68 | 6.56 | 7.34 | 5.38 | 6.28 | 0.06 | ** | ** | ** |
| Yeasts/(log10 CFU/g FM) | 3.76 | 3.28 | 2.56 | 4.46 | 3.90 | 7.13 | 6.07 | 0.09 | ** | ** | ** |
| Molds/(log10 CFU/g FM) | 3.92 | 2.80 | <2.00 | 3.08 | 3.51 | 6.24 | 4.68 | 0.09 | — | — | — |
| LA/(g·kg-1 DM) | 31.67 | 48.61 | 11.56 | 26.85 | 4.73 | 15.49 | 1.04 | ** | ** | ** | |
| AA/(g·kg-1 DM) | 18.32 | 15.88 | 11.55 | 12.52 | 7.43 | 10.41 | 0.63 | ** | ** | ** | |
| PA/(g·kg-1 DM) | 3.75 | 2.64 | 1.56 | 1.24 | 0.82 | 0.68 | 0.28 | * | ** | NS | |
| BA/(g·kg-1 DM) | 1.43 | 0.45 | 2.01 | 1.48 | 3.21 | 2.61 | 0.04 | ** | ** | ** | |
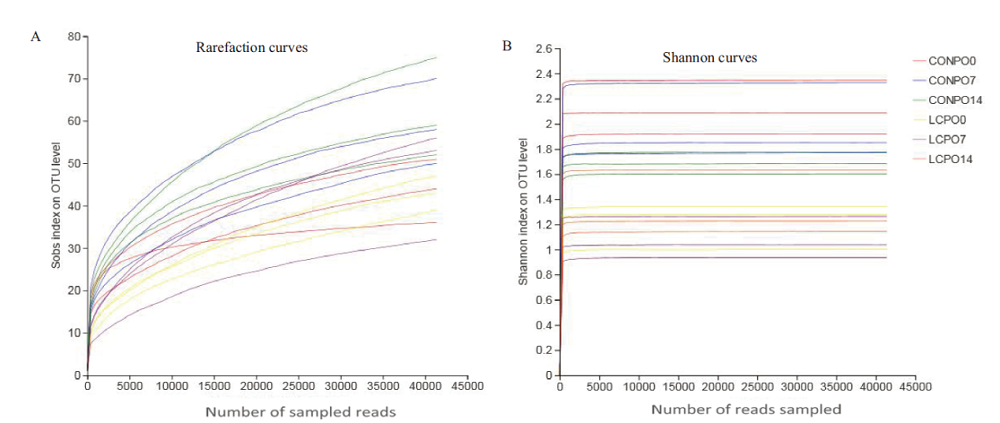
图1 青贮苜蓿菌群稀释曲线(A)和Shannon曲线(B) CONPO0、CONPO7和CONPO14分别表示对照组有氧暴露0 d、7 d和14 d;LCPPO0、LCPPO7和LCPPO14分别表示干酪乳杆菌接种处理组有氧暴露0 d、7 d和14 d Number of sampled reads
Fig.1 Rarefaction curves(A)and Shannon curves(B)of bacterial community in alfalfa silage CONPO0,CONPO7 and CONPO14 represent the control group aerobic exposure 0,7 and 14 d,respectively. LCPPO0,LCPPO7 and LCPPO14 represent the Lactobacillus casei inoculation treatment group aerobic exposure 0,7 and 14 d,respectively
| Sample\Estimators | Sobs | Shannon | Simpson | Ace | Chao |
|---|---|---|---|---|---|
| CONPO0_1 | 43.67 | 2.12 | 0.18 | 53.25 | 49.37 |
| CONPO7_1 | 59.33 | 1.98 | 0.22 | 71.99 | 67.07 |
| CONPO14_1 | 62.00 | 1.69 | 0.33 | 79.05 | 74.23 |
| LCPPO0_1 | 43.00 | 1.21 | 0.41 | 79.80 | 55.789 |
| LCPPO7_1 | 47.00 | 1.08 | 0.49 | 76.73 | 60.91 |
| LCPPO14_1 | 44.00 | 1.33 | 0.38 | 92.71 | 67.73 |
| SEM | 2.66 | 0.10 | 0.03 | 5.65 | 3.22 |
| P | 0.092 | 0.001 | 0.004 | 0.552 | 0.263 |
表2 青贮苜蓿有氧暴露期间菌群多样性指数
Table 2 Alpha-diversity of bacterial community in the silage alfalfa during aerobic exposure
| Sample\Estimators | Sobs | Shannon | Simpson | Ace | Chao |
|---|---|---|---|---|---|
| CONPO0_1 | 43.67 | 2.12 | 0.18 | 53.25 | 49.37 |
| CONPO7_1 | 59.33 | 1.98 | 0.22 | 71.99 | 67.07 |
| CONPO14_1 | 62.00 | 1.69 | 0.33 | 79.05 | 74.23 |
| LCPPO0_1 | 43.00 | 1.21 | 0.41 | 79.80 | 55.789 |
| LCPPO7_1 | 47.00 | 1.08 | 0.49 | 76.73 | 60.91 |
| LCPPO14_1 | 44.00 | 1.33 | 0.38 | 92.71 | 67.73 |
| SEM | 2.66 | 0.10 | 0.03 | 5.65 | 3.22 |
| P | 0.092 | 0.001 | 0.004 | 0.552 | 0.263 |
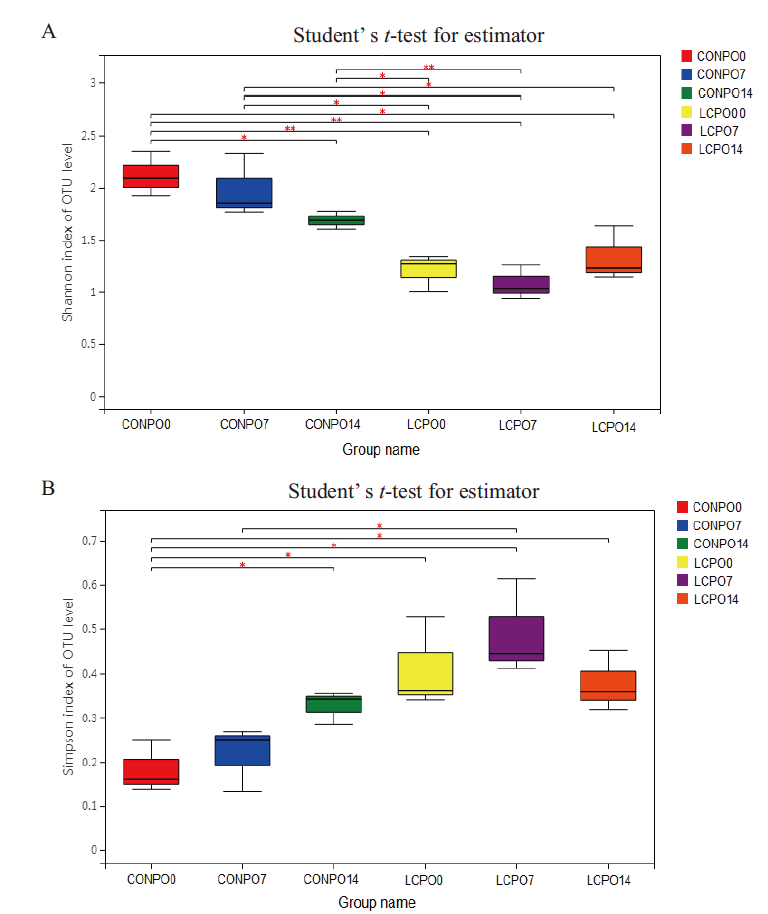
图2 青贮苜蓿菌群Shannon指数和 Simpsom指数差异显著性分析
Fig. 2 Analysis on the difference between Shannon index and Simpson indexes of bacterial community in the silage alfalfa
| Genus | OTU | CONPO0 | CONPO7 | CONPO14 | LCPPO0 | LCPPO7 | LCPPO14 |
|---|---|---|---|---|---|---|---|
| Acetobacter | OTU53 | 0 | 0.03 | 34.99 | 0 | 0.27 | 18.37 |
| Lactobacillus | OTU123 | 0 | 0 | 0 | 4.08 | 0.35 | 0.46 |
| Pediococcus | OTU58 | 0 | 0 | 0 | 0.52 | 1.2 | 0.13 |
| Weissella | OTU26 | 4.17 | 3.71 | 1.58 | 0 | 0.01 | 0 |
| Komagataeibacter | OTU125 | 0 | 0 | 0 | 0 | 5.58 | |
| Cedecea | OTU45 | 2.02 | 1.81 | 3.29 | 0 | 0 | 0 |
| Lactobacillus | OTU127 | 0.65 | 0.48 | 0.19 | 0 | 0.01 | 2.29 |
| Lactobacillus | OTU133 | 0.64 | 1.7 | 0.84 | 9.2 | 17.81 | 21.23 |
| Lactobacillus | OTU41 | 2.63 | 34.63 | 28.38 | 0.18 | 3.97 | |
| Lysinibacillus | OTU57 | 0 | 0 | 3.81 | 0 | 0 | 0 |
| ALCPaligenes | OTU35 | 0 | 0 | 0.36 | 0 | 0 | 0.03 |
| Sporolactobacillus | OTU29 | 1.1 | 0.12 | 0.03 | 0 | 0 | 0 |
| Lactobacillus | OTU27 | 8.52 | 10.71 | 8.47 | 0.06 | 0.05 | 0.13 |
| Lactobacillus | OTU153 | 0.32 | 0.05 | 52.99 | 65.39 | 42.11 | |
| Garciella | OTU11 | 7.74 | 0 | 0 | 0 | 0 | 0 |
| Lactobacillus | OTU54 | 4.3 | 3.17 | 2.54 | 0 | 0 | 0 |
| Enterobacter | OTU38 | 32.58 | 19.35 | 6.59 | 0.94 | 1.19 | 0.43 |
| Enterococcus | OTU21 | 13.12 | 6.84 | 3.19 | 0.04 | 0.03 | 0.03 |
| Lactobacillus | OTU104 | 3.74 | 1.19 | 0.27 | 26.65 | 9.07 | 3.81 |
| Lactobacillus | OTU76 | 1.23 | 8.69 | 1.47 | 0.07 | 0.05 | 0.06 |
| Lactobacillus | OTU148 | 0 | 0 | 0 | 1.98 | 1.74 | 0.2 |
| Lactobacillus | OTU115 | 0.01 | 0.01 | 0 | 2.54 | 1.69 | 0.85 |
| Lactobacillus | OTU71 | 2.95 | 4.36 | 1.68 | 0 | 0 | 0 |
| Enterococcus | OTU14 | 3.79 | 1.34 | 0.67 | 0 | 0 | 0 |
表3 青贮苜蓿有氧暴露各时段OTU组成
Table 3 OTU composition of the silage alfalfa in different exposure time and treatments(>1%)
| Genus | OTU | CONPO0 | CONPO7 | CONPO14 | LCPPO0 | LCPPO7 | LCPPO14 |
|---|---|---|---|---|---|---|---|
| Acetobacter | OTU53 | 0 | 0.03 | 34.99 | 0 | 0.27 | 18.37 |
| Lactobacillus | OTU123 | 0 | 0 | 0 | 4.08 | 0.35 | 0.46 |
| Pediococcus | OTU58 | 0 | 0 | 0 | 0.52 | 1.2 | 0.13 |
| Weissella | OTU26 | 4.17 | 3.71 | 1.58 | 0 | 0.01 | 0 |
| Komagataeibacter | OTU125 | 0 | 0 | 0 | 0 | 5.58 | |
| Cedecea | OTU45 | 2.02 | 1.81 | 3.29 | 0 | 0 | 0 |
| Lactobacillus | OTU127 | 0.65 | 0.48 | 0.19 | 0 | 0.01 | 2.29 |
| Lactobacillus | OTU133 | 0.64 | 1.7 | 0.84 | 9.2 | 17.81 | 21.23 |
| Lactobacillus | OTU41 | 2.63 | 34.63 | 28.38 | 0.18 | 3.97 | |
| Lysinibacillus | OTU57 | 0 | 0 | 3.81 | 0 | 0 | 0 |
| ALCPaligenes | OTU35 | 0 | 0 | 0.36 | 0 | 0 | 0.03 |
| Sporolactobacillus | OTU29 | 1.1 | 0.12 | 0.03 | 0 | 0 | 0 |
| Lactobacillus | OTU27 | 8.52 | 10.71 | 8.47 | 0.06 | 0.05 | 0.13 |
| Lactobacillus | OTU153 | 0.32 | 0.05 | 52.99 | 65.39 | 42.11 | |
| Garciella | OTU11 | 7.74 | 0 | 0 | 0 | 0 | 0 |
| Lactobacillus | OTU54 | 4.3 | 3.17 | 2.54 | 0 | 0 | 0 |
| Enterobacter | OTU38 | 32.58 | 19.35 | 6.59 | 0.94 | 1.19 | 0.43 |
| Enterococcus | OTU21 | 13.12 | 6.84 | 3.19 | 0.04 | 0.03 | 0.03 |
| Lactobacillus | OTU104 | 3.74 | 1.19 | 0.27 | 26.65 | 9.07 | 3.81 |
| Lactobacillus | OTU76 | 1.23 | 8.69 | 1.47 | 0.07 | 0.05 | 0.06 |
| Lactobacillus | OTU148 | 0 | 0 | 0 | 1.98 | 1.74 | 0.2 |
| Lactobacillus | OTU115 | 0.01 | 0.01 | 0 | 2.54 | 1.69 | 0.85 |
| Lactobacillus | OTU71 | 2.95 | 4.36 | 1.68 | 0 | 0 | 0 |
| Enterococcus | OTU14 | 3.79 | 1.34 | 0.67 | 0 | 0 | 0 |

图7 LEfSe分析青贮苜蓿组间丰度差异显著的物种(LAD>4.0) 不同颜色节点表示在对应组别中显著富集,且对组间差异存在显著影响的微生物类群;淡黄色节点表示在不同分组中均无显著差异,或对组间差异无显著影响的微生物类群
Fig. 7 LEfSe analysis of the bacteria taxon with significant difference between treatments(LAD>4.0) Nodes with different colors indicate microbial groups that are significantly enriched in the corresponding groups and have a significant impact on the differences between groups. Light yellow nodes indicate microbial groups that have no significant differences in different groups or have no significant impact on differences between groups
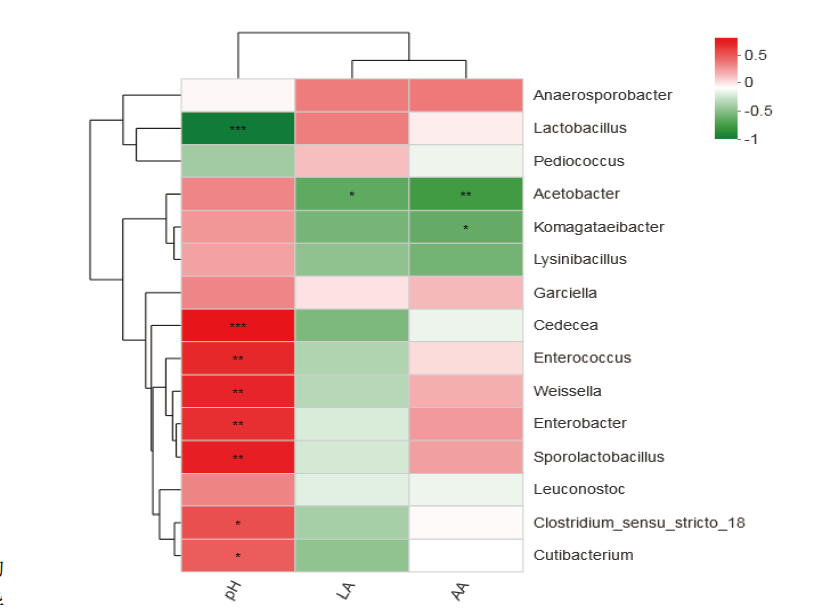
图8 青贮细菌菌群与青贮品质关联性热图分析 X轴和Y轴分别为环境因子和物种,通过计算获得相关性R值和P值。右侧图例是不同R值的颜色区间。*表示0.01< P≤0.05,**表示0.001<P ≤0.01,***表示P≤0.001
Fig. 8 Heatmap analysis of correlation between silage quality and bacterial community The X-axis and Y-axis are environmental factors and species,respectively,and the correlation R value and P value are obtained through calculation. The legend on the right is the color interval for different R values. * refers to 0.01<P≤0.05,** refers to 0.001<P≤0.01,*** refers to P≤0.001
| [1] |
Muck RE, Nadeau EMG, McAllister TA, et al. Silage review:Recent advances and future uses of silage additives[J]. J Dairy Sci, 2018, 101(5):3980-4000.
doi: S0022-0302(18)30322-9 pmid: 29685273 |
| [2] |
McAllister TA, Feniuk R, Mir Z, et al. Inoculants for alfalfa silage:Effects on aerobic stability, digestibility and the growth performance of feedlot steers[J]. Livest Prod Sci, 1998, 53(2):171-181.
doi: 10.1016/S0301-6226(97)00150-4 URL |
| [3] |
Dunière L, Sindou J, et al. Silage processing and strategies to prevent persistence of undesirable microorganisms[J]. Animal Feed Sci Technol, 2013, 182(1/2/3/4):1-15.
doi: 10.1016/j.anifeedsci.2013.04.006 URL |
| [4] |
Woolford MK. The detrimental effects of air on silage[J]. J Appl Bacteriol, 1990, 68(2):101-116.
doi: 10.1111/jam.1990.68.issue-2 URL |
| [5] | Basso FC, Lara EC, Assis F, et al. Fermentation characteristics and aerobic stability of corn silages inoculated with Bacillus subtilis[J]. Revista Brasileira de Saudee Producao Animal, 2012, 13(4):1009-1019. |
| [6] |
Hu ZF, Niu HX, Tong Q, et al. The microbiota dynamics of alfalfa silage during ensiling and after air exposure, and the metabolomics after air exposure are affected by Lactobacillus casei and cellulase addition[J]. Front Microbiol, 2020, 11:519121.
doi: 10.3389/fmicb.2020.519121 URL |
| [7] |
Filya I, Sucu E, Karabulut A. The effect of Lactobacillus buchneri on the fermentation, aerobic stability and ruminal degradability of maize silage[J]. J Appl Microbiol, 2006, 101(6):1216-1223.
pmid: 17105551 |
| [8] |
Mori H, Maruyama F, et al. Design and experimental application of a novel non-degenerate universal primer set that amplifies prokaryotic 16S rRNA genes with a low possibility to amplify eukaryotic rRNA genes[J]. DNA Res, 2014, 21(2):217-227.
doi: 10.1093/dnares/dst052 URL |
| [9] |
Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur:open-source, platform-independent, community-supported software for describing and comparing microbial communities[J]. Appl Environ Microbiol, 2009, 75(23):7537-7541.
doi: 10.1128/AEM.01541-09 URL |
| [10] |
Edgar RC, Haas BJ, Clemente JC, et al. UCHIME improves sensitivity and speed of chimera detection[J]. Bioinformatics, 2011, 27(16):2194-2200.
doi: 10.1093/bioinformatics/btr381 URL |
| [11] |
Liu QH, Dong ZH, Shao T. Effect of additives on fatty acid profile of high moisture alfalfa silage during ensiling and after exposure to air[J]. Animal Feed Sci Technol, 2018, 236:29-38.
doi: 10.1016/j.anifeedsci.2017.11.022 URL |
| [12] |
Zhang L, Zhou X, Gu Q, et al. Analysis of the correlation between bacteria and fungi in sugarcane tops silage prior to and after aerobic exposure[J]. Bioresour Technol, 2019, 291:121835.
doi: 10.1016/j.biortech.2019.121835 URL |
| [13] |
Zhang T, Li L, Wang XF, et al. Effects of Lactobacillus buchneri and Lactobacillus plantarum on fermentation, aerobic stability, bacteria diversity and ruminal degradability of alfalfa silage[J]. World J Microbiol Biotechnol, 2009, 25(6):965-971.
doi: 10.1007/s11274-009-9973-x URL |
| [14] |
Li XM, Chen F, Wang XK, et al. Impacts of low temperature and ensiling period on the bacterial community of oat silage by SMRT[J]. Microorganisms, 2021, 9(2):274.
doi: 10.3390/microorganisms9020274 URL |
| [15] |
Li Y, Nishino N. Bacterial and fungal communities of wilted Italian ryegrass silage inoculated with and without Lactobacillus rhamnosus or Lactobacillus buchneri[J]. Lett Appl Microbiol, 2011, 52(4):314-321.
doi: 10.1111/j.1472-765X.2010.03000.x pmid: 21204884 |
| [16] | 何轶群, 雷赵民, 等. 青贮饲料中优良乳酸菌的分离鉴定及其生物学特性研究[J]. 生物技术通报, 2013(5):177-183. |
| He YQ, Lei ZM, et al. Isolation and identification of excellent lactic acid bacteria from silage and its biological characteristics research[J]. Biotechnol Bull, 2013(5):177-183. | |
| [17] |
Carvalho BF, Sales GFC, Schwan RF, et al. Criteria for lactic acid bacteria screening to enhance silage quality[J]. J Appl Microbiol, 2021, 130(2):341-355.
doi: 10.1111/jam.v130.2 URL |
| [18] |
Spoelstra SF, Courtin MG, Beers JACV. Acetic acid bacteria can initiate aerobic deterioration of whole crop maize silage[J]. J Agric Sci, 1988, 111(1):127-132.
doi: 10.1017/S0021859600082915 URL |
| [19] |
Jiang FG, Cheng HJ, Liu D, et al. Treatment of whole-plant corn silage with lactic acid bacteria and organic acid enhances quality by elevating acid content, reducing pH, and inhibiting undesirable microorganisms[J]. Front Microbiol, 2020, 11:593088.
doi: 10.3389/fmicb.2020.593088 URL |
| [1] | 钟辉, 刘亚军, 王滨花, 和梦洁, 吴兰. 分析方法对细菌群落16S rRNA基因扩增测序分析结果的影响[J]. 生物技术通报, 2022, 38(6): 81-92. |
| [2] | 许广, 王梦姣, 邓百万, 郭苗苗. 不同植茶年限茶树根际土壤细菌多样性及群落结构研究[J]. 生物技术通报, 2020, 36(3): 124-132. |
| [3] | 王永妍, 赵炳赫, 梁广钰, 李云, 徐仰仓. 不同季节使用微生态制剂后养殖海水细菌群落特征[J]. 生物技术通报, 2020, 36(2): 126-133. |
| [4] | 张世伟, 陈曦, 钟其顶, 黄占斌, 孟镇, 罗金学, 石玲, 白志辉. 不同品种酿酒葡萄表皮微生物群落多样性分析[J]. 生物技术通报, 2017, 33(3): 128-137. |
| [5] | 位光山, 张嘉炜, 李明聪, 高峥. 黄河入海口水体细菌群落多样性及分布特征[J]. 生物技术通报, 2017, 33(10): 199-208. |
| [6] | 于蕊,左芳雷,陈喜玲,魏艳杰,陈尚武. 重组粪肠球菌gshF基因对副干酪乳杆菌(Lactobacillus paracasei)L14抗逆性能的影响[J]. 生物技术通报, 2014, 0(9): 149-156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||