








生物技术通报 ›› 2022, Vol. 38 ›› Issue (1): 86-97.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0144
唐嘉城1( ), 梁毅珉1, 马葭思1, 彭桂香2, 谭志远1
), 梁毅珉1, 马葭思1, 彭桂香2, 谭志远1
收稿日期:2021-02-04
出版日期:2022-01-26
发布日期:2022-02-22
作者简介:唐嘉城,男,硕士研究生,研究方向:分子生物学;E-mail: 基金资助:
TANG Jia-cheng1( ), LIANG Yi-min1, MA Jia-si1, PENG Gui-xiang2, TAN Zhi-yuan1
), LIANG Yi-min1, MA Jia-si1, PENG Gui-xiang2, TAN Zhi-yuan1
Received:2021-02-04
Published:2022-01-26
Online:2022-02-22
摘要:
内生菌可为植物提供营养成分,也可以通过代谢产物促进植物生长,目前很少出现关于百香果(Passiflora edulia Sims)内生菌的研究。百香果是西番莲科西番莲属的一种草质藤本植物,主要生长于亚热带与热带地区,对其内生菌进行分离纯化,依据插入序列指纹图谱(IS-PCR)结果对所得菌株聚类,经16S rRNA基因测序并进行系统发育分析,之后对聚类得到的各类群代表菌株进行固氮酶活性、产生长素能力、溶磷能力、解钾能力、产蛋白酶能力与生理生化特性试验。共分离纯化得到51株百香果内生细菌,经聚类分为12个类群,分别属于Bacillus altitudinis、Bacillus circulans、Lysinibacillus macroides、Brevibacillus antibioticus、Paenibacillus illinoisensis、Microbacterium zeae、Rhizobium pusense、Beijerinckia fluminensis、Achromobacter mucicolens、Stenotrophomonas maltophilia、Klebsiella michiganensis和Klebsiella pneumoniae,对分离得到的内生菌进行促生特性试验,结果表明分离所得的百香果内生菌各类群代表菌株具有丰富的功能:其中10株具有分泌生长素的能力,5株具有固氮功能,6株具溶磷功能,7株具有解钾能力,3株具有分泌铁载体功能,5株具有产蛋白酶能力。本研究表明,从百香果中分离得到的内生菌不但具有种群多样性,而且具有功能多样性,这些具有不同特性的菌株有望应用在农业生产中以发挥其价值。
唐嘉城, 梁毅珉, 马葭思, 彭桂香, 谭志远. 百香果内生细菌多样性及促生长特性[J]. 生物技术通报, 2022, 38(1): 86-97.
TANG Jia-cheng, LIANG Yi-min, MA Jia-si, PENG Gui-xiang, TAN Zhi-yuan. Diversity and Growth Promotion of Endophytic Bacteria Isolated from Passiflora edulia Sims[J]. Biotechnology Bulletin, 2022, 38(1): 86-97.
| 类群 Group | 代表菌株(GenBank登录号) Representative strain(GenBank accession number) | 最相似菌株名称(GenBank登录号) Closely related strain(GenBank accession number) | 相似性 Similarity/% |
|---|---|---|---|
| Ⅰ | BXG95(MW714902) | Bacillus altitudinis 41KF2bT(NR_042337) | 98.20 |
| Ⅱ | BXG129(MW714912) | Bacillus circulans strain NBRC 13626T(NR_112632) | 97.99 |
| Ⅲ | BXG111(MW714903) | Lysinibacillus macroides strain LMG 18474T(NR_114920) | 98.42 |
| Ⅳ | BXG92(MW714901) | Brevibacillus antibioticus strain TGS2-1T(NR_165725) | 98.70 |
| Ⅴ | BXY92(MW714907) | Paenibacillus illinoisensis strain NBRC 15959T(NR_113828) | 97.49 |
| Ⅵ | BXJ201(MW714905) | Microbacterium zeae strain 1204T(NR_149816) | 97.61 |
| Ⅶ | BXG81(MW714904) | Rhizobium pusense strain NRCPB10T(NR_116874) | 99.00 |
| Ⅷ | BXG212(MW714911) | Beijerinckia fluminensis strain UQM 1685T(NR_116306) | 98.57 |
| Ⅸ | BXG101(MW714910) | Achromobacter mucicolens strain R-46658T(NR_117613) | 98.13 |
| Ⅹ | BXG201(MW714909) | Stenotrophomonas maltophilia strain ATCC 19861T(NR_040804) | 98.29 |
| Ⅺ | BXJ71(MW714906) | Klebsiella michiganensis strain W14T(NR_118335) | 97.13 |
| Ⅻ | BXG53(MW714908) | Klebsiella pneumoniae strain DSM 30104T(NR_117683) | 98.62 |
表2 百香果各类群代表菌株16S rRNA基因序列相似性比对结果
Table 2 Comparison results of 16S rRNA gene sequence similarity of representative strains of various groups of P. edulia Sims
| 类群 Group | 代表菌株(GenBank登录号) Representative strain(GenBank accession number) | 最相似菌株名称(GenBank登录号) Closely related strain(GenBank accession number) | 相似性 Similarity/% |
|---|---|---|---|
| Ⅰ | BXG95(MW714902) | Bacillus altitudinis 41KF2bT(NR_042337) | 98.20 |
| Ⅱ | BXG129(MW714912) | Bacillus circulans strain NBRC 13626T(NR_112632) | 97.99 |
| Ⅲ | BXG111(MW714903) | Lysinibacillus macroides strain LMG 18474T(NR_114920) | 98.42 |
| Ⅳ | BXG92(MW714901) | Brevibacillus antibioticus strain TGS2-1T(NR_165725) | 98.70 |
| Ⅴ | BXY92(MW714907) | Paenibacillus illinoisensis strain NBRC 15959T(NR_113828) | 97.49 |
| Ⅵ | BXJ201(MW714905) | Microbacterium zeae strain 1204T(NR_149816) | 97.61 |
| Ⅶ | BXG81(MW714904) | Rhizobium pusense strain NRCPB10T(NR_116874) | 99.00 |
| Ⅷ | BXG212(MW714911) | Beijerinckia fluminensis strain UQM 1685T(NR_116306) | 98.57 |
| Ⅸ | BXG101(MW714910) | Achromobacter mucicolens strain R-46658T(NR_117613) | 98.13 |
| Ⅹ | BXG201(MW714909) | Stenotrophomonas maltophilia strain ATCC 19861T(NR_040804) | 98.29 |
| Ⅺ | BXJ71(MW714906) | Klebsiella michiganensis strain W14T(NR_118335) | 97.13 |
| Ⅻ | BXG53(MW714908) | Klebsiella pneumoniae strain DSM 30104T(NR_117683) | 98.62 |
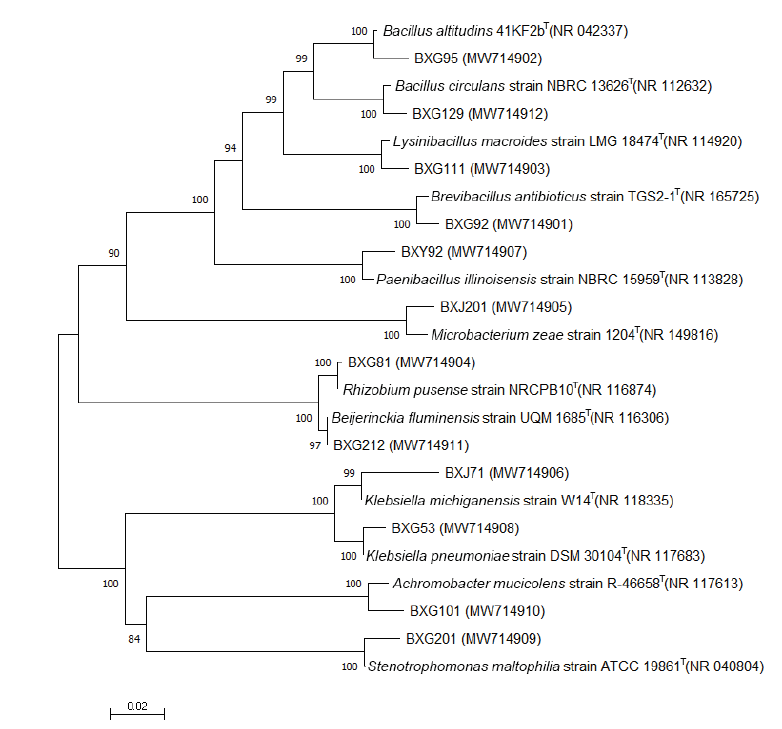
图3 各类群代表菌株16S rRNA基因序列系统发育树(邻接法) 设置步长检验次数为1 000次,分支处为bootstrap值,标尺代表核苷酸碱基差异为2%
Fig.3 Phylogenetic dendrogram of 16S rRNA gene sequences for representative strains(Neighbor-joining method) The number of random sampling calculations is 1 000,the numbers at nodes are bootstrap values(%),and the bar is 2% in nucleotide substitution
| 特性 Property | BXG95 | BXG129 | BXG111 | BXG92 | BXY92 | BXJ201 | BXG81 | BXG212 | BXG101 | BXG201 | BXJ71 | BXG53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 革兰氏染色 Gram stain | + | + | - | + | + | + | - | - | - | - | - | - |
| 过氧化氢酶 Catalase | + | + | + | + | + | + | + | + | + | + | + | + |
| 甲基红 Methyl red | + | + | - | + | + | + | + | + | - | - | - | + |
| 产氨 Ammonia production | - | + | - | + | - | + | + | + | + | + | + | + |
| 脲酶 Urease | - | + | - | - | - | + | + | + | + | + | - | + |
| 乙酰甲基甲醇实验 V.P. test | - | - | + | - | - | - | - | - | + | + | + | - |
| NO3-还原测定 Nitrate reduction | - | + | - | + | - | - | + | - | + | + | + | + |
| 明胶液化 Gelatin hydrolysis | + | + | - | - | + | - | - | - | - | + | + | - |
| 固氮酶活性 Nitrogenase activity /(nmol C2H4/(mL·h)) | 0 | 0 | 0 | 0 | 0 | 0 | 23.97±0.43 | 100.94±0.89 | 0 | 21.11±0.66 | 477.93±3.85 | 191.33±1.27 |
表3 各代表菌株生理生化特性
Table 3 Physiological and biochemical properties of the representative strains
| 特性 Property | BXG95 | BXG129 | BXG111 | BXG92 | BXY92 | BXJ201 | BXG81 | BXG212 | BXG101 | BXG201 | BXJ71 | BXG53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 革兰氏染色 Gram stain | + | + | - | + | + | + | - | - | - | - | - | - |
| 过氧化氢酶 Catalase | + | + | + | + | + | + | + | + | + | + | + | + |
| 甲基红 Methyl red | + | + | - | + | + | + | + | + | - | - | - | + |
| 产氨 Ammonia production | - | + | - | + | - | + | + | + | + | + | + | + |
| 脲酶 Urease | - | + | - | - | - | + | + | + | + | + | - | + |
| 乙酰甲基甲醇实验 V.P. test | - | - | + | - | - | - | - | - | + | + | + | - |
| NO3-还原测定 Nitrate reduction | - | + | - | + | - | - | + | - | + | + | + | + |
| 明胶液化 Gelatin hydrolysis | + | + | - | - | + | - | - | - | - | + | + | - |
| 固氮酶活性 Nitrogenase activity /(nmol C2H4/(mL·h)) | 0 | 0 | 0 | 0 | 0 | 0 | 23.97±0.43 | 100.94±0.89 | 0 | 21.11±0.66 | 477.93±3.85 | 191.33±1.27 |
| 特性Characteristics | BXG95 | BXG129 | BXG111 | BXG92 | BXY92 | BXJ201 | BXG81 | BXG212 | BXG101 | BXG201 | BXJ71 | BXG53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 可溶性磷含量 Soluble P content/(mg·L-1) | - | - | 9.75 | - | 12.46 | - | - | 12.93 | 13.64 | - | 12.38 | 23.47 |
| 可溶性钾含量 Soluble K content/(mg·L-1) | - | 22.4 | - | - | 45.2 | 42.7 | 29 | 23.6 | - | - | 41 | 65.6 |
| 生长素 IAA/(mg·L-1) | 24.32 | 28.71 | - | 3.36 | 12.38 | - | 7.55 | 8.91 | 20.97 | 11.46 | 14.85 | 17.13 |
| 产铁载体 Siderophore production | - | - | - | - | - | - | + | - | - | - | + | + |
| 产蛋白酶 Proteinase production | 3.61 | 1.97 | - | - | 2.68 | - | - | - | - | 3.60 | 2.36 | - |
表4 各代表菌株促生长特性
Table 4 Growth-promoting characteristics of representative strains
| 特性Characteristics | BXG95 | BXG129 | BXG111 | BXG92 | BXY92 | BXJ201 | BXG81 | BXG212 | BXG101 | BXG201 | BXJ71 | BXG53 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 可溶性磷含量 Soluble P content/(mg·L-1) | - | - | 9.75 | - | 12.46 | - | - | 12.93 | 13.64 | - | 12.38 | 23.47 |
| 可溶性钾含量 Soluble K content/(mg·L-1) | - | 22.4 | - | - | 45.2 | 42.7 | 29 | 23.6 | - | - | 41 | 65.6 |
| 生长素 IAA/(mg·L-1) | 24.32 | 28.71 | - | 3.36 | 12.38 | - | 7.55 | 8.91 | 20.97 | 11.46 | 14.85 | 17.13 |
| 产铁载体 Siderophore production | - | - | - | - | - | - | + | - | - | - | + | + |
| 产蛋白酶 Proteinase production | 3.61 | 1.97 | - | - | 2.68 | - | - | - | - | 3.60 | 2.36 | - |
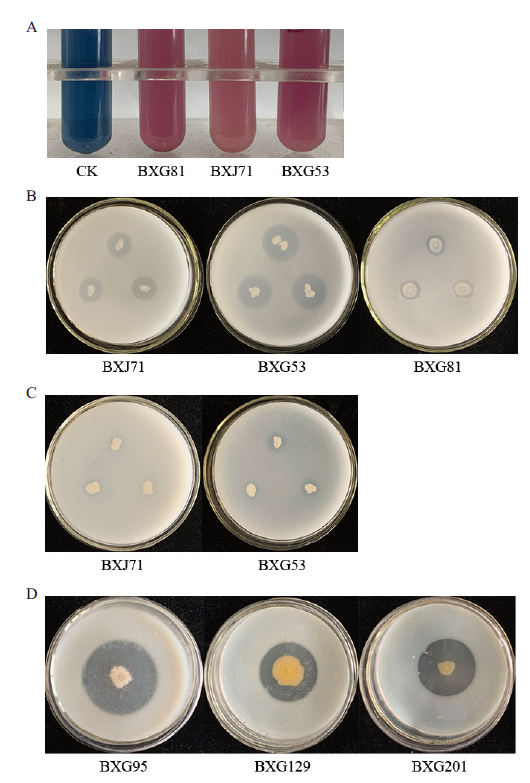
图5 部分代表菌株促生特性 A:产铁载体试验图;B:溶磷试验图;C:解钾试验图;D:产蛋白酶试验图
Fig.5 Growth-promoting characteristics of some repres-entative strains A:Siderophore production test chart. B:Soluble P test chart. C:Soluble K test chart. D:Protease production test chart
| [1] |
Hallmann J, Quadt-Hallmann A, Mahaffee WF, et al. Bacterial endophytes in agricultural crops[J]. Can J Microbiol, 1997, 43(10):895-914.
doi: 10.1139/m97-131 URL |
| [2] | 黄敬瑜, 张楚军, 姚瑜龙, 等. 植物内生菌生物抗菌活性物质研究进展[J]. 生物工程学报, 2017, 33(2):178-186. |
| Huang JY, Zhang CJ, Yao YL, et al. Progress in antimicrobial substances of endophytes[J]. Chin J Biotechnol, 2017, 33(2):178-186. | |
| [3] |
Hanada RE, Pomella AWV, Costa HS, et al. Endophytic fungal diversity in Theobroma cacao(cacao)and T. grandiflorum(cupuaçu)trees and their potential for growth promotion and biocontrol of black-pod disease[J]. Fungal Biol, 2010, 114(11/12):901-910.
doi: 10.1016/j.funbio.2010.08.006 URL |
| [4] |
Singh M, Kumar A, Singh R, et al. Endophytic bacteria:a new source of bioactive compounds[J]. 3 Biotech, 2017, 7(5):315.
doi: 10.1007/s13205-017-0942-z URL |
| [5] | 阳洁, 秦莹溪, 王晓甜, 等. 广西药用野生稻内生细菌多样性及促生作用[J]. 生态学杂志, 2015, 34(11):3094-3100. |
| Yang J, Qin YX, Wang XT, et al. Diversity and growth promotion of endophytic bacteria isolated from Oryza officinalis in Guangxi[J]. Chin J Ecol, 2015, 34(11):3094-3100. | |
| [6] | 刘丽辉, 蒋慧敏, 区宇程, 等. 南方野生稻内生细菌的分离鉴定及促生作用[J]. 应用与环境生物学报, 2020, 26(5):1051-1058. |
| Liu LH, Jiang HM, Ou YC, et al. Identification and growth promotion of endophytic bacteria isolated from Oryza meridionalis[J]. Chin J Appl Environ Biol, 2020, 26(5):1051-1058. | |
| [7] | 谭志远, 彭桂香, 徐培智, 等. 普通野生稻(Oryza rufipogon)内生固氮菌多样性及高固氮酶活性[J]. 科学通报, 2009, 54(13):1885-1893. |
| Tan ZY, Peng GX, Xu PZ, et al. Diversity of Endophytic Nitrogen-fixing Bacteria and High Nitrogenase Activity in Oryza rufipogon[J]. Chin Sci Bull, 2009, 54(13):1885-1893. | |
| [8] | 彭桂香, 王华荣, 张国霞, 等. 糖蜜草内生固氮菌IS-PCR和16S rRNA基因全序列分析[J]. 华南农业大学学报, 2005, 26(4):73-76. |
| Peng GX, Wang HR, Zhang GX, et al. Molecular study of endophytic nitrogen fixing bacteria isolated from Melinis minutiflora[J]. J South China Agric Univ, 2005, 26(4):73-76. | |
| [9] | 原红娟, 严慧, 杨芳, 等. 澳洲野生稻(Oryza australiensis)内生固氮菌的分子鉴定及发育分析[J]. 应用与环境生物学报, 2014, 20(4):571-577. |
| Yuan HJ, Yan H, Yang F, et al. Molecular characterization and phylogenetic analysis of endophytic nitrogenfixing bacteria in Oryza australiensis[J]. Chin J Appl Environ Biol, 2014, 20(4):571-577. | |
| [10] | 周德庆, 徐德强, 胡宝龙. 微生物学实验教程[M].3版. 北京: 高等教育出版社, 2013. |
| Zhou DQ, Xu DQ, Hu BL. Experimental microbiology[M]. Beijing: Higher Education Press, 2013. | |
| [11] |
Zehr JP, Mellon MT, Zani S. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase(nifH)genes[J]. Appl Environ Microbiol, 1998, 64(12):5067.
doi: 10.1128/AEM.64.12.5067-5067.1998 URL |
| [12] | 赵龙飞, 徐亚军, 曹冬建, 等. 溶磷性大豆根瘤内生菌的筛选、抗性及系统发育和促生[J]. 生态学报, 2015, 35(13):4425-4435. |
| Zhao LF, Xu YJ, Cao DJ, et al. Screening, resistance, phylogeny and growth promoting of phosphorus solubilizing bacteria isolated from soybean root nodules[J]. Acta Ecol Sin, 2015, 35(13):4425-4435. | |
| [13] | 冉广芬, 马海州, 孟瑞英, 等. 四苯硼钠—季铵盐容量法快速测钾[J]. 盐湖研究, 2009, 17(2):39-42. |
| Ran GF, Ma HZ, Meng RY, et al. Rapid determination of potassium content by sodium tetraphenylboron-quaternary ammonium salt volumetric method[J]. J Salt Lake Res, 2009, 17(2):39-42. | |
| [14] | Ripa FA, Cao WD, Tong S, et al. Assessment of plant growth promoting and abiotic stress tolerance properties of wheat endophytic fungi[J]. Biomed Res Int, 2019, 2019:6105865. |
| [15] |
Beckers B, Op De Beeck M, Weyens N, et al. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees[J]. Microbiome, 2017, 5(1):25.
doi: 10.1186/s40168-017-0241-2 pmid: 28231859 |
| [16] |
Shivaji S, Chaturvedi P, Suresh K, et al. Bacillus aerius sp. nov., Bacillus aerophilus sp. nov., Bacillus stratosphericus sp. nov. and Bacillus altitudinis sp. nov., isolated from cryogenic tubes used for collecting air samples from high altitudes[J]. Int J Syst Evol Microbiol, 2006, 56(7):1465-1473.
doi: 10.1099/ijs.0.64029-0 URL |
| [17] |
Baliyan N, Dhiman S, Dheeman S, et al. Optimization of indole-3-acetic acid using response surface methodology and its effect on vegetative growth of chickpea[J]. Rhizosphere, 2021, 17:100321.
doi: 10.1016/j.rhisph.2021.100321 URL |
| [18] |
Shida O, Takagi H, Kadowaki K, et al. Emended description of Paenibacillus amylolyticus and description of Paenibacillus illinoisensis sp. nov. and Paenibacillus chibensis sp. nov[J]. Int J Syst Bacteriol, 1997, 47(2):299-306.
pmid: 9103613 |
| [19] |
Ash C, Priest FG, Collins MD. Molecular identification of rRNA group 3 bacilli(Ash, Farrow, Wallbanks and Collins)using a PCR probe test[J]. Antonie Van Leeuwenhoek, 1993, 64(3/4):253-260.
doi: 10.1007/BF00873085 URL |
| [20] |
Liu D, Yang Q, Ge K, et al. Promotion of iron nutrition and growth on peanut by Paenibacillus illinoisensis and Bacillus sp. strains in calcareous soil[J]. Braz J Microbiol, 2017, 48(4):656-670.
doi: S1517-8382(16)30635-9 pmid: 28645648 |
| [21] |
Panday D, Schumann P, Das SK. Rhizobium pusense sp. nov., isolated from the rhizosphere of chickpea(Cicer arietinum L.)[J]. Int J Syst Evol Microbiol, 2011, 61(Pt 11):2632-2639.
doi: 10.1099/ijs.0.028407-0 URL |
| [22] |
Soberón-Chávez G, Nájera R. Isolation from soil of Rhizobium leguminosarum lacking symbiotic information[J]. Can J Microbiol, 1989, 35(4):464-468.
doi: 10.1139/m89-071 URL |
| [23] |
Quigley PE, Cunningham PJ, Hannah M, et al. Symbiotic effectiveness of Rhizobium leguminosarum bv. trifolii collected from pastures in south-western Victoria[J]. Aust J Exp Agric, 1997, 37(6):623.
doi: 10.1071/EA96089 URL |
| [24] |
Segovia L, Piñero D, Palacios R, et al. Genetic structure of a soil population of nonsymbiotic Rhizobium leguminosarum[J]. Appl Environ Microbiol, 1991, 57(2):426-433.
doi: 10.1128/aem.57.2.426-433.1991 URL |
| [25] | Yanni YG, Rizk RY, Corich V, et al. Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth[M]// Opportunities for Biological Nitrogen Fixation in Rice and Other Non-Legumes. Dordrecht:Springer Netherlands, 1997:99-114. |
| [26] |
Rosenblueth M, Martínez-Romero E. Rhizobium etli maize populations and their competitiveness for root colonization[J]. Arch Microbiol, 2004, 181(5):337-344.
pmid: 15024554 |
| [27] | 李艳梅, 王琼瑶, 涂卫国, 等. 镍胁迫下产铁载体细菌对花生的促生性[J]. 微生物学通报, 2017, 44(8):1882-1890. |
| Li YM, Wang QY, Tu WG, et al. Growth promoting activity of siderophore secreting bacteria for peanut plant under nickel stress[J]. Microbiol China, 2017, 44(8):1882-1890. | |
| [28] | 杨鸿儒, 袁博, 赵霞, 等. 三种荒漠灌木根际可培养固氮细菌类群及其固氮和产铁载体能力[J]. 微生物学通报, 2016, 43(11):2366-2373. |
| Yang HR, Yuan B, Zhao X, et al. Cultivable diazotrophic community in the rhizosphere of three desert shrubs and their nitrogen-fixation and siderophore-producing capabilities[J]. Microbiol China, 2016, 43(11):2366-2373. | |
| [29] | 毛得奖, 朱亚玲, 韩宁. 假单胞菌铁载体及色素研究[J]. 微生物学通报, 2013, 40(3):500-516. |
| Mao DJ, Zhu YL, Han N. Siderophores and pigments produced by Pseudomonas bacteria[J]. Microbiol China, 2013, 40(3):500-516. | |
| [30] |
Oggerin M, Arahal DR, Rubio V, et al. Identification of Beijerinckia fluminensis strains CIP 106281T and UQM 1685T as Rhizobium radiobacter strains, and proposal of Beijerinckia doebereinerae sp. nov. to accommodate Beijerinckia fluminensis LMG 2819[J]. INTERNATIONAL J SYSTEMATIC EVOLUTIONARY MICROBIOLOGY, 2009, 59(9):2323-2328.
doi: 10.1099/ijs.0.006593-0 URL |
| [31] | Ramanuj K, Shelat H. Plant growth promoting potential of bacterial endophytes from medicinal plants[J]. Adv Res, 2018, 13(6):1-15. |
| [32] |
Saha R, Farrance CE, Verghese B, et al. Klebsiella michiganensis sp. nov., A New Bacterium Isolated from a Tooth Brush Holder[J]. Curr Microbiol, 2013, 66(1):72-78.
doi: 10.1007/s00284-012-0245-x URL |
| [33] |
Palus JA, Borneman J, Ludden PW, et al. A diazotrophic bacterial endophyte isolated from stems of Zea mays L. and Zea luxurians Iltis and Doebley[J]. Plant Soil, 1996, 186(1):135-142.
doi: 10.1007/BF00035067 URL |
| [34] | 谭泽文, 谭志远, 黄慧灵, 等. 梧县药用野生稻内生固氮菌分离鉴定与系统发育分析[J]. 应用与环境生物学报, 2017, 23(4):622-627. |
| Tan ZW, Tan ZY, Huang HL, et al. Isolation and phylogenetic analysis of endophytic nitrogen-fixing bacteria from Oryza officinalis in Wuxian[J]. Chin J Appl Environ Biol, 2017, 23(4):622-627. | |
| [35] | 谭志远, 傅琴梅, 彭桂香, 等. 青香茅和五节芒内生固氮菌的分离与生理生化鉴定[J]. 应用与环境生物学报, 2013, 19(4):643-649. |
| Tan ZY, Fu QM, Peng GX, et al. Identification and characterization of endophytic diazotrophs isolated from Cymbopogon caesius and Miscanthus floridulus[J]. Chin J Appl Environ Biol, 2013, 19(4):643-649. | |
| [36] | 付思远, 席雨晴, 赵鹏菲, 等. 泓森槐可培养内生固氮细菌多样性与潜在促生长特性评价[J]. 微生物学通报, 2020, 47(8):2458-2470. |
| Fu SY, Xi YQ, Zhao PF, et al. Evaluating diversity and potential growth promoting characteristics of the culturable endophytic diazotrophic bacteria isolated from Robinia pseudoacacia ‘Hongsen’[J]. Microbiol China, 2020, 47(8):2458-2470. | |
| [37] | 龚凤娟, 恩特马克·布拉提白, 张宇凤, 等. 具有ACC脱氨酶活性的杜仲内生细菌的分离鉴定及其抗菌活性[J]. 微生物学通报, 2011, 38(10):1526-1532. |
| Gong FJ, Borrathybay Entomack, Zhang YF, et al. Isolation and antibacterial activity of ACC deaminase-containing endophytic bacteria from Eucommia ulmoides Oliver[J]. Microbiol China, 2011, 38(10):1526-1532. |
| [1] | 吴巧茵, 施友志, 李林林, 彭政, 谭再钰, 刘利平, 张娟, 潘勇. 类胡萝卜素降解菌株的原位筛选及其在雪茄提质增香中的应用[J]. 生物技术通报, 2023, 39(9): 192-201. |
| [2] | 方澜, 黎妍妍, 江健伟, 成胜, 孙正祥, 周燚. 盘龙参内生真菌胞内细菌7-2H的分离鉴定和促生特性研究[J]. 生物技术通报, 2023, 39(8): 272-282. |
| [3] | 王羽, 尹铭绅, 尹晓燕, 奚家勤, 杨建伟, 牛秋红. 烟草甲体内烟碱降解菌的筛选、鉴定及降解特性[J]. 生物技术通报, 2023, 39(6): 308-315. |
| [4] | 李善家, 雷雨昕, 孙梦格, 刘海锋, 王兴敏. 种子内生细菌多样性与植物互馈作用研究进展[J]. 生物技术通报, 2023, 39(4): 166-175. |
| [5] | 高晓蓉, 丁尧, 吕军. 芘降解菌Pseudomonas sp. PR3的植物促生特性及其对芘胁迫下水稻生长的影响[J]. 生物技术通报, 2022, 38(9): 226-236. |
| [6] | 张昊, 刘苗苗, 刘晓娜, 李宗谕, 赵丽丽, 杨清香. 内生菌影响药用植物产生药理活性化合物的研究进展[J]. 生物技术通报, 2022, 38(8): 41-51. |
| [7] | 徐重新, 仲建锋, 高美静, 卢莉娜, 刘贤金, 沈燕. 植物内生菌在食用农产品质量安全与营养品质调控中的研究进展[J]. 生物技术通报, 2022, 38(5): 215-227. |
| [8] | 杜佳慧, 徐伟芳, 杨晓冬, 谭松, 尹登科, 刘园旭. 多花黄精产吲哚乙酸内生菌的分离筛选及其对黄精种子萌发的影响[J]. 生物技术通报, 2022, 38(12): 223-232. |
| [9] | 伊帕热·帕尔哈提, 祖力胡玛尔·肉孜, 田永芝, 朱艳蕾, 李远婷, 马晓林. 荒漠植物内生菌多样性及其增强农作物抗旱和耐盐性的研究进展[J]. 生物技术通报, 2022, 38(12): 88-99. |
| [10] | 王志山, 黎妮, 王伟平, 刘洋. 水稻种子内生细菌研究进展[J]. 生物技术通报, 2022, 38(1): 236-246. |
| [11] | 王楠, 苏誉, 刘文杰, 封明, 毛瑜, 张新国. 植物内生菌中抗耐药微生物活性成分的研究进展[J]. 生物技术通报, 2021, 37(8): 263-274. |
| [12] | 梁振霆, 唐婷. 内生菌对植物次生代谢产物的生物合成影响和抗逆功能研究[J]. 生物技术通报, 2021, 37(8): 35-45. |
| [13] | 马勤, 雷瑞峰, 迪力热巴·阿不都肉苏力, 穆耶赛尔·奥斯曼, 祖力胡玛尔·肉孜, 安登第. 环境胁迫下内生菌与宿主代谢相互作用研究进展[J]. 生物技术通报, 2021, 37(3): 153-161. |
| [14] | 徐秀倩, 吴小芹, 吴天宇, 曾梦嫚. 林木根际细菌JYZ-SD5的促生抗逆性能及种类鉴定[J]. 生物技术通报, 2019, 35(3): 31-38. |
| [15] | 王霞, 薛林贵, 张晓华, 何小燕, 范桃桃, 尚海. 菘蓝内生细菌的分离、筛选和鉴定[J]. 生物技术通报, 2018, 34(3): 163-169. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||