








生物技术通报 ›› 2024, Vol. 40 ›› Issue (3): 296-304.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0902
王梦雅( ), 刘家齐, 姜海霖, 李菁华, 赵春燕, 黄红兰(
), 刘家齐, 姜海霖, 李菁华, 赵春燕, 黄红兰( )
)
收稿日期:2023-09-19
出版日期:2024-03-26
发布日期:2024-04-08
通讯作者:
黄红兰,女,博士,教授,研究方向:微生物感染与免疫;E-mail: hhl@jlu.edu.cn作者简介:王梦雅,女,硕士研究生,研究方向:噬菌体相关研究; E-mail: 2462860228@qq.com
基金资助:
WANG Meng-ya( ), LIU Jia-qi, JIANG Hai-lin, LI Jing-hua, ZHAO Chun-yan, HUANG Hong-lan(
), LIU Jia-qi, JIANG Hai-lin, LI Jing-hua, ZHAO Chun-yan, HUANG Hong-lan( )
)
Received:2023-09-19
Published:2024-03-26
Online:2024-04-08
摘要:
【目的】 筛选并分离出能裂解肠侵袭性大肠埃希菌噬菌体,分析其生物学特性,并探究其对污染猪肉的杀菌作用。【方法】 采用双层平板法分离鉴定噬菌体,并测定其最佳感染复数,一步生长曲线等,对其基因组进行测序分析以及裂解功效的测定。【结果】 从医院污水中分离鉴定能特异裂解肠侵袭性大肠埃希菌(Enteroinvasive Escherichia coli coli,EIEC)的噬菌体并命名为DK-13,其呈典型的蝌蚪状外形,包含一个正二十面体的头部和一个可收缩的螺旋对称的尾部,属于肌尾噬菌体科(Myoviridae)噬菌体;生物学特性表明:最佳感染复数为0.01,潜伏期约为10 min,裂解期约为70 min,噬菌体DK-13能在50℃,pH 5.0-10.0条件下存活。全基因组测序表明,噬菌体基因组长约172 275 bp,GC含量为40.18%,预测其共有293个开放阅读框(open reading frames, ORF),未发现已知耐药基因和毒力基因。应用试验表明:被污染的猪肉中表现的杀菌效果良好,宿主菌的数量明显减少。【结论】 分离并鉴定一株新的烈性噬菌体DK-13,DK-13具有潜伏期短、裂解效率高等优势,在食品安全方面具有很大应用潜力。
王梦雅, 刘家齐, 姜海霖, 李菁华, 赵春燕, 黄红兰. 肠侵袭性大肠埃希菌噬菌体DK-13的生物学特性及应用[J]. 生物技术通报, 2024, 40(3): 296-304.
WANG Meng-ya, LIU Jia-qi, JIANG Hai-lin, LI Jing-hua, ZHAO Chun-yan, HUANG Hong-lan. Biological Characteristics and Application of Enteroinvasive Escherichia coli Phage DK-13[J]. Biotechnology Bulletin, 2024, 40(3): 296-304.

图1 噬菌体DK-13的形态特征 A:噬菌体形态;B:透射电镜形态
Fig. 1 Morphological characteristics of phage DK-13 A: Morphology of phage DK-13. B: Transmission electron micrograph of phage DK-13

图2 噬菌体DK-13的生物学特性 A:噬菌体DK-13的MOI;B:噬菌体DK-13的一步生长曲线。不同小写字母表示处理组别间有显著差异(P<0.05)。下同
Fig. 2 Biological characters of phage DK-13 A: MOI of phage DK-13. B: Step growth curve of phage DK-13. Different lower letters indicate significant difference among each group (P<0.05). The same below
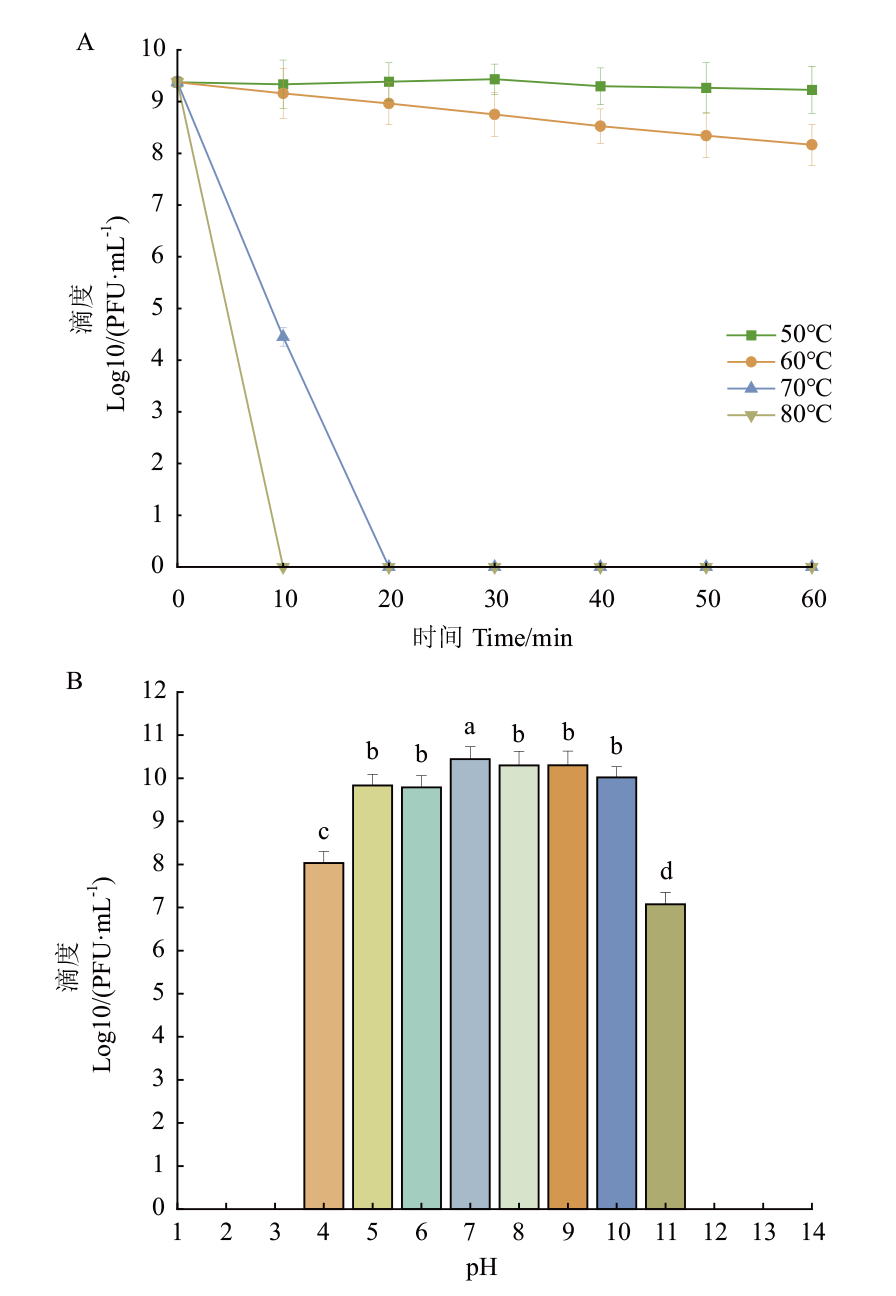
图3 噬菌体DK-13的生物学特性 A:噬菌体DK-13的热稳定性;B:噬菌体DK-13的酸碱稳定性
Fig. 3 Biological characteristics of phage DK-13 A: Temperature resistance of phage DK-13. B: pH resistance of phage DK-13
| 菌株名称 Strain name | 菌株类型 Strain type | 噬菌体DK-13的裂解性 Phage DK-13 lytic ability |
|---|---|---|
| 大肠埃希菌A1 Escherichia coli A1 | O157:H7 | - |
| 大肠埃希菌A2 E. coli A2 | EPEC | - |
| 大肠埃希菌A3 E. coli A3 | O26:H11 | - |
| 大肠埃希菌A4 E. coli A4 | O26:H11 | - |
| 大肠埃希菌A5 E. coli A5 | O26:H11 | - |
| 大肠埃希菌A6 E. coli A6 | O26:H11 | - |
| 大肠埃希菌A7 E. coli A7 | O26:H11 | - |
| 大肠埃希菌A8 E. coli A8 | O46:H38 | - |
| 大肠埃希菌A9 E. coli A9 | O11:NM | - |
| 大肠埃希菌A10 E. coli A10 | O157:H7 | - |
| 大肠埃希菌A11 E. coli A11 | O157:H7 | - |
| 大肠埃希菌A12 E. coli A12 | O157:H7 | - |
| 大肠埃希菌A13 E. coli A13 | O157:H7 | - |
| 大肠埃希菌A14 E. coli A14 | O157:H7 | - |
| 大肠埃希菌A15 E. coli A15 | O157:H7 | - |
| 蜡样芽孢杆菌L1 Bacillus cereus L1 | * | - |
| 蜡样芽孢杆菌L2 B. cereus L2 | * | - |
| 金黄色葡萄球菌B1 Streptococcus aureus B1 | * | - |
| 金黄色葡萄球菌B2 S. aureus B2 | * | - |
| 金黄色葡萄球菌B3 S. aureus B3 | * | - |
| 大肠埃希菌(宿主菌)D1 E. coli D1 | EIEC | + |
表1 噬菌体DK-13宿主范围
Table 1 Host range of DK-13 phage
| 菌株名称 Strain name | 菌株类型 Strain type | 噬菌体DK-13的裂解性 Phage DK-13 lytic ability |
|---|---|---|
| 大肠埃希菌A1 Escherichia coli A1 | O157:H7 | - |
| 大肠埃希菌A2 E. coli A2 | EPEC | - |
| 大肠埃希菌A3 E. coli A3 | O26:H11 | - |
| 大肠埃希菌A4 E. coli A4 | O26:H11 | - |
| 大肠埃希菌A5 E. coli A5 | O26:H11 | - |
| 大肠埃希菌A6 E. coli A6 | O26:H11 | - |
| 大肠埃希菌A7 E. coli A7 | O26:H11 | - |
| 大肠埃希菌A8 E. coli A8 | O46:H38 | - |
| 大肠埃希菌A9 E. coli A9 | O11:NM | - |
| 大肠埃希菌A10 E. coli A10 | O157:H7 | - |
| 大肠埃希菌A11 E. coli A11 | O157:H7 | - |
| 大肠埃希菌A12 E. coli A12 | O157:H7 | - |
| 大肠埃希菌A13 E. coli A13 | O157:H7 | - |
| 大肠埃希菌A14 E. coli A14 | O157:H7 | - |
| 大肠埃希菌A15 E. coli A15 | O157:H7 | - |
| 蜡样芽孢杆菌L1 Bacillus cereus L1 | * | - |
| 蜡样芽孢杆菌L2 B. cereus L2 | * | - |
| 金黄色葡萄球菌B1 Streptococcus aureus B1 | * | - |
| 金黄色葡萄球菌B2 S. aureus B2 | * | - |
| 金黄色葡萄球菌B3 S. aureus B3 | * | - |
| 大肠埃希菌(宿主菌)D1 E. coli D1 | EIEC | + |

图4 噬菌体DK-13基因组注释图谱 箭头方向代表基因方向。红色:结构基因模块;棕色:包装基因模块;蓝色:核苷酸代谢基因模块;橙色:复制/重组基因模块;粉色:转录/翻译基因模块;绿色:其他功能模块;灰色:假定蛋白;黑色:GC含量
Fig. 4 Annotation diagram of the genome of DK-13 phage The direction of arrow indicates the direction of gene. Red: The structural gene module. Brown: The packaged genetic module. Blue: The nucleotide metabolism gene module. Orange: Duplication/ Recombination gene module. Pink: Transcription/ Translation gene module. Green: Other function module. Grey: Hypothetical protein. Black: GC content
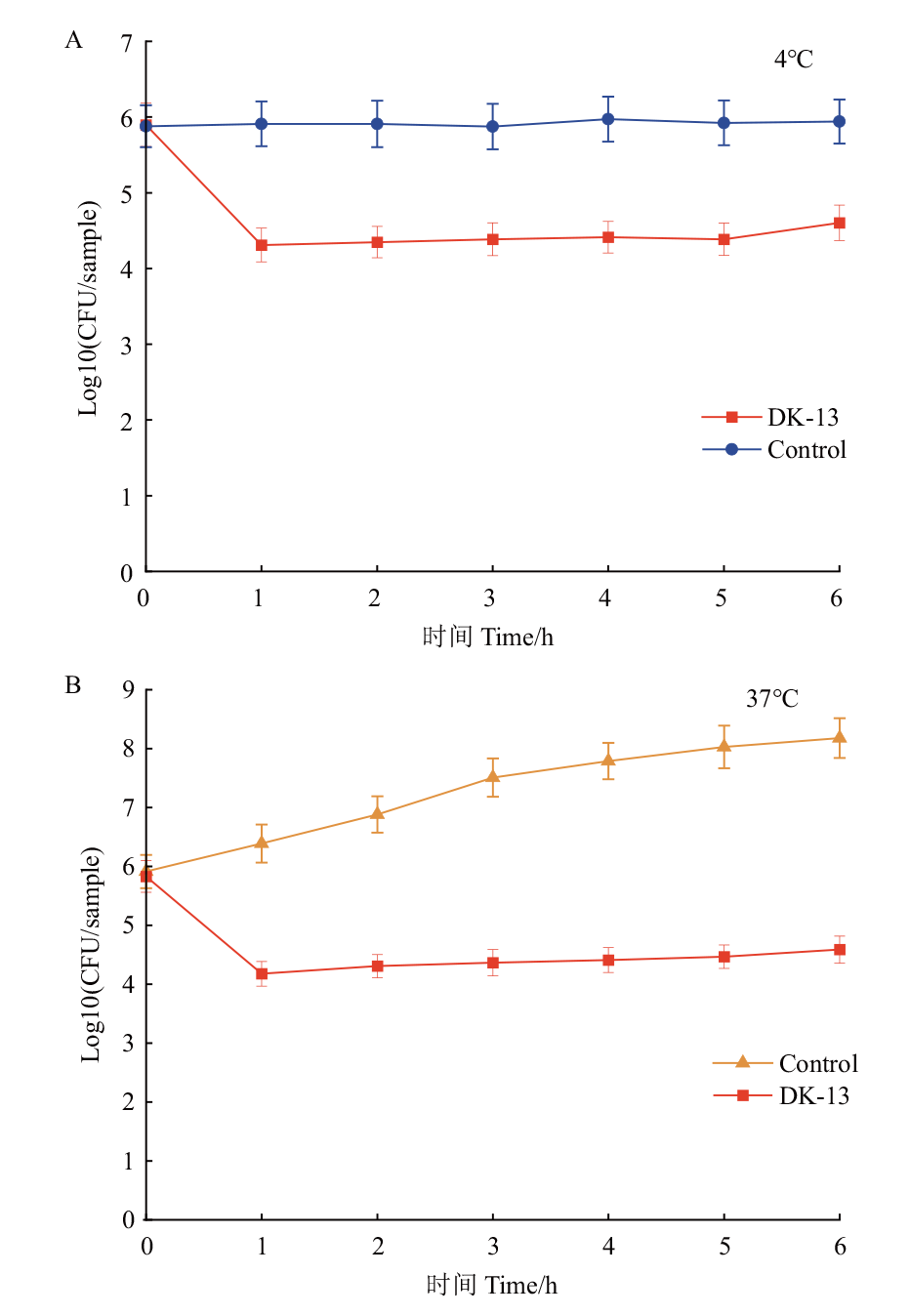
图6 不同温度下噬菌体DK-13对污染猪肉的杀菌效果 A: 4℃下噬菌体的杀菌效果;B:37℃下噬菌体的杀菌效果
Fig. 6 Sterilization effect of phage DK-13 at different temperatures on contaminated pork A: Sterilization effect of phage DK-13 at 4℃. B: Sterilization effect of phage DK-13 at 37℃
| [1] |
陈亚强, 彭津津, 廖明, 等. 土鸡屠宰过程中大肠杆菌毒力基因检测及耐药性分析[J]. 中国畜牧兽医, 2020, 47(8): 2615-2624.
doi: 10.16431/j.cnki.1671-7236.2020.08.031 |
| CHEN Y Q, PENG J J, L LIAO M, et al. Virulence genes detection and antibiotic resistance analysis of Escherichia coli isolated from slaughtering process of free-range chicken[J]. China Animal Husbandry & Veterinary Medicine, 2020 47(8):2615-2624. | |
| [2] |
Fricke W F, McDermott P F, Mammel M K, et al. Antimicrobial resistance-conferring plasmids with similarity to virulence plasmids from avian pathogenic Escherichia coli strains in Salmonella enterica serovar kentucky isolates from poultry[J]. Applied and Environmental Microbiology, 2009, 75(18): 5963-5971.
doi: 10.1128/AEM.00786-09 URL |
| [3] | Van Den Beld M J C, Reubsaet F A. G. Differentiation between Shigella, enteroinvasive Escherichia coli(EIEC)and noninvasive Escherichia coli[J]. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology, 2012, 31(6): 899-904. |
| [4] |
Zhang K, Ge H, He J, et al. Salmonella typhimurium st34 isolate was more resistant than the st19 isolate in china, 2007-2019[J]. Foodborne Pathogens and Disease, 2022, 19(1): 62-69.
doi: 10.1089/fpd.2021.0047 URL |
| [5] |
Ge H, Fu S, Guo H, et al. Application and challenge of bacteriophage in the food protection[J]. International Journal of Food Microbiology, 2022, 380: 109872.
doi: 10.1016/j.ijfoodmicro.2022.109872 URL |
| [6] |
Rehman S, Ali Z, Khan M, et al. The dawn of phage therapy[J]. Reviews in Medical Virology, 2019, 29(4): e2041.
doi: 10.1002/rmv.2041 |
| [7] |
Harada L K, Silva E C, Campos W F, et al. Biotechnological applications of bacteriophages: state of the art[J]. Microbiological Research, 2018, 212-213: 38-58.
doi: S0944-5013(18)30133-2 pmid: 29853167 |
| [8] |
Henry M, Debarbieux L. Tools from viruses: bacteriophage successes and beyond[J]. Virology, 2012, 434(2): 151-161.
doi: 10.1016/j.virol.2012.09.017 pmid: 23063405 |
| [9] |
Moye Z D, Woolston J, Sulakvelidze A. Bacteriophage applications for food production and processing: 4[J]. Viruses, 2018, 10(4): 205.
doi: 10.3390/v10040205 URL |
| [10] |
Kahn L H, Bergeron G, Bourassa M W, et al. From farm management to bacteriophage therapy: strategies to reduce antibiotic use in animal agriculture[J]. Annals of the New York Academy of Sciences, 2019, 1441(1): 31-39.
doi: 10.1111/nyas.14034 pmid: 30924542 |
| [11] |
Magnone J P, Marek P J, Sulakvelidze A, et al. Additive approach for inactivation of escherichia coli o157:h7, salmonella, and shigella spp. on contaminated fresh fruits and vegetables using bacteriophage cocktail and produce wash[J]. Journal of Food Protection, 2013, 76(8): 1336-1341.
doi: 10.4315/0362-028X.JFP-12-517 pmid: 23905788 |
| [12] |
Vikram A, Tokman J I, Woolston J, et al. Phage biocontrol improves food safety by significantly reducing the level and prevalence of escherichia coli o157:h7 in various foods[J]. Journal of Food Protection, 2020, 83(4): 668-676.
doi: 10.4315/0362-028X.JFP-19-433 pmid: 32221572 |
| [13] |
Rahimzadeh G, Saeedi M, Moosazadeh M, et al. Encapsulation of bacteriophage cocktail into chitosan for the treatment of bacterial diarrhea: 1[J]. Scientific Reports, 2021, 11(1): 15603.
doi: 10.1038/s41598-021-95132-1 pmid: 34341399 |
| [14] | 李兆雪, 兰冠达, 范聪聪, 等. 肠出血性大肠埃希菌O157: H7噬菌体FEC14和FEC19特性及污染牛肉杀菌潜在应用探究[J]. 微生物学通报, 2022, 49(7): 2741-2752. |
| Li ZX, Lan GD, Fan CC, et al. Characterization of enterohaemorrhagic Escherichia coli O157: H7 phages FEC14 and FEC19 and their potential use in contaminated beef[J]. Microbiol China, 2022, 49(7): 2741-2752. | |
| [15] | 李载平. 《分子克隆实验指南》(第3版)[J]. 科学通报, 2002, 47(24): 1888. |
|
Li ZP. Experimental guide to molecular cloning(3rd edition)[J]. Chin Sci Bull, 2002, 47(24): 1888.
doi: 10.1360/csb2002-47-24-1888 URL |
|
| [16] |
Li S, Konoval HM, Marecek S, et al. Salmonella spp. response to lytic bacteriophage and lactic acid on marinated and tenderized raw pork loins[J]. Foods, 2022, 11(6): 879.
doi: 10.3390/foods11060879 URL |
| [17] |
Abidin AU, Asmara AA, Asmarany A, et al. A linkage of personal, food, and environmental hygiene to presence of E. coli in Warmindo Food Stall[J]. Gac Sanit, 2021, 35(Suppl 2): S107-S111.
doi: 10.1016/j.gaceta.2021.10.008 URL |
| [18] | 冯杰. 猪、肉制品及人源耐碳青霉烯类肠杆菌科(CRE)细菌的耐药性和传播特性分析[D]. 杨凌: 西北农林科技大学, 2022. |
| Feng J. Antibiotic resistance and transmission characteristics of carbapenem-resistant Enterobacteriaceae(CRE)strains from pig, retail meat products and human[D]. Yangling: Northwest A&F University, 2022. | |
| [19] |
Sarhan WA, Azzazy HME. Phage approved in food, why not as a therapeutic?[J]. Expert Rev Anti Infect Ther, 2015, 13(1): 91-101.
doi: 10.1586/14787210.2015.990383 pmid: 25488141 |
| [20] |
Hatfull GF. Bacteriophage genomics[J]. Curr Opin Microbiol, 2008, 11(5): 447-453.
doi: 10.1016/j.mib.2008.09.004 pmid: 18824125 |
| [21] | Sulakvelidze A, Barrow P. Phage therapy in animals and agribusiness[M]// Bacteriophages. CRC Press, 2004 |
| [22] |
Bajovic B, Bolumar T, Heinz V. Quality considerations with high pressure processing of fresh and value added meat products[J]. Meat Sci, 2012, 92(3): 280-289.
doi: 10.1016/j.meatsci.2012.04.024 pmid: 22608831 |
| [23] |
Schmelcher M, Loessner MJ. Bacteriophage endolysins: applications for food safety[J]. Curr Opin Biotechnol, 2016, 37: 76-87.
doi: 10.1016/j.copbio.2015.10.005 URL |
| [24] |
Han SH, Byun KH, Mizan MFR, et al. Bacteriophage and their lysins: a new era of biocontrol for inactivation of pathogenic bacteria in poultry processing and production—a review[J]. Food Contr, 2022, 137: 108976.
doi: 10.1016/j.foodcont.2022.108976 URL |
| [25] |
Gutiérrez D, Rodríguez-Rubio L, Martínez B, et al. Bacteriophages as weapons against bacterial biofilms in the food industry[J]. Front Microbiol, 2016, 7: 825.
doi: 10.3389/fmicb.2016.00825 pmid: 27375566 |
| [26] |
Perera MN, Abuladze T, Li MR, et al. Bacteriophage cocktail significantly reduces or eliminates Listeria monocytogenes contamination on lettuce, apples, cheese, smoked salmon and frozen foods[J]. Food Microbiol, 2015, 52: 42-48.
doi: 10.1016/j.fm.2015.06.006 pmid: 26338115 |
| [27] |
Yuan XM, Zhang SH, Wang J, et al. Isolation and characterization of a novel Escherichia coli Kayfunavirus phage DY1[J]. Virus Res, 2021, 293: 198274.
doi: 10.1016/j.virusres.2020.198274 URL |
| [28] |
Nicolas M, Trotereau A, Culot A, et al. Isolation and characterization of a novel phage collection against avian-pathogenic Escherichia coli[J]. Microbiol Spectr, 2023, 11(3): e0429622.
doi: 10.1128/spectrum.04296-22 URL |
| [29] |
Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics[J]. Trends Biotechnol, 2010, 28(12): 591-595.
doi: 10.1016/j.tibtech.2010.08.001 pmid: 20810181 |
| [30] |
Kaliniene L, Klausa V, Truncaite L. Low-temperature T4-like coliphages vB_EcoM-VR5, vB_EcoM-VR7 and vB_EcoM-VR20[J]. Arch Virol, 2010, 155(6): 871-880.
doi: 10.1007/s00705-010-0656-6 pmid: 20361343 |
| [31] |
Hong Y, Pan Y, Ebner PD. Meat Science and Muscle Biology Symposium: development of bacteriophage treatments to reduce Escherichia coli O157: H7 contamination of beef products and produce[J]. J Anim Sci, 2014, 92(4): 1366-1377.
doi: 10.2527/jas.2013-7272 pmid: 24492574 |
| [32] |
Figueiredo ACL, Almeida RCC. Antibacterial efficacy of nisin, bacteriophage P100 and sodium lactate against Listeria monocytogenes in ready-to-eat sliced pork ham[J]. Braz J Microbiol, 2017, 48(4): 724-729.
doi: S1517-8382(16)30056-9 pmid: 28641956 |
| [33] |
Soffer N, Woolston J, Li M, et al. Bacteriophage preparation lytic for shigella significantly reduces shigella sonnei contamination in various foods[J]. PLoS One, 2017, 12(3): e0175256.
doi: 10.1371/journal.pone.0175256 URL |
| [34] | 孙新城, 赵成鑫, 胡旭阳, 等. 噬菌体在食品安全领域中的应用[J]. 中国病原生物学杂志, 2022, 17(4): 479-482. |
| Sun XC, Zhao CX, Hu XY, et al. Application of bacteriophage in food safety[J]. J Pathog Biol, 2022, 17(4): 479-482. |
| [1] | 李托, 李陇平, 屈雷. 有尾噬菌体的结构及其受体研究进展[J]. 生物技术通报, 2023, 39(6): 88-101. |
| [2] | 陈晓琳, 刘洋儿, 许文涛, 郭明璋, 刘慧琳. 合成生物学细胞传感技术在食品安全快速检测中的应用[J]. 生物技术通报, 2023, 39(1): 137-149. |
| [3] | 胡雪莹, 张越, 郭雅杰, 仇天雷, 高敏, 孙兴滨, 王旭明. 不同施肥处理农田土壤中噬菌体与细菌携带抗生素抗性基因的比较[J]. 生物技术通报, 2022, 38(9): 116-126. |
| [4] | 文畅, 刘晨, 卢诗韵, 许忠兵, 艾超凡, 廖汉鹏, 周顺桂. 一株新的多重耐药福氏志贺菌噬菌体生物学特性及基因组分析[J]. 生物技术通报, 2022, 38(9): 127-135. |
| [5] | 徐重新, 张霄, 刘媛, 仲建锋, 谢雅晶, 卢莉娜, 高美静, 刘贤金. 靶向模拟Bt Cry1C蛋白抗虫功能的人源化基因工程抗体筛选及鉴定[J]. 生物技术通报, 2022, 38(5): 191-200. |
| [6] | 王加利, 和似琦, 康子茜, 王建勋. 噬菌体抗体展示技术及其在抗新冠病毒抗体发现中的应用[J]. 生物技术通报, 2022, 38(5): 248-256. |
| [7] | 张雅涵, 朱丽霞, 胡静, 朱亚静, 张雪婧, 曹叶中. 草甘膦在我国生物育种产业化应用中的机遇与挑战[J]. 生物技术通报, 2022, 38(11): 1-9. |
| [8] | 张俊锋, 李孟珂, 吴志浩, 崔晓龙, 肖炜, 张仕颖. 噬菌体DCEAV-31和DCEIV-9对溶藻菌溶藻特性的影响[J]. 生物技术通报, 2022, 38(11): 250-257. |
| [9] | 黄景晓, 尚俊康, 陈慧敏, 沈嘉旻, 黎圆圆, 喻玉立, 倪进东, 林伯坤. 一株烈性沙门氏菌噬菌体的生物学特性及基因组分析[J]. 生物技术通报, 2021, 37(6): 136-146. |
| [10] | 王孝芳, 侯玉刚, 杨可铭, 王佳宁, 韦中, 徐阳春, 沈其荣. 一株青枯菌专性噬菌体的分离及应用效果研究[J]. 生物技术通报, 2020, 36(9): 194-201. |
| [11] | 王琦, 颜春蕾, 高洪伟, 吴薇, 杨庆利. 基于核酸适配体传感器检测食品致病菌的研究进展[J]. 生物技术通报, 2020, 36(11): 245-258. |
| [12] | 吴亚, 徐智辉, 张彪, 赵冬芳, 曹文欣, 张兴平. 核酸适配体光学生物传感器在卡那霉素检测中的研究进展[J]. 生物技术通报, 2020, 36(1): 193-201. |
| [13] | 戚家明, 杨娜, 孙杉杉, 明艳超, 郭亮, 张东旭, 徐志文. 一株具有噬菌体抗性的芽孢杆菌BS-2的鉴定及葡萄糖流加工艺优化[J]. 生物技术通报, 2019, 35(3): 210-216. |
| [14] | 耿慧君, 邹伟, 崔惠敬, 李晓宇, 王丽丽, 徐永平. 基于转录组学的金黄色葡萄球菌噬菌体安全性评估[J]. 生物技术通报, 2019, 35(12): 64-75. |
| [15] | 汤初美, 朱龙佼, 郭顺堂, 苗敬, 许文涛. 食源性致病菌安全事故处置与警示——以“2011年德国肠出血性大肠杆菌感染暴发疫情”为例[J]. 生物技术通报, 2018, 34(8): 204-214. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||