








生物技术通报 ›› 2021, Vol. 37 ›› Issue (12): 160-168.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0335
包林珠1,2( ), 时灿1,2, 卢玲儿1,2, 徐行1,2, 周泽斌1,2, 任建峰1,2, 李伟明3, 张庆华1,2(
), 时灿1,2, 卢玲儿1,2, 徐行1,2, 周泽斌1,2, 任建峰1,2, 李伟明3, 张庆华1,2( )
)
收稿日期:2021-03-18
出版日期:2021-12-26
发布日期:2022-01-19
作者简介:包林珠,女,硕士研究生,研究方向:分子生物学;E-mail: 基金资助:
BAO Lin-zhu1,2( ), SHI Can1,2, LU Ling-er1,2, XU Xing1,2, ZHOU Ze-bin1,2, REN Jian-feng1,2, LI Wei-ming3, ZHANG Qing-hua1,2(
), SHI Can1,2, LU Ling-er1,2, XU Xing1,2, ZHOU Ze-bin1,2, REN Jian-feng1,2, LI Wei-ming3, ZHANG Qing-hua1,2( )
)
Received:2021-03-18
Published:2021-12-26
Online:2022-01-19
摘要:
旨在研究斑马鱼(Danio rerio)mapk1基因对tp53基因的调控作用。通过生信网站分析斑马鱼tp53启动子序列与mapk1基因CDS序列,构建斑马鱼tp53启动子荧光素酶报告基因质粒pGL3-tp53-Luc和 mapk1表达质粒pCMV-Tag2B-mapk1。利用Luciferase实验验证tp53启动子报告基因的活性,以及mapk1基因对tp53基因的活性影响。结果显示,斑马鱼tp53基因启动子区无CpG岛位点,包含 Oct-1(TATGTAAAGC)、Sp-1(AGATCCCGCC)、c-Jun(CTGACGTCAC)、CREB(CTGACGTCAC)、CRE-BP1(CTGACGTCAC)、NF-kappa B1(AGGGGAATCC)和RAR-alpha1(TTGAACTTTT)共7个转录因子结合位点;斑马鱼与人和小鼠MAPK1氨基酸的一致性分别为91.33%和90.79%。pGL3-tp53-Luc在哺乳动物细胞系和鱼类细胞系中均具有很高的活性,分别是对照组的17倍和9倍。在HEK293T细胞中过表达pCMV-Tag2B-mapk1 质粒后pGL3-tp53-Luc的luciferase活性是对照组的3倍左右。实验验证了斑马鱼mapk1基因可促进tp53基因的表达。
包林珠, 时灿, 卢玲儿, 徐行, 周泽斌, 任建峰, 李伟明, 张庆华. 斑马鱼(Danio rerio)mapk1基因对tp53基因调控研究[J]. 生物技术通报, 2021, 37(12): 160-168.
BAO Lin-zhu, SHI Can, LU Ling-er, XU Xing, ZHOU Ze-bin, REN Jian-feng, LI Wei-ming, ZHANG Qing-hua. Regulation of Gene mapk1 in Danio rerio on Gene tp53[J]. Biotechnology Bulletin, 2021, 37(12): 160-168.
| 体系System | 体积Volume |
|---|---|
| 上游引物Forward primer | 0.5 μL |
| 下游引物Reverse primer | 0.5 μL |
| DNA模板DNA template | 10 ng |
| 高保真酶Prime STAR® Max DNA polymerase | 12.5 μL |
| 灭菌水ddH2O | Up to 25 μL |
表1 PCR试剂及体系
Table 1 PCR reagents and systems
| 体系System | 体积Volume |
|---|---|
| 上游引物Forward primer | 0.5 μL |
| 下游引物Reverse primer | 0.5 μL |
| DNA模板DNA template | 10 ng |
| 高保真酶Prime STAR® Max DNA polymerase | 12.5 μL |
| 灭菌水ddH2O | Up to 25 μL |
| 引物Primer | 序列Sequence(5'-3') | 用途Usage |
|---|---|---|
| tp53-F1 | CCCATGATGTGGGGACACAA | 启动子序列扩增 Amplification of promoter sequences |
| tp53-R1 | TCAAACGATCCCGGCAAGTA | |
| tp53-F2 | CGGGGTACCCCCATGATGTGGGGACACAA | 报告基因载体构建 Construction of reporter gene vector |
| tp53-R2 | CCGCTCGAGTCAAACGATCCCGGCAAGTA | |
| mapk1-F1 | ATGGCGACAGCTGCGGTTTC | CDS序列扩增 Amplification of CDS sequences |
| mapk1-R1 | CTATGGTCTGTAGCCTGGCT | |
| mapk1-F2 | CCGGAATTCATGGCGACAGCTGCGGTTTC | 表达载体构建 Construction of expression vector |
| mapk1-F2 | CCGCTCGAGCTATGGTCTGTAGCCTGGCT |
表2 实验所用的引物序列
Table 2 Primer sequences used in the experiment
| 引物Primer | 序列Sequence(5'-3') | 用途Usage |
|---|---|---|
| tp53-F1 | CCCATGATGTGGGGACACAA | 启动子序列扩增 Amplification of promoter sequences |
| tp53-R1 | TCAAACGATCCCGGCAAGTA | |
| tp53-F2 | CGGGGTACCCCCATGATGTGGGGACACAA | 报告基因载体构建 Construction of reporter gene vector |
| tp53-R2 | CCGCTCGAGTCAAACGATCCCGGCAAGTA | |
| mapk1-F1 | ATGGCGACAGCTGCGGTTTC | CDS序列扩增 Amplification of CDS sequences |
| mapk1-R1 | CTATGGTCTGTAGCCTGGCT | |
| mapk1-F2 | CCGGAATTCATGGCGACAGCTGCGGTTTC | 表达载体构建 Construction of expression vector |
| mapk1-F2 | CCGCTCGAGCTATGGTCTGTAGCCTGGCT |
| 组别Group | pGL3-Enhancer/ng | pGL3-tp53-Luc/ng | phRL-T/ng | Opti-MEM/μL | Fu GENE HD/μL |
|---|---|---|---|---|---|
| pGL3-Enhancer | 200 | 0 | 10 | 50 | 0.63 |
| pGL3-tp53-Luc | 0 | 200 | 10 | 50 | 0.63 |
表3 检测tp53启动子报告基因活性的转染体系
Table 3 Transfection system for detecting tp53 promoter reporter gene activity
| 组别Group | pGL3-Enhancer/ng | pGL3-tp53-Luc/ng | phRL-T/ng | Opti-MEM/μL | Fu GENE HD/μL |
|---|---|---|---|---|---|
| pGL3-Enhancer | 200 | 0 | 10 | 50 | 0.63 |
| pGL3-tp53-Luc | 0 | 200 | 10 | 50 | 0.63 |
| 组别Group | pCMV-Tag2B-mapk1/ng | pCMV-Tag2B/ng | pGL3-tp53-Luc/ng | phRL-TK/ng | Opti-MEM/μL | Fu GENE HD/μL |
|---|---|---|---|---|---|---|
| 对照Control | 0 | 200 | 100 | 10 | 50 | 0.93 |
| 共转 Co-transfect | 200 | 0 | 100 | 10 | 50 | 0.93 |
表4 转染复合物配制表
Table 4 Formulation table of transfected compound
| 组别Group | pCMV-Tag2B-mapk1/ng | pCMV-Tag2B/ng | pGL3-tp53-Luc/ng | phRL-TK/ng | Opti-MEM/μL | Fu GENE HD/μL |
|---|---|---|---|---|---|---|
| 对照Control | 0 | 200 | 100 | 10 | 50 | 0.93 |
| 共转 Co-transfect | 200 | 0 | 100 | 10 | 50 | 0.93 |

图3 斑马鱼tp53启动子特征序列分析 黑色下划线的碱基为启动子区上下游引物;红色碱基为相对应的转录因子结合位点;7个蓝色碱基TATATAA为TATA-box;单独蓝色碱基A为转录起始位点;3个绿色碱基ATG为起始密码子
Fig. 3 Analysis of the characteristic sequence of the tp53 promoter in zebrafish The bases underlined in black are the upstream and downstream primers of the promoter region. The red bases are corresponding transcription factor binding sites. The seven blue bases TATATAA is TATA-box. Single blue base A is the transcription start site(TSS). The three green bases,ATG,are the starting codons
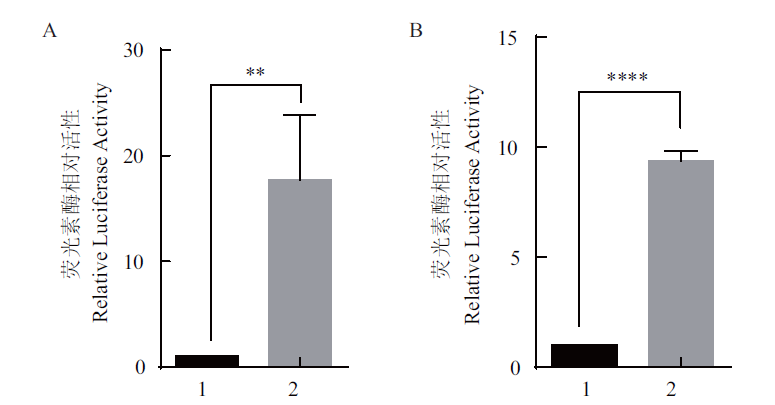
图6 pGL3-tp53-Luc活性 A:HEK293T 细胞;B:ZF4 细胞。1:pGL3-Enhancer,2:pGL3-tp53-Luc;误差用 mean ± SE 表示(n=3,** P<0.01,***P<0.001)
Fig. 6 Activity of pGL3-tp53-Luc A:HEK293T cells. B:ZF4 cells. 1:pGL3-Enhancer. 2:pGL3-tp53-Luc.The error is expressed as mean ± SE(n=3,** P<0.01,***P<0.001)
| 物种 Species | 序列来源 Sequence source | 一致性 Consistency/% |
|---|---|---|
| 人 Homo spaiens | NP_002736.3 | 91.33 |
| 小鼠Mus musculus | NP_001033752.1 | 90.79 |
表5 斑马鱼与人和小鼠mapk1基因氨基酸序列对比
Table 5 Comparison of amino acid sequences of mapk1 gene between zebrafish and human and mouse
| 物种 Species | 序列来源 Sequence source | 一致性 Consistency/% |
|---|---|---|
| 人 Homo spaiens | NP_002736.3 | 91.33 |
| 小鼠Mus musculus | NP_001033752.1 | 90.79 |
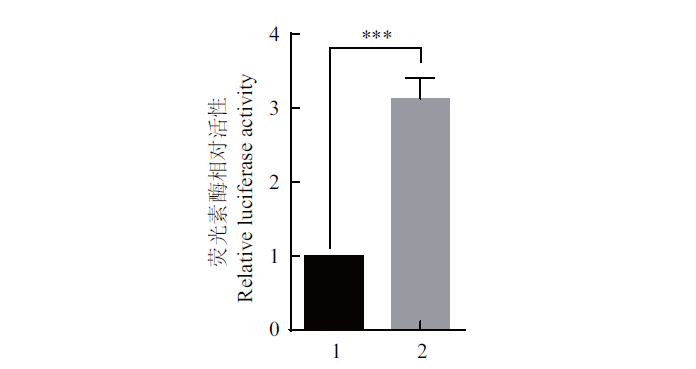
图8 pCMV-Tag2B-mapk1和pGL3-tp53-Luc共同转染 HEK293T 细胞后荧光素酶相对表达量 1:pCMV-Tag2B;2:共转;误差用 mean ± SE 表示(n=3,*** P<0.001)
Fig. 8 Relative expression of luciferase in the HEK293T cells co-transfected with pCMV-Tag2B-mapk1 and pGL3-tp53-Luc plasmids 1:pCMV-Tag2B. 2:Co-transfect. The error is expressed as mean ± SE(n=3,***P<0.001)
| [1] | 李帅, 张炳东. 细胞凋亡途径的研究进展[J]. 山东医药, 2017, 57(37):103-106. |
| Li S, Zhang BD. Research progress of apoptosis pathway[J]. Shandong Med J, 2017, 57(37):103-106. | |
| [2] | 施一公, 孙兵法. 细胞凋亡的结构生物学研究进展[J]. 生命科学, 2010, 22(3):224-228. |
| SHI YG, Sun BF. Mechanisms of programmed cell death through structural biology[J]. Chin Bull Life Sci, 2010, 22(3):224-228. | |
| [3] |
Bertram JS. The molecular biology of cancer[J]. Mol Aspects Med, 2000, 21(6):167-223.
pmid: 11173079 |
| [4] |
Gagliardi M, Pitner MK, Park J, et al. Differential functions of ERK1 and ERK2 in lung metastasis processes in triple-negative breast cancer[J]. Sci Rep, 2020, 10(1):8537.
doi: 10.1038/s41598-020-65250-3 pmid: 32444778 |
| [5] |
Roskoski R. Targeting ERK1/2 protein-serine/threonine kinases in human cancers[J]. Pharmacol Res, 2019, 142:151-168.
doi: S1043-6618(19)30086-6 pmid: 30794926 |
| [6] |
Vousden KH. p53:death star[J]. Cell, 2000, 103(5):691-694.
pmid: 11114324 |
| [7] |
Greenblatt MS, Bennett WP, Hollstein M, et al. Mutations in the p53 tumor suppressor gene:clues to cancer etiology and molecular pathogenesis[J]. Cancer Res, 1994, 54(18):4855-4878.
pmid: 8069852 |
| [8] |
Berghmans S, Murphey RD, Wienholds E, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors[J]. PNAS, 2005, 102(2):407-412.
pmid: 15630097 |
| [9] |
Bates S, Vousden KH. Mechanisms of p53-mediated apoptosis[J]. Cell Mol Life Sci, 1999, 55(1):28-37.
pmid: 10065149 |
| [10] |
Haupt S, Berger M, Goldberg Z, et al. Apoptosis - the p53 network[J]. J Cell Sci, 2003, 116(20):4077-4085.
doi: 10.1242/jcs.00739 URL |
| [11] | Cheng R, Ford BL, O’Neal PE, et al. Zebrafish(Danio rerio)p53 tumor suppressor gene:cDNA sequence and expression during embryogenesis[J]. Mol Mar Biol Biotechnol, 1997, 6(2):88-97. |
| [12] | 顾颖, 李延, 陈蔚丰, 等. 斑马鱼p53基因结构比对分析及其在生态毒理学中的应用[J]. 生态毒理学报, 2006, 1(1):45-51. |
| Gu Y, Li Y, Chen WF, et al. Structure alignments of zebrafish p53 gene and its application in ecotoxicology[J]. Asian J Ecotoxicol, 2006, 1(1):45-51. | |
| [13] | Storer NY, Zon LI. Zebrafish models of p53 functions[J]. Cold Spring Harb Perspect Biol, 2010, 2(8):a001123. |
| [14] |
Langheinrich U, Hennen E, Stott G, et al. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling[J]. Curr Biol, 2002, 12(23):2023-2028.
pmid: 12477391 |
| [15] |
Cohen P. Protein kinases——the major drug targets of the twenty-first century?[J]. Nat Rev Drug Discov, 2002, 1(4):309-315.
doi: 10.1038/nrd773 URL |
| [16] |
Shih A, Lin HY, Davis FB, et al. Thyroid hormone promotes serine phosphorylation of p53 by mitogen-activated protein kinase[J]. Biochemistry, 2001, 40(9):2870-2878.
pmid: 11258898 |
| [17] |
Li DW, Liu JP, Mao YW, et al. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation[J]. Mol Biol Cell, 2005, 16(9):4437-4453.
doi: 10.1091/mbc.e05-01-0010 URL |
| [18] | 曾文军, 王柳均, 樊翌明, 等. 细胞凋亡的流式细胞仪检测技术研究进展[J]. 医学文选, 2005(3):425-428. |
| Zeng WJ, Wang LJ, Fan YM, et al. Research progress of flow cytometry detection technology for cell apoptosis[J]. Anthol Med, 2005(3):425-428. | |
| [19] |
Vaux DL, Korsmeyer SJ. Cell death in development[J]. Cell, 1999, 96(2):245-254.
pmid: 9988219 |
| [20] |
Watanabe M, Naraba H, Sakyo T, et al. DNA damage-induced modulation of GLUT3 expression is mediated through p53-independent extracellular signal-regulated kinase signaling in HeLa cells[J]. Mol Cancer Res, 2010, 8(11):1547-1557.
doi: 10.1158/1541-7786.MCR-10-0011 pmid: 20870738 |
| [21] |
Pan HC, Jiang Q, Yu Y, et al. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells[J]. Neurochem Int, 2015, 80:60-71.
doi: 10.1016/j.neuint.2014.12.001 URL |
| [22] | Cha JH, Choi YJ, Cha SH, et al. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway[J]. Oncol Rep, 2012, 28(1):41-48. |
| [23] |
Persons DL, Yazlovitskaya EM, Pelling JC. Effect of extracellular signal-regulated kinase on p53 accumulation in response to cisplatin[J]. J Biol Chem, 2000, 275(46):35778-35785.
pmid: 10958792 |
| [24] |
Foletta VC. Transcription factor AP-1, and the role of Fra-2[J]. Immunol Cell Biol, 1996, 74(2):121-133.
pmid: 8723999 |
| [25] |
Black AR, Black JD, Azizkhan-Clifford J. Sp1 and krüppel-like factor family of transcription factors in cell growth regulation and cancer[J]. J Cell Physiol, 2001, 188(2):143-160.
pmid: 11424081 |
| [26] |
Yu Y, Zhang D, Huang H, et al. NF-κB1 p50 promotes p53 protein translation through miR-190 downregulation of PHLPP1[J]. Oncogene, 2014, 33(8):996-1005.
doi: 10.1038/onc.2013.8 pmid: 23396362 |
| [27] |
Eferl R, Ricci R, Kenner L, et al. Liver tumor development:c-Jun antagonizes the proapoptotic activity of p53[J]. Cell, 2003, 112(2):181-192.
doi: 10.1016/S0092-8674(03)00042-4 URL |
| [28] |
Qiu YY, Chen Y, Zeng TH, et al. Hypoxia-induced apoptosis and mitochondrial dysfunction in chondrocytes arising from CREB phosphorylation reduction[J]. Genet Mol Res, 2016, 15(2). DOI: 10.4238/gmr.15027755.
doi: 10.4238/gmr.15027755 |
| [29] |
Vogt PK. Fortuitous convergences:the beginnings of JUN[J]. Nat Rev Cancer, 2002, 2(6):465-469.
doi: 10.1038/nrc818 URL |
| [30] |
Dong Q, Jie YX, Ma J, et al. Renal tubular cell death and inflammation response are regulated by the MAPK-ERK-CREB signaling pathway under hypoxia-reoxygenation injury[J]. J Recept Signal Transduct, 2019, 39(5/6):383-391.
doi: 10.1080/10799893.2019.1698050 URL |
| [1] | 吴坤坤, 徐行, 季策, 任建峰, 李伟明, 张庆华. 斑马鱼notch3基因真核表达载体的构建及其表达分析[J]. 生物技术通报, 2022, 38(1): 179-186. |
| [2] | 徐琨, 杨爱江, 胡霞, 邹海洮, 李彬, 刘吉. 锑在斑马鱼不同组织中的积累及其对抗氧化系统的影响[J]. 生物技术通报, 2021, 37(4): 145-154. |
| [3] | 岳鑫, 杨爱江, 徐鹏, 胡霞, 朱桓毅, 包欣. 锑胁迫对斑马鱼酶活性的影响研究[J]. 生物技术通报, 2019, 35(6): 99-106. |
| [4] | 吕鹏, 徐嘉擎, 王森, 闫艳春. 杀菌剂苯并异噻唑啉酮对斑马鱼胚胎的急性毒性和氧化应激效应研究[J]. 生物技术通报, 2018, 34(1): 172-182. |
| [5] | 吴玉琼, 陈莹, 胡永乐, 左正宏, 王重刚. 四种新型农药对斑马鱼胚胎发育的毒性效应[J]. 生物技术通报, 2017, 33(6): 155-161. |
| [6] | 刘帅军,沈丹,钟继汉,陈伟,王伟,张丽,陈才,杨昆仑,高波,宋成义. SB转座子介导的斑马鱼增强子捕获注解分析[J]. 生物技术通报, 2017, 33(5): 153-158. |
| [7] | 林金杏, 杨迟, 冯丽萍, 胡建华. 斑马鱼嗜水气单胞菌的鉴定和人工感染组织病理学研究[J]. 生物技术通报, 2016, 32(9): 239-245. |
| [8] | 何丽番,高海. Mecp2在斑马鱼胚胎神经发育中对NOTCH信号通路的调控[J]. 生物技术通报, 2016, 32(4): 228-233. |
| [9] | 曹守莹, 白长存. PiggyBac转座系统在转mCherry基因斑马鱼上的应用[J]. 生物技术通报, 2016, 32(2): 185-191. |
| [10] | 杨川, 胡敏. 斑马鱼SFPQ蛋白的原核表达及纯化[J]. 生物技术通报, 2016, 32(1): 163-168. |
| [11] | 王冬梅, 谷从友, 刘铜, 张琼宇, 李培, 胡晓军. 诺氟沙星对斑马鱼胚胎发育的毒性作用及对TGF-β1的影响[J]. 生物技术通报, 2016, 32(1): 169-173. |
| [12] | 余凯敏, 冯为民, 李国超, 张家禹, 刘丽丽, 闫艳春. 毒死蜱的环境生物学效应分析[J]. 生物技术通报, 2015, 31(8): 225-230. |
| [13] | 李国超, 余凯敏, 冯为民, 刘丽丽, 张家禹, 闫艳春. 17β-雌二醇对斑马鱼性别分化的影响[J]. 生物技术通报, 2015, 31(6): 200-208. |
| [14] | 袁梦,何志旭,舒莉萍,袁家侃,刘丰,李燕. 基于CRISPR/Cas9系统对斑马鱼hoxb4基因的编辑及其突变体基因型的初筛[J]. 生物技术通报, 2015, 31(10): 249-254. |
| [15] | 陶然, 常玉梅, 梁利群, 唐然, 窦新杰, 王楠, 李明云. Tgf2转座子多克隆位点的克隆与表达[J]. 生物技术通报, 2014, 0(2): 124-129. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||