








生物技术通报 ›› 2023, Vol. 39 ›› Issue (5): 112-119.doi: 10.13560/j.cnki.biotech.bull.1985.2022-1247
潘国强1( ), 吴思源1,2, 刘璐1, 郭惠明1, 程红梅1, 苏晓峰1(
), 吴思源1,2, 刘璐1, 郭惠明1, 程红梅1, 苏晓峰1( )
)
收稿日期:2022-10-10
出版日期:2023-05-26
发布日期:2023-06-08
通讯作者:
苏晓峰,男,博士,副研究员,研究方向:生物化学与分子生物学;E-mail: suxiaofeng@caas.cn作者简介:潘国强,男,硕士研究生,研究方向:生物化学与分子生物学;E-mail: 15704610584@163.com吴思源为本文共同第一作者
基金资助:
PAN Guo-qiang1( ), WU Si-yuan1,2, LIU Lu1, GUO Hui-ming1, CHENG Hong-mei1, SU Xiao-feng1(
), WU Si-yuan1,2, LIU Lu1, GUO Hui-ming1, CHENG Hong-mei1, SU Xiao-feng1( )
)
Received:2022-10-10
Published:2023-05-26
Online:2023-06-08
摘要:
为了更加快捷地筛选大丽轮枝菌致病关键基因,利用聚乙二醇介导的原生质体转化法构建病原菌随机插入突变体库,对部分阳性转化子的生长表型指标和致病力进行分析,筛选生长发育与致病缺陷突变体并进行靶标基因定位。结果表明,本研究共获得13 030多个阳性转化子,随机挑选5个转化子与V991野生型菌株进行比较分析,发现其中一个突变体在PDA和不同碳源培养基上的菌落直径、产孢量以及致病力均显著降低。侧翼序列测序结果结合Blast比对分析表明,该缺陷突变体中的潮霉素抗性基因表达盒定位于大丽轮枝菌3号染色体1 528 782 bp处,属于内切葡聚糖酶1基因(VDAG_04017)。综上所述,本研究通过大丽轮枝菌插入突变体库构建、突变体生长表型指标和致病力鉴定及靶标基因定位等一系列分子生物学手段,可初步鉴定病原菌致病相关基因,为从全基因组层面深入研究大丽轮枝菌致病机制奠定了一定基础。
潘国强, 吴思源, 刘璐, 郭惠明, 程红梅, 苏晓峰. 大丽轮枝菌(Verticillim dahliae)突变体库的构建与分析[J]. 生物技术通报, 2023, 39(5): 112-119.
PAN Guo-qiang, WU Si-yuan, LIU Lu, GUO Hui-ming, CHENG Hong-mei, SU Xiao-feng. Construction and Preliminary Analysis of Verticillim dahliae Mutant Library[J]. Biotechnology Bulletin, 2023, 39(5): 112-119.
| 引物名称Primer name | 序列 Sequence(5'-3') |
|---|---|
| VdrDNA-qPCR-F | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| VdrDNA-qPCR-R | CGCCTGCGGGACTCCGATGCGAGCTGTAAC |
| NbActin-qPCR-F | GGCTTCCTCAAGGTCGGCTATG |
| NbActin-qPCR-R | GCTGCATGTCATCCCACTTCTTC |
表1 qPCR检测烟草根部大丽轮枝菌生物量引物序列
Table 1 Primer sequences for detection of biomass of V. dahliae in Nicotiana benthamiana root by qPCR
| 引物名称Primer name | 序列 Sequence(5'-3') |
|---|---|
| VdrDNA-qPCR-F | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| VdrDNA-qPCR-R | CGCCTGCGGGACTCCGATGCGAGCTGTAAC |
| NbActin-qPCR-F | GGCTTCCTCAAGGTCGGCTATG |
| NbActin-qPCR-R | GCTGCATGTCATCCCACTTCTTC |
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| AP1 | Provided by Takara Genome Walking Kit, unknown sequence |
| SP1 | ATCGTTGGTGTCGATGTCAGCTCC |
| SP2 | GCGTTTCGGGTTTACCTCTTCCAG |
| SP3 | CGAGATCAAGCAGATCAACGGTCG |
表2 插入位点侧翼序列克隆引物序列
Table 2 Primer sequences for the amplification of flanking sequences at insertion site
| 引物名称 Primer name | 序列 Sequence(5'-3') |
|---|---|
| AP1 | Provided by Takara Genome Walking Kit, unknown sequence |
| SP1 | ATCGTTGGTGTCGATGTCAGCTCC |
| SP2 | GCGTTTCGGGTTTACCTCTTCCAG |
| SP3 | CGAGATCAAGCAGATCAACGGTCG |
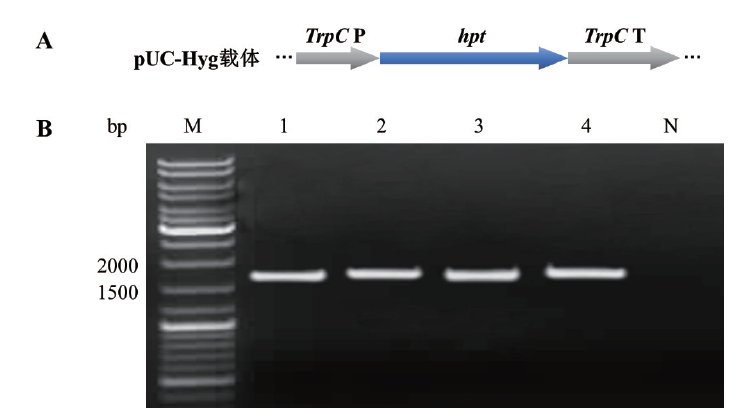
图1 载体pUC-Hyg潮霉素抗性基因表达盒的示意图和PCR扩增 A:载体pUC-Hyg的潮霉素抗性基因表达盒示意图。B:潮霉素抗性基因表达盒片段PCR扩增,M:DNA分子量大小标识;1-4:潮霉素抗性基因表达盒片段;N:阴性对照(ddH2O)
Fig. 1 Schematic diagram and PCR amplification of hygromycin resistance gene expression cassette in pUC-Hyg A: Schematic diagram of hygromycin resistance gene expression cassette in pUC-Hyg. B: PCR amplification of hygromycin resistance gene expression cassette fragment. M: DNA marker. 1-4: Hygromycin resistance gene expression cassette fragment. N: Negative control(ddH2O)

图2 大丽轮枝菌随机插入突变体PCR鉴定 M:DNA分子量大小标识;1-5:1-5号转化子基因组DNA PCR鉴定产物;WT:V991基因组DNA PCR鉴定产物;N:阴性对照(ddH2O)
Fig. 2 PCR identification of randomly inserted mutants of V. dahlia M: DNA marker. 1-5: PCR identification products of transformant 1-5 genomic DNA. WT: PCR identification products of V991 genomic DNA. N: Negative control(ddH2O)

图3 随机插入突变体在PDA和不同碳源培养基上的菌落生长表型 A:大丽轮枝菌V991和5个随机插入突变体在PDA和不同碳源培养基上培养12 d的菌落形态;B:大丽轮枝菌V991和5个随机插入突变体在PDA和不同碳源培养基上培养12 d的菌落直径;C:大丽轮枝菌V991和5个随机插入突变体在PDA和不同碳源培养基上培养12 d的产孢量。误差线表示标准差,不同的字母表示P<0.05时有显著差异
Fig. 3 Colony growth phenotype of randomly inserted mutants on PDA and different carbon source medium A: Colony morphology of V. dahliae V991 and 5 randomly inserted mutants were cultured on PDA and different carbon source medium for 12 d. B: Colony diameters of V. dahliae V991 and 5 randomly inserted mutants were cultured on PDA and different carbon source medium for 12 d. C: Spore production of V. dahliae V991 and 5 randomly inserted mutants cultured on PDA and different carbon source medium for 12 d. Error bars indicate the standard deviations and different letters indicate significant differences at P<0.05
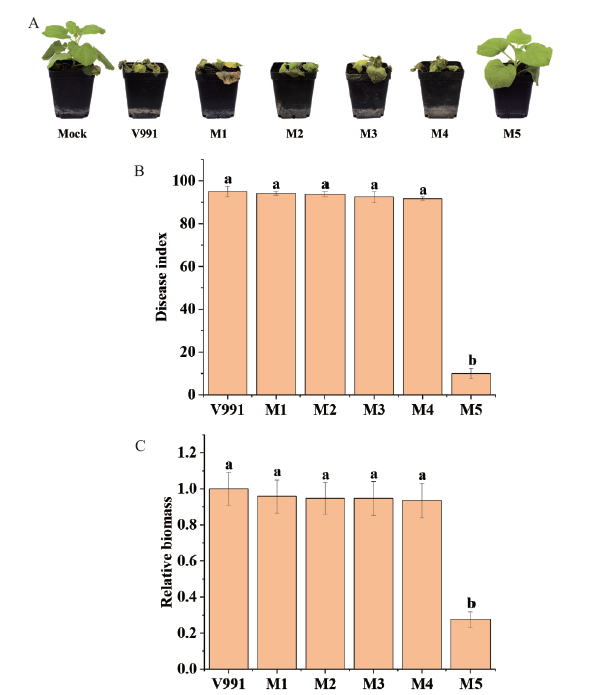
图4 大丽轮枝菌随机插入突变体对烟草的致病力 A:大丽轮枝菌V991和5个随机插入突变体菌株接种烟草12 d后发病情况,Mock:未接种大丽轮枝菌的烟草植株。B:大丽轮枝菌V991和5个随机插入突变体菌株接种烟草12 d后病情指数。C:大丽轮枝菌V991和5个随机插入突变体菌株接种烟草12 d后烟草根部组织中真菌生物量。误差线表示标准差,不同的字母表示P<0.05时有显著差异
Fig. 4 Pathogenicity of randomly inserted mutants of V. dahliae to N. benthamiana A: Disease incidence of N. benthamiana plants after inoculation with V. dahliae V991 and 5 randomly inserted mutants for 12 d. Mock: N. benthamiana plants that were not inoculated with V. dahliae. B: Disease index of N. benthamiana plants after inoculation with V. dahliae V991 and 5 randomly inserted mutants for 12 d. C: Fungal biomass in the root tissues of N. benthamiana after inoculation with V. dahliae V991 and 5 randomly inserted mutants for 12 d. Error bars indicate the standard deviations and different letters indicate significant differences at P<0.05
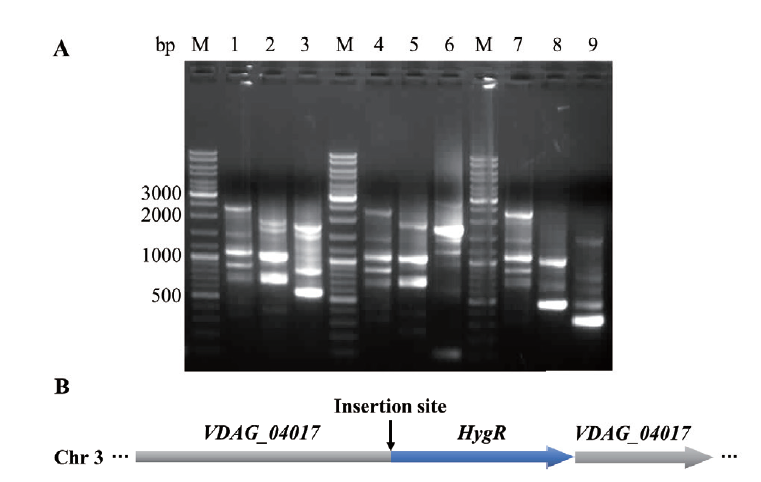
图5 随机插入突变体M5插入位点侧翼序列分析 A:潮霉素抗性基因表达盒片段插入位点侧翼序列检测(M:DNA分子量大小标识;1-9:三轮PCR反应扩增产物);B:潮霉素抗性基因表达盒片段插入位点示意图
Fig. 5 Flanking sequence analysis of insertion site for randomly inserted mutant M5 A: Flanking sequence detection of the insertion site of hygromycin resistance gene expression cassette fragment(M: DNA marker. 1-9: three rounds of PCR reaction amplification products). B: Schematic diagram of insertion site of hygromycin resistance gene expression cassette fragment
| [1] |
Ali F, Qanmber G, Li YH, et al. Genome-wide identification of Gossypium INDETERMINATE DOMAIN genes and their expression profiles in ovule development and abiotic stress responses[J]. J Cotton Res, 2019, 2: 3-18.
doi: 10.1186/s42397-019-0021-6 |
| [2] |
Wendel JF. New world tetraploid cottons contain old world cytoplasm[J]. Proc Natl Acad Sci USA, 1989, 86(11): 4132-4136.
pmid: 16594050 |
| [3] |
Shaban M, Miao YH, Ullah A, et al. physiological and molecular mechanism of defense in cotton against Verticillium dahliae[J]. Plant Physiol Biochem, 2018, 125: 193-204.
doi: 10.1016/j.plaphy.2018.02.011 URL |
| [4] |
Daayf F. Verticillium wilts in crop plants: Pathogen invasion and host defence responses[J]. Can J Plant Pathol, 2015, 37(1): 8-20.
doi: 10.1080/07060661.2014.989908 URL |
| [5] |
Gui YJ, Chen JY, Zhang DD, et al. Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1[J]. Environ Microbiol, 2017, 19(5): 1914-1932.
doi: 10.1111/1462-2920.13695 URL |
| [6] |
Zhang Y, Gao YH, Wang HL, et al. Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1-mediated ethylene biosynthesis[J]. Mol Plant, 2021, 14(11): 1901-1917.
doi: 10.1016/j.molp.2021.07.014 pmid: 34303024 |
| [7] |
Su XF, Lu GQ, Li XK, et al. Host-induced gene silencing of an adenylate kinase gene involved in fungal energy metabolism improves plant resistance to Verticillium dahliae[J]. Biomolecules, 2020, 10(1): 127-142.
doi: 10.3390/biom10010127 URL |
| [8] |
Zhang T, Jin Y, Zhao JH, et al. Host-induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae[J]. Mol Plant, 2016, 9(6): 939-942.
doi: 10.1016/j.molp.2016.02.008 pmid: 26925819 |
| [9] |
Klimes A, Dobinson KF. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae[J]. Fungal Genet Biol, 2006, 43(4): 283-294.
doi: 10.1016/j.fgb.2005.12.006 pmid: 16488633 |
| [10] |
Chi MH, Park SY, Kim S, et al. A novel pathogenicity gene is required in the rice blast fungus to suppress the basal defenses of the host[J]. PLoS Pathog, 2009, 5(4): e1000401-e1000416.
doi: 10.1371/journal.ppat.1000401 URL |
| [11] |
Chou TH, Tzean SS. Protoplasting, regeneration and transformation of medicinal mushroom Ganoderma multipileum using succinate dehydrogenase mutation gene as a selection marker[J]. Ann Microbiol, 2016, 66(1): 111-120.
doi: 10.1007/s13213-015-1087-0 URL |
| [12] |
Rehman L, Su XF, Guo HM, et al. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae[J]. BMC Biotechnol, 2016, 16(1): 57-65.
doi: 10.1186/s12896-016-0287-4 URL |
| [13] |
Su XF, Rehman L, Guo HM, et al. The oligosaccharyl transferase subunit STT3 mediates fungal development and is required for virulence in Verticillium dahliae[J]. Curr Genet, 2018, 64(1): 235-246.
doi: 10.1007/s00294-017-0729-0 URL |
| [14] |
Wei F. Effects of individual and combined use of bio-fumigation-derived products on the viability of Verticillium dahliae microsclerotia in soil[J]. Crop Prot, 2016, 79: 170-176.
doi: 10.1016/j.cropro.2015.09.008 URL |
| [15] |
Faino L, de Jonge R, Thomma BPHJ. The transcriptome of Verticillium dahliae-infected Nicotiana benthamiana determined by deep RNA sequencing[J]. Plant Signal Behav, 2012, 7(9): 1065-1069.
doi: 10.4161/psb.21014 URL |
| [16] |
Klosterman SJ, Subbarao KV, Kang S, et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens[J]. PLoS Pathog, 2011, 7(7): e1002137-e1002155.
doi: 10.1371/journal.ppat.1002137 URL |
| [17] |
Dobinson KF, Grant SJ, Kang S. Cloning and targeted disruption, via Agrobacterium tumefaciens-mediated transformation, of a trypsin protease gene from the vascular wilt fungus Verticillium dahliae[J]. Curr Genet, 2004, 45(2): 104-110.
doi: 10.1007/s00294-003-0464-6 URL |
| [18] |
Mullins ED, Chen X, Romaine P, et al. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer[J]. Phytopathology, 2001, 91(2): 173-180.
doi: 10.1094/PHYTO.2001.91.2.173 pmid: 18944391 |
| [19] |
Amey RC. PEG-mediated and Agrobacterium-mediated transformation in the mycopathogen Verticillium fungicola[J]. Mycol Res, 2002, 106(1): 4-11.
doi: 10.1017/S0953756201005251 URL |
| [20] |
Dobrowolska A, Staczek P. Development of transformation system for Trichophyton rubrum by electroporation of germinated conidia[J]. Curr Genet, 2009, 55(5): 537-542.
doi: 10.1007/s00294-009-0264-8 pmid: 19629488 |
| [21] |
Wang D, Chen JY, Song J, et al. Cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae[J]. Plant Physiol, 2021, 187(1): 409-429.
doi: 10.1093/plphys/kiab274 pmid: 34618145 |
| [22] |
Zhang J, Zhang YY, Yang JF, et al. The α-1, 6-mannosyltransferase VdOCH1 plays a major role in microsclerotium formation and virulence in the soil-borne pathogen Verticillium dahliae[J]. Fungal Biol, 2019, 123(7): 539-546.
doi: 10.1016/j.funbio.2019.05.007 URL |
| [23] |
Gao F, Zhou BJ, Li GY, et al. A glutamic acid-rich protein identified in Verticillium dahliae from an insertional mutagenesis affects microsclerotial formation and pathogenicity[J]. PLoS One, 2010, 5(12): e15319-e15328.
doi: 10.1371/journal.pone.0015319 URL |
| [24] |
Zhang DD, Wang XY, Chen JY, et al. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae[J]. Sci Rep, 2016, 6: 27979-27990.
doi: 10.1038/srep27979 |
| [25] |
Maruthachalam K, Klosterman SJ, Kang S, et al. Identification of pathogenicity-related genes in the vascular wilt fungus Verticillium dahliae by Agrobacterium tumefaciens-mediated T-DNA insertional mutagenesis[J]. Mol Biotechnol, 2011, 49(3): 209-221.
doi: 10.1007/s12033-011-9392-8 pmid: 21424547 |
| [26] |
Yang C, Liu R, Pang JH, et al. Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice[J]. Nat Commun, 2021, 12(1): 2178-2190.
doi: 10.1038/s41467-021-22456-x |
| [1] | 田李, 李俊娇, 戴小枫, 张丹丹, 陈捷胤. 从功能基因到生物学性状:大丽轮枝菌致病性形成的分子基础[J]. 生物技术通报, 2022, 38(1): 51-69. |
| [2] | 雒丽丽, 张昊, 杨美欣, 王云飞, 许景升, 徐进, 姚强, 冯洁. 黄淮与东北麦区小麦赤霉菌温度相关的致病力分化研究[J]. 生物技术通报, 2021, 37(4): 47-55. |
| [3] | 赵小强, 陈志荣, 何芳, 沈楠, 高峰, 黄家风. 大丽轮枝菌原生质体的制备及再生[J]. 生物技术通报, 2018, 34(7): 166-173. |
| [4] | 张梦恬, 裴娟 ,李国 ,赵辉 ,陈建权 ,祝建波, 王爱英. 新疆石河子地区棉花黄萎病菌分离鉴定及其致病力分析[J]. 生物技术通报, 2018, 34(6): 73-78. |
| [5] | 谢成建. 大丽轮枝菌致病及微菌核形成相关基因研究进展[J]. 生物技术通报, 2018, 34(4): 51-59. |
| [6] | 姜岚, 庞金环, 肖伟烈, 张国丽, 刘俊, 杨超. 56种中药提取物对棉花黄萎病的防治效果研究[J]. 生物技术通报, 2018, 34(2): 128-134. |
| [7] | 郭云峰, 安邦. 橡胶树胶孢炭疽菌NADPH氧化酶功能研究[J]. 生物技术通报, 2018, 34(10): 165-171. |
| [8] | 王炳楠;杨秀芬;曾洪梅;邱德文;. 大丽轮枝菌分泌蛋白激发子的分离纯化及生物功能研究[J]. , 2011, 0(11): 166-171. |
| [9] | 简桂良;卢美光;赵磊;王深正;. 继代对棉花黄萎病落叶型菌系致病力的影响[J]. , 2009, 0(S1): 165-168. |
| [10] | 李思经;. 政府承包商寻求研究AIDS与癌症的关系[J]. , 1990, 0(11): 18-19. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||