








生物技术通报 ›› 2022, Vol. 38 ›› Issue (3): 246-255.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0642
张国宁1( ), 冯婧娴1, 杨颖博2, 陈万生1,3(
), 冯婧娴1, 杨颖博2, 陈万生1,3( ), 肖莹1(
), 肖莹1( )
)
收稿日期:2021-05-17
出版日期:2022-03-26
发布日期:2022-04-06
作者简介:张国宁,男,硕士研究生,研究方向:天然产物生物合成与代谢调控;E-mail: 基金资助:
ZHANG Guo-ning1( ), FENG Jing-xian1, YANG Ying-bo2, CHEN Wan-sheng1,3(
), FENG Jing-xian1, YANG Ying-bo2, CHEN Wan-sheng1,3( ), XIAO Ying1(
), XIAO Ying1( )
)
Received:2021-05-17
Published:2022-03-26
Online:2022-04-06
摘要:
环糊精葡萄糖基转移酶(cyclodextrin glucosyltransferase,CGTase)是一种可催化淀粉或多糖中α-1, 4键断裂并环化形成环糊精(cyclodextrins,CDs)的α-淀粉酶。CGTase在工业上主要用于制造环糊精,近年来利用其转糖基作用改造天然产物的性质取得了令人瞩目的研究进展,正成为CGTase应用颇有前途的发展方向。本文结合CGTase的来源、蛋白结构及催化机制等相关研究,重点介绍近年来CGTase在天然产物糖基化修饰中的应用,为CGTase在该领域的深入研究及应用提供参考。
张国宁, 冯婧娴, 杨颖博, 陈万生, 肖莹. 环糊精葡萄糖基转移酶在天然产物糖基化修饰中的应用[J]. 生物技术通报, 2022, 38(3): 246-255.
ZHANG Guo-ning, FENG Jing-xian, YANG Ying-bo, CHEN Wan-sheng, XIAO Ying. Application of Cyclodextrin Glucosyltransferase in the Glycosylation Modification of Natural Products[J]. Biotechnology Bulletin, 2022, 38(3): 246-255.
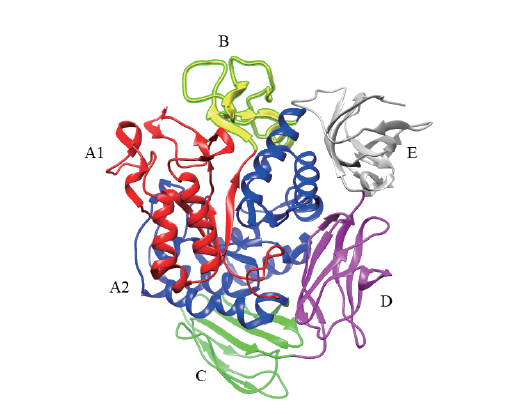
图1 CGTase蛋白结构(PDB登录码:4JCL) A1:红色;B:黄色;A2:蓝色;C:绿色;D:紫色;E:暗灰色
Fig. 1 CGTase protein structure(PDB accession number:4JCL) A1:Red. B:Yellow. A2:Blue. C:Green. D:Purple. E:Dark grey

图2 CGTase催化4种反应类型 A:环化反应;B:偶合反应;C:歧化反应;D:水解反应
Fig. 2 CGTase catalyzes four types of reactions A:Cyclization. B:Coupling. C:Disproportionation. D:Hydrolysis
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 木犀草素 | 黄酮类 | Bacillus circulans | α-环糊精 | 水溶性增加,增强了其在医疗实践中的应用 | [ |
| 柚皮苷 | 黄酮类 | Alkalophilic Bacillus sp | 麦芽煳精 | 溶解度提高1.0×103倍,扩大其在食品工业中的应用范围 | [ |
| 新橙皮苷 | 黄酮类 | Alkalophilic Bacillus | β-环糊精 | 溶解度提高1.5×103倍,扩大其在食品工业中的应用范围 | [ |
| 芦丁 | 黄酮醇类 | Bacillus macerans | 糊精 | 溶解度提高3×104倍 | [ |
| 苯并[H]喹唑啉类化合物 | 杂环化合物 | Thermophilic Bacterial | γ-环糊精 | 增高水溶性,提高制剂生物活性和生物利用度 | [ |
| 槐糖苷 | 异黄酮类 | Paenibacillus macerans | α-环糊精或麦芽糖 | 糖链增长水溶性增加,扩大在食品和制药行业中的应用范围 | [ |
| 槲皮素-3-葡萄糖;低聚葡萄糖基柚皮素-7-葡萄糖;低聚葡萄糖基橙皮素-7-葡萄糖 | 黄酮醇、二氢黄酮 | B. macerans | α-环糊精 α-cyclodextrin | 糖苷产物水溶性增强,扩大在食品和制药行业中的应用范围 | [ |
| (-)-表儿茶素(EC) | 黄烷-3醇类 | Paenibacillus sp. | β-环糊精(最佳);淀粉;麦芽七糖(G7) | 糖苷产物水溶性增强,扩大在食品行业中的应用范围 | [ |
| 没食子儿茶素没食子酸(EGCG) | 黄烷-3醇类 | Thermoanaerobacter sp | 淀粉 | 植物多酚糖基化,提高溶解度和生物利用度 | [ |
| 橙皮苷 | 二氢黄酮 | A. Bacillus | 可溶性淀粉 | 水溶性提高300倍,扩大应用范围 | [ |
| 葛根素 | 异黄酮 | Bacillus licheniformis | α-cyclodextrin | PU-G、PU-2G以及PU-3G与PU相比,溶解度分别增强16.5、100.9和179.1倍 | [ |
表1 CGTase糖基化修饰提高天然产物溶解度的应用
Table 1 Application of CGTase glycosylation modification to improve the solubility of natural products
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 木犀草素 | 黄酮类 | Bacillus circulans | α-环糊精 | 水溶性增加,增强了其在医疗实践中的应用 | [ |
| 柚皮苷 | 黄酮类 | Alkalophilic Bacillus sp | 麦芽煳精 | 溶解度提高1.0×103倍,扩大其在食品工业中的应用范围 | [ |
| 新橙皮苷 | 黄酮类 | Alkalophilic Bacillus | β-环糊精 | 溶解度提高1.5×103倍,扩大其在食品工业中的应用范围 | [ |
| 芦丁 | 黄酮醇类 | Bacillus macerans | 糊精 | 溶解度提高3×104倍 | [ |
| 苯并[H]喹唑啉类化合物 | 杂环化合物 | Thermophilic Bacterial | γ-环糊精 | 增高水溶性,提高制剂生物活性和生物利用度 | [ |
| 槐糖苷 | 异黄酮类 | Paenibacillus macerans | α-环糊精或麦芽糖 | 糖链增长水溶性增加,扩大在食品和制药行业中的应用范围 | [ |
| 槲皮素-3-葡萄糖;低聚葡萄糖基柚皮素-7-葡萄糖;低聚葡萄糖基橙皮素-7-葡萄糖 | 黄酮醇、二氢黄酮 | B. macerans | α-环糊精 α-cyclodextrin | 糖苷产物水溶性增强,扩大在食品和制药行业中的应用范围 | [ |
| (-)-表儿茶素(EC) | 黄烷-3醇类 | Paenibacillus sp. | β-环糊精(最佳);淀粉;麦芽七糖(G7) | 糖苷产物水溶性增强,扩大在食品行业中的应用范围 | [ |
| 没食子儿茶素没食子酸(EGCG) | 黄烷-3醇类 | Thermoanaerobacter sp | 淀粉 | 植物多酚糖基化,提高溶解度和生物利用度 | [ |
| 橙皮苷 | 二氢黄酮 | A. Bacillus | 可溶性淀粉 | 水溶性提高300倍,扩大应用范围 | [ |
| 葛根素 | 异黄酮 | Bacillus licheniformis | α-cyclodextrin | PU-G、PU-2G以及PU-3G与PU相比,溶解度分别增强16.5、100.9和179.1倍 | [ |
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 熊果苷arbutin | 苯酚类 | Bacillus macerans | 淀粉 | 对人酪氨酸酶抑制活性增强 | [ |
| α-生育酚-β-葡萄糖苷、δ-生育酚-β-葡萄糖苷 | 多酚类 | 不明确 | 淀粉 | 对大鼠腹膜肥大细胞产生IgE抗体和增强组胺释放抑制作用 | [ |
| 对苯二酚(HQ) | 苯酚类 | Thermoanaerobacter sp | 麦芽糊精 | 抗褐变能力增强 | [ |
| α-L-鼠李糖 | 糖类 | Bacillus circulans 251 | 麦芽糖糊精 | 可能应用于针对细菌性痢疾的合成候选疫苗 | [ |
| 槲皮素-3-葡萄糖;低聚葡萄糖基柚皮素-7-葡萄糖;低聚葡萄糖基橙皮素-7-葡萄糖 | 黄酮醇、二氢黄酮 | B. macerans | α-环糊精 | 对Cu2+氧化降解的抵抗能力大大增强 | [ |
| (+)儿茶素 | 黄烷-3醇类 | B. macerans | 淀粉 | 抑制蘑菇中酪氨酸酶的活性 | [ |
| 辣木提取物中的多酚类化合物 | 多酚类 | Trichoderma viride | 小麦淀粉 | 抗氧化和清除自由基能力增强 | [ |
| 月桂酸蔗糖 | 糖类 | 不明确 | 糊精 | 潜在的抗肿瘤和杀虫活性 | [ |
| 橙皮素苷(3'-, 5-,and 7-O-glucosides) | 二氢黄酮 | 不明确 | 淀粉 | 对IgE抗体和大鼠嗜中性白细胞产生O2具有抑制作用 | [ |
| 辣椒素的β葡萄糖苷 | 芳香烃类 | 不明确 | 淀粉 | 抑制IgE抗体的形成 | [ |
| 白藜芦醇 | 多酚类 | Thermoanaerobacter sp | 淀粉 | 具有表面活性剂性质 | [ |
| 水杨醇 | 酚类 | B. macerans | α-环糊精 | 促进吸收,并可作为温和有效的解热镇痛药前体 | [ |
| (-)-表儿茶素(EC) | 黄烷-3醇类 | Paenibacillus sp. | β-环糊精(最佳);淀粉;麦芽七糖(G7) | 糖苷产物抗紫外线褐变能力增强 | [ |
| 橙皮苷 | 二氢黄酮类 | Alkalophilic Bacillus | 可溶性淀粉 | 糖苷产物吸收紫外线以稳定食品中的色素 | [ |
| Anhydro-D-fructose | 糖类 | 不明确 | β-环糊精 | 降低了与牛血清白蛋白的氨基羰基反应活性 | [ |
| 1, 5-Anhydro-D-fructose | 糖类 | Bacillus stearothermophilus | β-环糊精 | 为提高食物蛋白溶解性和稳定性以及蛋白酶抗性提供新的手段 | [ |
| D-pinitol L-chiro-inositol D-chiro-Inositol muco-inositol allo-inositol | 脂环醇 | Thermoanaerobacter sp | β-环糊精 | 肌醇糖基化衍生物在细胞功能中具有多种生理作用 | [ |
| 肌醇(myoinositol) | Bacillus ohbensis. |
表2 CGTase糖基化修饰增强或改善天然产物生物活性的应用
Table 2 Application of CGTase glycosylation modification to improve the bioactivity of natural products
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 熊果苷arbutin | 苯酚类 | Bacillus macerans | 淀粉 | 对人酪氨酸酶抑制活性增强 | [ |
| α-生育酚-β-葡萄糖苷、δ-生育酚-β-葡萄糖苷 | 多酚类 | 不明确 | 淀粉 | 对大鼠腹膜肥大细胞产生IgE抗体和增强组胺释放抑制作用 | [ |
| 对苯二酚(HQ) | 苯酚类 | Thermoanaerobacter sp | 麦芽糊精 | 抗褐变能力增强 | [ |
| α-L-鼠李糖 | 糖类 | Bacillus circulans 251 | 麦芽糖糊精 | 可能应用于针对细菌性痢疾的合成候选疫苗 | [ |
| 槲皮素-3-葡萄糖;低聚葡萄糖基柚皮素-7-葡萄糖;低聚葡萄糖基橙皮素-7-葡萄糖 | 黄酮醇、二氢黄酮 | B. macerans | α-环糊精 | 对Cu2+氧化降解的抵抗能力大大增强 | [ |
| (+)儿茶素 | 黄烷-3醇类 | B. macerans | 淀粉 | 抑制蘑菇中酪氨酸酶的活性 | [ |
| 辣木提取物中的多酚类化合物 | 多酚类 | Trichoderma viride | 小麦淀粉 | 抗氧化和清除自由基能力增强 | [ |
| 月桂酸蔗糖 | 糖类 | 不明确 | 糊精 | 潜在的抗肿瘤和杀虫活性 | [ |
| 橙皮素苷(3'-, 5-,and 7-O-glucosides) | 二氢黄酮 | 不明确 | 淀粉 | 对IgE抗体和大鼠嗜中性白细胞产生O2具有抑制作用 | [ |
| 辣椒素的β葡萄糖苷 | 芳香烃类 | 不明确 | 淀粉 | 抑制IgE抗体的形成 | [ |
| 白藜芦醇 | 多酚类 | Thermoanaerobacter sp | 淀粉 | 具有表面活性剂性质 | [ |
| 水杨醇 | 酚类 | B. macerans | α-环糊精 | 促进吸收,并可作为温和有效的解热镇痛药前体 | [ |
| (-)-表儿茶素(EC) | 黄烷-3醇类 | Paenibacillus sp. | β-环糊精(最佳);淀粉;麦芽七糖(G7) | 糖苷产物抗紫外线褐变能力增强 | [ |
| 橙皮苷 | 二氢黄酮类 | Alkalophilic Bacillus | 可溶性淀粉 | 糖苷产物吸收紫外线以稳定食品中的色素 | [ |
| Anhydro-D-fructose | 糖类 | 不明确 | β-环糊精 | 降低了与牛血清白蛋白的氨基羰基反应活性 | [ |
| 1, 5-Anhydro-D-fructose | 糖类 | Bacillus stearothermophilus | β-环糊精 | 为提高食物蛋白溶解性和稳定性以及蛋白酶抗性提供新的手段 | [ |
| D-pinitol L-chiro-inositol D-chiro-Inositol muco-inositol allo-inositol | 脂环醇 | Thermoanaerobacter sp | β-环糊精 | 肌醇糖基化衍生物在细胞功能中具有多种生理作用 | [ |
| 肌醇(myoinositol) | Bacillus ohbensis. |
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 新橙皮苷 | 黄酮类 | Alkalophilic Bacillus | 可溶性淀粉 | 苦味降低10倍 | [ |
| Neohesperidin | Flavonoid | Soluble starch | 10 times lower of bitterness | ||
| 甜叶悬钩子苷 | 二萜类 | Bacillus circulans | 可溶性淀粉 | 甜度增加 | [ |
| Rubusoside | Diterpenoid | Soluble starch | Increased sweetness | ||
| 罗汉果V Mogroside V | 四环三萜类 Tetracyclic triterpenoid | Paenibacillus macerans Geobacillus sp. Thermoanaerobacter sp. | 麦芽糊精 Maltodextrin | 甜度增加 Increased sweetness | [ |
| 甘油 Glycerin | 脂肪醇 Fatty alcohol | Geobacillus(Bacillus)stearothermophilus(效果较好 Good effect) Thermoanaerobacter sp.(效果较好 Good effect)Bacillus circulans | 淀粉Starch | 有望应用于食品以改善口感It is expected to be used to improve the taste of food | [ |
表3 CGTase糖基化修饰改善天然产物口感的应用
Table 3 Application of CGTase glycosylation modification to improve the taste of natural products
| 底物 Substrate | 结构类型 Structure type | CGTase来源 CGTase source | 糖基供体 Glycosyl donor | 糖苷化产物应用 Application of glycosylation products | 参考文献 Reference |
|---|---|---|---|---|---|
| 新橙皮苷 | 黄酮类 | Alkalophilic Bacillus | 可溶性淀粉 | 苦味降低10倍 | [ |
| Neohesperidin | Flavonoid | Soluble starch | 10 times lower of bitterness | ||
| 甜叶悬钩子苷 | 二萜类 | Bacillus circulans | 可溶性淀粉 | 甜度增加 | [ |
| Rubusoside | Diterpenoid | Soluble starch | Increased sweetness | ||
| 罗汉果V Mogroside V | 四环三萜类 Tetracyclic triterpenoid | Paenibacillus macerans Geobacillus sp. Thermoanaerobacter sp. | 麦芽糊精 Maltodextrin | 甜度增加 Increased sweetness | [ |
| 甘油 Glycerin | 脂肪醇 Fatty alcohol | Geobacillus(Bacillus)stearothermophilus(效果较好 Good effect) Thermoanaerobacter sp.(效果较好 Good effect)Bacillus circulans | 淀粉Starch | 有望应用于食品以改善口感It is expected to be used to improve the taste of food | [ |
| [1] |
van de Manakker F, Vermonden T, van Nostrum CF, et al. Cyclodextrin-based polymeric materials:synjournal, properties, and pharmaceutical/biomedical applications[J]. Biomacromolecules, 2009, 10(12):3157-3175.
doi: 10.1021/bm901065f pmid: 19921854 |
| [2] |
Biwer A, Antranikian G, Heinzle E. Enzymatic production of cyclodextrins[J]. Appl Microbiol Biotechnol, 2002, 59(6):609-617.
pmid: 12226716 |
| [3] |
Sonnendecker C, Zimmermann W. Change of the product specificity of a cyclodextrin glucanotransferase by semi-rational mutagenesis to synthesize large-ring cyclodextrins[J]. Catalysts, 2019, 9(3):242.
doi: 10.3390/catal9030242 URL |
| [4] |
Han R, Li J, Shin HD, et al. Recent advances in discovery, heterologous expression, and molecular engineering of cyclodextrin glycosyltransferase for versatile applications[J]. Biotechnol Adv, 2014, 32(2):415-428.
doi: 10.1016/j.biotechadv.2013.12.004 URL |
| [5] |
Leemhuis H, Kelly RM, Dijkhuizen L. Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications[J]. Appl Microbiol Biotechnol, 2010, 85(4):823-835.
doi: 10.1007/s00253-009-2221-3 pmid: 19763564 |
| [6] |
Kim MH, Sohn CB, Oh TK. Cloning and sequencing of a cyclodextrin glycosyltransferase gene from Brevibacillus brevis CD162 and its expression in Escherichia coli[J]. FEMS Microbiol Lett, 1998, 164(2):411-418.
pmid: 9682490 |
| [7] |
Nazir S, Sulistyo J, Hashmi MI, et al. Enzymatic synjournal of polyphenol glycosides catalyzed by transglycosylation reaction of cyclodextrin glucanotransferase derived from Trichoderma viride[J]. J Food Sci Technol, 2018, 55(8):3026-3034.
doi: 10.1007/s13197-018-3223-x URL |
| [8] |
Bautista V, Esclapez J, Pérez-Pomares F, et al. Cyclodextrin glycosyltransferase:a key enzyme in the assimilation of starch by the halophilic archaeon Haloferax mediterranei[J]. Extremophiles, 2012, 16(1):147-159.
doi: 10.1007/s00792-011-0414-z pmid: 22134680 |
| [9] |
Lim CH, Rasti B, Sulistyo J, et al. Comprehensive study on transglycosylation of CGTase from various sources[J]. Heliyon, 2021, 7(2):e06305.
doi: 10.1016/j.heliyon.2021.e06305 URL |
| [10] |
Tachibana Y, Kuramura A, Shirasaka N, et al. Purification and characterization of an extremely thermostable cyclomaltodextrin glucanotransferase from a newly isolated hyperthermophilic archaeon, a Thermococcus sp[J]. Appl Environ Microbiol, 1999, 65(5):1991-1997.
doi: 10.1128/AEM.65.5.1991-1997.1999 URL |
| [11] |
Svensson B. Protein engineering in the alpha-amylase family:catalytic mechanism, substrate specificity, and stability[J]. Plant Mol Biol, 1994, 25(2):141-157.
pmid: 8018865 |
| [12] |
Klein C, Schulz GE. Structure of cyclodextrin glycosyltransferase refined at 2. 0 A resolution[J]. J Mol Biol, 1991, 217(4):737-750.
pmid: 1826034 |
| [13] |
Wind RD, Buitelaar RM, Dijkhuizen L. Engineering of factors determining alpha-amylase and cyclodextrin glycosyltransferase specificity in the cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1[J]. Eur J Biochem, 1998, 253(3):598-605.
pmid: 9654055 |
| [14] |
Janeček Š. Parallel β/α-barrels of α-amylase, cyclodextrin glycosyltransferase and oligo-1, 6-glucosidase versus the barrel of β-amylase:Evolutionary distance is a reflection of unrelated sequences[J]. FEBS Lett, 1994, 353(2):119-123.
doi: 10.1016/0014-5793(94)01019-6 URL |
| [15] |
Sonnendecker C, Zimmermann W. Domain shuffling of cyclodextrin glucanotransferases for tailored product specificity and thermal stability[J]. FEBS Open Bio, 2019, 9(2):384-395.
doi: 10.1002/2211-5463.12588 pmid: 30761262 |
| [16] |
Leemhuis H, Rozeboom HJ, Dijkstra BW, et al. Improved thermostability of Bacillus circulans cyclodextrin glycosyltransferase by the introduction of a salt bridge[J]. Proteins, 2004, 54(1):128-134.
doi: 10.1002/prot.10516 URL |
| [17] |
Knegtel RM, Strokopytov B, Penninga D, et al. Crystallographic studies of the interaction of cyclodextrin glycosyltransferase from Bacillus circulans strain 251 with natural substrates and products[J]. J Biol Chem, 1995, 270(49):29256-29264.
doi: 10.1074/jbc.270.49.29256 pmid: 7493956 |
| [18] |
Rimphanitchayakit V, Tonozuka T, Sakano Y. Construction of chimeric cyclodextrin glucanotransferases from Bacillus circulans A11 and Paenibacillus macerans IAM1243 and analysis of their product specificity[J]. Carbohydr Res, 2005, 340(14):2279-2289.
doi: 10.1016/j.carres.2005.07.013 URL |
| [19] |
Zhou J, Feng Z, Liu S, et al. CGTase, a novel antimicrobial protein from Bacillus cereus YUPP-10, suppresses Verticillium dahliae and mediates plant defence responses[J]. Mol Plant Pathol, 2021, 22(1):130-144.
doi: 10.1111/mpp.13014 URL |
| [20] |
Li Z, Huang M, Gu Z, et al. Asp577 mutations enhance the catalytic efficiency of cyclodextrin glycosyltransferase from Bacillus circulans[J]. Int J Biol Macromol, 2016, 83:111-116.
doi: 10.1016/j.ijbiomac.2015.11.042 URL |
| [21] |
Wang L, Duan XG, Wu J. Enhancing the α-cyclodextrin specificity of cyclodextrin glycosyltransferase from Paenibacillus macerans by mutagenesis masking subsite -7[J]. Appl Environ Microbiol, 2016, 82(8):2247-2255.
doi: 10.1128/AEM.03535-15 URL |
| [22] |
Li ZF, Ban XF, Gu ZB, et al. Mutations enhance β-cyclodextrin specificity of cyclodextrin glycosyltransferase from Bacillus circulans[J]. Carbohydr Polym, 2014, 108:112-117.
doi: 10.1016/j.carbpol.2014.03.015 URL |
| [23] |
Chen FJ, Xie T, Yue Y, et al. Molecular dynamic analysis of mutant Y195I α-cyclodextrin glycosyltransferase with switched product specificity from α-cyclodextrin to γ-cyclodextrin[J]. J Mol Modeling, 2015, 21(8):1-9.
doi: 10.1007/s00894-014-2561-5 URL |
| [24] | Tao X, Wang T, Su L, et al. Enhanced 2- o-alpha-d-glucopyranosyl-l-ascorbic acid synjournal through iterative saturation mutagenesis of acceptor subsite residues in Bacillus stearothermophilus NO2 cyclodextrin glycosyltransferase. J Agric Food Chem[J], 2018, 66(34):9052-9060. |
| [25] |
Han R, Liu L, Shin HD, et al. Iterative saturation mutagenesis of -6 subsite residues in cyclodextrin glycosyltransferase from Paenibacillus macerans to improve maltodextrin specificity for 2-O-D-glucopyranosyl-L-ascorbic acid synjournal[J]. Appl Environ Microbiol, 2013, 79(24):7562-7568.
doi: 10.1128/AEM.02918-13 URL |
| [26] |
Gudiminchi RK, Towns A, Varalwar S, et al. Enhanced synjournal of 2-O-α-d-glucopyranosyl-l-ascorbic acid from α-cyclodextrin by a highly disproportionating CGTase[J]. ACS Catal, 2016, 6(3):1606-1615.
doi: 10.1021/acscatal.5b02108 URL |
| [27] |
Uitdehaag JCM, van der Veen BA, Dijkhuizen L, et al. Catalytic mechanism and product specificity of cyclodextrin glycosyltransferase, a prototypical transglycosylase from the α-amylase family[J]. Enzyme Microb Technol, 2002, 30(3):295-304.
doi: 10.1016/S0141-0229(01)00498-7 URL |
| [28] |
Strokopytov B, Penninga D, Rozeboom HJ, et al. X-ray structure of cyclodextrin glycosyltransferase complexed with acarbose. implications for the catalytic mechanism of glycosidases[J]. Biochemistry, 1995, 34(7):2234-2240.
pmid: 7857935 |
| [29] |
Uitdehaag JC, Mosi R, Kalk KH, et al. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the alpha-amylase family[J]. Nat Struct Biol, 1999, 6(5):432-436.
pmid: 10331869 |
| [30] |
Choung WJ, Hwang SH, Ko DS, et al. Enzymatic synjournal of a novel kaempferol-3-O-β-d-glucopyranosyl-(1→4)-O-α-d-glucopyranoside using cyclodextrin glucanotransferase and its inhibitory effects on aldose reductase, inflammation, and oxidative stress[J]. J Agric Food Chem, 2017, 65(13):2760-2767.
doi: 10.1021/acs.jafc.7b00501 URL |
| [31] |
Marié T, Willig G, Teixeira ARS, et al. Enzymatic synjournal of resveratrol α-glycosides from β-cyclodextrin-resveratrol complex in water[J]. ACS Sustainable Chem Eng, 2018, 6(4):5370-5380.
doi: 10.1021/acssuschemeng.8b00176 URL |
| [32] | Radu O L, Armand S, Lenouvel F. La glycosylation de la luteoline, en milieux de solventes organiques, par l’action catalytique de la cyclodextrine glycosyltransferase de Bacillus circulans. Revue Roumaine de Chimie[J], 2006, 51(2):147-152. |
| [33] |
Kometani T, Nishimura T, Nakae T, et al. Synjournal of neohesperidin glycosides and naringin glycosides by cyclodextrin glucanotransferase from an alkalophilic Bacillus species[J]. Biosci Biotechnol Biochem, 1996, 60(4):645-649.
doi: 10.1271/bbb.60.645 URL |
| [34] |
Suzuki Y, Suzuki K. Enzymatic formation of 4G-alpha-D-glucopyranosyl-rutin[J]. Agric Biol Chem, 1991, 55(1):181-187.
pmid: 1368662 |
| [35] |
Markosyan AA, Abelyan LA, Markosyan AI, et al. Transglycosylation of benzo[h]quinazolines[J]. Appl Biochem Microbiol, 2009, 45(2):130-136.
doi: 10.1134/S0003683809020033 URL |
| [36] | Han RZ, Ni J, Zhou JY, et al. Engineering of cyclodextrin glycosyltransferase reveals pH-regulated mechanism of enhanced long-chain glycosylated sophoricoside specificity[J]. Appl Environ Microbiol, 2020, 86(7):e00004-20. |
| [37] |
Lee YS, Woo JB, Ryu SI, et al. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability[J]. Food Chem, 2017, 229:75-83.
doi: 10.1016/j.foodchem.2017.02.057 URL |
| [38] |
Aramsangtienchai P, Chavasiri W, Ito K, et al. Synjournal of epicatechin glucosides by a β-cyclodextrin glycosyltransferase[J]. J Mol Catal B:Enzym, 2011, 73(1/2/3/4):27-34.
doi: 10.1016/j.molcatb.2011.07.013 URL |
| [39] |
Gonzalez-Alfonso JL, Leemans L, Poveda A, et al. Efficient α-glucosylation of epigallocatechin gallate catalyzed by cyclodextrin glucanotransferase from Thermoanaerobacter species[J]. J Agric Food Chem, 2018, 66(28):7402-7408.
doi: 10.1021/acs.jafc.8b02143 URL |
| [40] |
Kometani T, Terada Y, Nishimura T, et al. Transglycosylation to hesperidin by cyclodextrin glucanotransferase from an alkalophilic Bacillus species in alkaline pH and properties of hesperidin glycosides[J]. Biosci Biotechnol Biochem, 1994, 58(11):1990-1994.
doi: 10.1271/bbb.58.1990 URL |
| [41] |
Huang W, He Q, Zhou ZR, et al. Enzymatic synjournal of puerarin glucosides using cyclodextrin glucanotransferase with enhanced antiosteoporosis activity[J]. ACS Omega, 2020, 5(21):12251-12258.
doi: 10.1021/acsomega.0c00950 pmid: 32548408 |
| [42] |
Sugimoto K, Nishimura T, Nomura K, et al. Syntheses of arbutin-alpha-glycosides and a comparison of their inhibitory effects with those of alpha-arbutin and arbutin on human tyrosinase[J]. Chem Pharm Bull:Tokyo, 2003, 51(7):798-801.
doi: 10.1248/cpb.51.798 URL |
| [43] |
Shimoda K, Akagi M, Hamada H. Production of β-maltooligosaccharides of α- and δ-tocopherols by Klebsiella pneumoniae and cyclodextrin glucanotransferase as anti-allergic agents[J]. Molecules, 2009, 14(8):3106-3114.
doi: 10.3390/molecules14083106 pmid: 19701147 |
| [44] |
Mathew S, Adlercreutz P. Regioselective glycosylation of hydroquinone to α-arbutin by cyclodextrin glucanotransferase from Thermoanaerobacter sp[J]. Biochem Eng J, 2013, 79:187-193.
doi: 10.1016/j.bej.2013.08.001 URL |
| [45] |
Kitao S, Sekine H. Α-D-glucosyl transfer to phenolic compounds by sucrose phosphorylase from Leuconostoc mesenteroides and production of α-arbutin[J]. Biosci Biotechnol Biochem, 1994, 58(1):38-42.
doi: 10.1271/bbb.58.38 URL |
| [46] |
Urbach C, Halila S, Guerreiro C, et al. CGTase-catalysed Cis-glucosylation of L-rhamnosides for the preparation of Shigella flexneri 2a and 3a haptens[J]. Chembiochem, 2014, 15(2):293-300.
doi: 10.1002/cbic.201300597 URL |
| [47] |
Funayama M, Nishino T, Hirota A, et al. Enzymatic synjournal of(+)catechin-α-glucoside and its effect on tyrosinase activity[J]. Biosci Biotechnol Biochem, 1993, 57(10):1666-1669.
doi: 10.1271/bbb.57.1666 URL |
| [48] |
Okada K, Zhao HS, Izumi M, et al. Glucosylation of sucrose laurate with cyclodextrin glucanotransferase[J]. Biosci Biotechnol Biochem, 2007, 71(3):826-829.
doi: 10.1271/bbb.60646 URL |
| [49] |
Shimoda K, Hamada H. Production of hesperetin glycosides by Xanthomonas campestris and cyclodextrin glucanotransferase and their anti-allergic activities[J]. Nutrients, 2010, 2(2):171-180.
doi: 10.3390/nu2020171 pmid: 22254014 |
| [50] |
Shimoda K, Kubota N, Akagi M. Synjournal of capsaicin oligosaccharides and their anti-allergic activity—synjournal of capsaicin oligosaccharides as anti-allergic food-additives[J]. Adv Chem Eng Sci, 2012, 2(1):45-49.
doi: 10.4236/aces.2012.21006 URL |
| [51] |
Torres P, Poveda A, Jimenez-Barbero J, et al. Enzymatic synjournal of α-glucosides of resveratrol with surfactant activity[J]. Adv Synth Catal, 2011, 353(7):1077-1086.
doi: 10.1002/adsc.201000968 URL |
| [52] |
Yoon SH, Bruce Fulton D, Robyt JF. Enzymatic synjournal of two salicin analogues by reaction of salicyl alcohol with Bacillus macerans cyclomaltodextrin glucanyltransferase and Leuconostoc mesenteroides B-742CB dextransucrase[J]. Carbohydr Res, 2004, 339(8):1517-1529.
doi: 10.1016/j.carres.2004.03.018 URL |
| [53] |
Yoshinaga K, Abe J, Tanimoto T, et al. Preparation and reactivity of a novel disaccharide, glucosyl 1, 5-anhydro-D-fructose(1, 5-anhydro-3-O-alpha-glucopyranosyl-D-fructose)[J]. Carbohydr Res, 2003, 338(21):2221-2225.
doi: 10.1016/S0008-6215(03)00341-0 URL |
| [54] |
Yoshinaga K, Abe J, Tanimoto T, et al. Preparation and reactivity of a novel disaccharide, glucosyl 1, 5-anhydro-D-fructose(1, 5-anhydro-3-O-alpha-glucopyranosyl-D-fructose)[J]. Carbohydr Res, 2003, 338(21):2221-2225.
doi: 10.1016/S0008-6215(03)00341-0 URL |
| [55] |
Miranda-Molina A, Marquina-Bahena S, López-Munguía A, et al. Regioselective glucosylation of inositols catalyzed by Thermoanaerobacter sp. CGTase[J]. Carbohydr Res, 2012, 360:93-101.
doi: 10.1016/j.carres.2012.08.002 URL |
| [56] |
Jaitak V, Kaul VK, Bandna, et al. Simple and efficient enzymatic transglycosylation of stevioside by β-cyclodextrin glucanotransferase from Bacillus firmus[J]. Biotechnol Lett, 2009, 31(9):1415-1420.
doi: 10.1007/s10529-009-0020-7 pmid: 19466564 |
| [57] |
Abelyan VA, Balayan AM, Ghochikyan VT, et al. Transglycosylation of stevioside by cyclodextrin glucanotransferases of various groups of microorganisms[J]. Appl Biochem Microbiol, 2004, 40(2):129-134.
doi: 10.1023/B:ABIM.0000018914.08571.50 URL |
| [58] |
Yu XJ, Yang JS, Li BZ, et al. High efficiency transformation of stevioside into a single mono-glycosylated product using a cyclodextrin glucanotransferase from Paenibacillus sp. CGMCC 5316[J]. World J Microbiol Biotechnol, 2015, 31(12):1983-1991.
doi: 10.1007/s11274-015-1947-6 URL |
| [59] |
Kim YH, Lee YG, Choi KJ, et al. Transglycosylation to ginseng saponins by cyclomaltodextrin glucanotransferases[J]. Biosci Biotechnol Biochem, 2001, 65(4):875-883.
doi: 10.1271/bbb.65.875 URL |
| [60] |
Ohtani K, Aikawa Y, Ishikawa H, et al. Further study on the 1, 4-. ALPHA. -transglucosylation of rubusoside, a sweet steviol-bisglucoside from Rubus suavissimus[J]. Agric Biol Chem, 1991, 55(2):449-453.
pmid: 1368695 |
| [61] |
Muñoz-Labrador A, Azcarate S, Lebrón-Aguilar R, et al. High-yield synjournal of transglycosylated mogrosides improves the flavor profile of monk fruit extract sweeteners[J]. J Agric Food Chem, 2021, 69(3):1011-1019.
doi: 10.1021/acs.jafc.0c07267 URL |
| [62] |
Yoshikawa S, Murata Y, Sugiura M, et al. Transglycosylation of mogroside V, a triterpene glycoside in Siraitia grosvenori, by cyclodextrin glucanotransferase and improvement of the qualities of sweetness[J]. J Appl Glycosci, 2005, 52(3):247-252.
doi: 10.5458/jag.52.247 URL |
| [63] |
Nakano H, Kiso T, Okamoto K, et al. Synjournal of glycosyl glycerol by cyclodextrin glucanotransferases[J]. J Biosci Bioeng, 2003, 95(6):583-588.
doi: 10.1016/S1389-1723(03)80166-4 URL |
| [64] |
Han R, Liu L, Shin HD, et al. Site-saturation engineering of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans to enhance substrate specificity towards maltodextrin for enzymatic synjournal of 2-O-D-glucopyranosyl-L-ascorbic acid(AA-2G)[J]. Appl Microbiol Biotechnol, 2013, 97(13):5851-5860.
doi: 10.1007/s00253-012-4514-1 URL |
| [65] |
Han RZ, Liu L, Li JH, et al. Functions, applications and production of 2-O-d-glucopyranosyl-l-ascorbic acid[J]. Appl Microbiol Biotechnol, 2012, 95(2):313-320.
doi: 10.1007/s00253-012-4150-9 URL |
| [66] |
Martı́n MT, Angeles Cruces M, Alcalde M, et al. Synjournal of maltooligosyl fructofuranosides catalyzed by immobilized cyclodextrin glucosyltransferase using starch as donor[J]. Tetrahedron, 2004, 60(3):529-534.
doi: 10.1016/j.tet.2003.10.113 URL |
| [67] |
Moon S, Lee H, Mathiyalagan R, et al. Synjournal of a novel α-glucosyl ginsenoside F1 by cyclodextrin glucanotransferase and its in vi-tro cosmetic applications[J]. Biomolecules, 2018, 8(4):142.
doi: 10.3390/biom8040142 URL |
| [68] |
Tao XM, Su LQ, Wang L, et al. Improved production of cyclodextrin glycosyltransferase from Bacillus stearothermophilus NO2 in Escherichia coli via directed evolution[J]. Appl Microbiol Biotechnol, 2020, 104(1):173-185.
doi: 10.1007/s00253-019-10249-8 URL |
| [69] |
Chen S, Xiong Y, Su L, et al. Position 228 in Paenibacillus macerans cyclodextrin glycosyltransferase is critical for 2-O-d-glucopyranosyl-l-ascorbic acid synjournal[J]. J Biotechnol, 2017, 247:18-24.
doi: 10.1016/j.jbiotec.2017.02.011 URL |
| [1] | 周璐祺, 崔婷茹, 郝楠, 赵雨薇, 赵斌, 刘颖超. 化学蛋白质组学在天然产物分子靶标鉴定中的应用[J]. 生物技术通报, 2023, 39(9): 12-26. |
| [2] | 周闪闪, 黄远龙, 黄建忠, 李善仁. 溶杆菌中活性天然产物的研究进展[J]. 生物技术通报, 2023, 39(10): 41-49. |
| [3] | 刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105. |
| [4] | 成温玉, 张博昕, 赵鸿远, 陈艳, 谢娟平. 天然产物抗猪流行性腹泻病毒研究进展[J]. 生物技术通报, 2022, 38(12): 127-136. |
| [5] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [6] | 赵鸿远, 王朝, 成温玉, 马宁宁, 李曼, 魏小丽. 抗非洲猪瘟病毒制剂的研究进展[J]. 生物技术通报, 2021, 37(5): 174-181. |
| [7] | 陈鹏. 活性天然产物蛋白靶点的快速筛选策略[J]. 生物技术通报, 2020, 36(11): 180-187. |
| [8] | 崔红利, 陈军, 侯义龙, 吴海歌, 秦松. 真核微藻蓝光受体及其功能研究进展[J]. 生物技术通报, 2017, 33(4): 51-62. |
| [9] | 申晓林, 袁其朋. 生物合成芳香族氨基酸及其衍生物的研究进展[J]. 生物技术通报, 2017, 33(1): 24-34. |
| [10] | 王丽苹, 罗云孜. 合成生物学在天然产物研究中的应用[J]. 生物技术通报, 2017, 33(1): 35-47. |
| [11] | 匡雪君, 邹丽秋, 孙超, 陈士林. 天然产物合成生物学体系的优化策略[J]. 生物技术通报, 2017, 33(1): 48-57. |
| [12] | 杨玉路, 王蕾, 陈晟, 吴敬. 重组β-环糊精葡萄糖基转移酶生产β-环糊精的工艺条件优化[J]. 生物技术通报, 2014, 0(8): 175-181. |
| [13] | 王慧, 罗成波, 贺纛, 孔令萍, 周碧君, 文明, 程振涛, 王开功. 绵羊肺炎支原体DnaK B细胞抗原表位预测及其蛋白结构分析[J]. 生物技术通报, 2014, 0(3): 159-164. |
| [14] | 吕学泽;梁玉荣;贺云霞;张培君;龚玉梅;王宏俊;. 副鸡禽杆菌aroA基因克隆及序列分析[J]. , 2012, 0(08): 83-87. |
| [15] | 邓会群;王惠利;杨红;洪华珠;李爱英;. 天然产物的C-糖基化研究进展[J]. , 2009, 0(05): 27-30. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||