








生物技术通报 ›› 2023, Vol. 39 ›› Issue (9): 12-26.doi: 10.13560/j.cnki.biotech.bull.1985.2023-0188
周璐祺1( ), 崔婷茹2, 郝楠1, 赵雨薇1, 赵斌1(
), 崔婷茹2, 郝楠1, 赵雨薇1, 赵斌1( ), 刘颖超1(
), 刘颖超1( )
)
收稿日期:2023-03-03
出版日期:2023-09-26
发布日期:2023-10-24
通讯作者:
赵斌,男,博士,副教授,研究方向:新农药作用机理;E-mail: bdzhaobin@126.com;作者简介:周璐祺,男,硕士研究生,研究方向:井冈霉素调控伏马毒素合成的分子作用机理;E-mail: luqizhou_hbnd@126.com
基金资助:
ZHOU Lu-qi1( ), CUI Ting-ru2, HAO Nan1, ZHAO Yu-wei1, ZHAO Bin1(
), CUI Ting-ru2, HAO Nan1, ZHAO Yu-wei1, ZHAO Bin1( ), LIU Ying-chao1(
), LIU Ying-chao1( )
)
Received:2023-03-03
Published:2023-09-26
Online:2023-10-24
摘要:
绿色新农药的开发和利用有利于农业的可持续发展,基于天然产物进行活性先导发现及作用机制研究是重要的新农药创制策略,然而其作用靶标和作用机制难以确定,阻碍了其在新农药中的应用。因此发现化合物新靶点对于新农药创制来说是一项既重要又艰巨的任务。化学蛋白质组学作为后基因组时代的新技术,目前已经成为研究药物靶点的重要手段之一。本文对基于化学蛋白组学的化合物作用分子靶点发现方法和典型案例进行探析,介绍这些技术的主要原理、应用以及各自的优点和局限性,旨在阐述基于化学蛋白质组学发现药物作用靶标的最新方法,并为天然产物靶点及新农药创制研究提供参考。
周璐祺, 崔婷茹, 郝楠, 赵雨薇, 赵斌, 刘颖超. 化学蛋白质组学在天然产物分子靶标鉴定中的应用[J]. 生物技术通报, 2023, 39(9): 12-26.
ZHOU Lu-qi, CUI Ting-ru, HAO Nan, ZHAO Yu-wei, ZHAO Bin, LIU Ying-chao. Application of Chemical Proteomics in Identifying the Molecular Targets of Natural Products[J]. Biotechnology Bulletin, 2023, 39(9): 12-26.
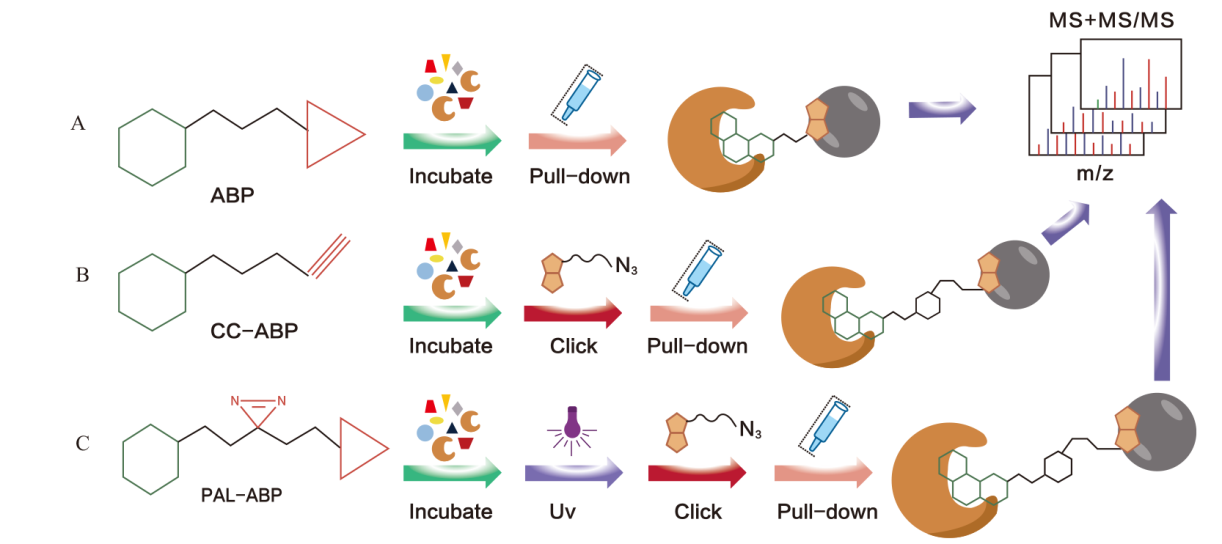
图2 基于亲和蛋白质组分析策略 A:与生物素偶联结合;B:基于生物正交反应基团(炔基)的探针;C:基于光亲和基团的生物探针
Fig. 2 Analysis strategies based on affinity proteomic A: Conjugated with biotin. B: Probes based on the bioorthogonal reactive groups(alkynyl). C: Biological probes based on photoaffinity groups
| 小分子化合物 Small molecule compound | 基于活性的探针结构 ABPs | 靶蛋白 Protein target |
|---|---|---|
| Thieno[2,3-d]pyrimidine derivatives[ | 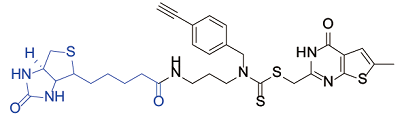 | Tubulin |
| Ebselen[ | 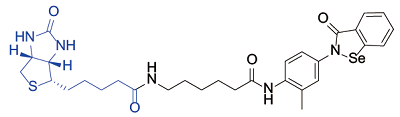 | β-lactoglobulin A |
| Clavulanic acid[ | 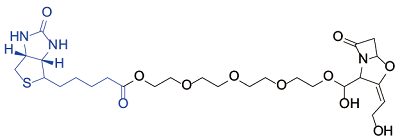 | Human serum albumin; Ig gamma-1 chain C region human; Haptoglobin human; Ig kappa chain C region human |
| Dihydroartemisinin[ |  | DNA methyltransferase 1 |
| 1,2,4-Oxadiazole derivatives[ |  | Rpn6 |
| Prazosin[ | 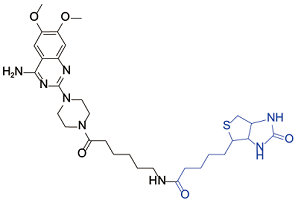 | Flagellum Attachment Zone 1 |
表1 生物素探针用于小分子化合物的靶点鉴定实例
Table 1 Examples of biotin probes used for the target identification of small molecular compounds
| 小分子化合物 Small molecule compound | 基于活性的探针结构 ABPs | 靶蛋白 Protein target |
|---|---|---|
| Thieno[2,3-d]pyrimidine derivatives[ |  | Tubulin |
| Ebselen[ |  | β-lactoglobulin A |
| Clavulanic acid[ |  | Human serum albumin; Ig gamma-1 chain C region human; Haptoglobin human; Ig kappa chain C region human |
| Dihydroartemisinin[ |  | DNA methyltransferase 1 |
| 1,2,4-Oxadiazole derivatives[ |  | Rpn6 |
| Prazosin[ |  | Flagellum Attachment Zone 1 |
| 小分子化合物 Small molecule compound | 基于活性的探针结构 ABPs | 靶蛋白 Protein target |
|---|---|---|
| Catechol estrogens[ | 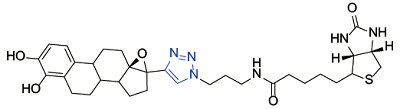 | Cytochrome c; superoxide dismutase |
| Calenduloside E[ | 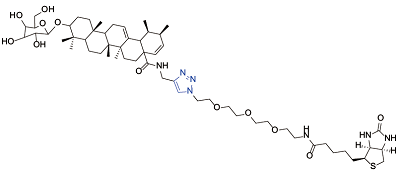 | Hsp90 |
| Swertiamarin[ | 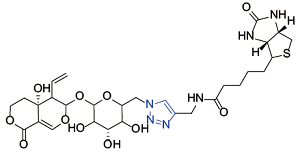 | AKT-PH |
| Salinipostin A[ | 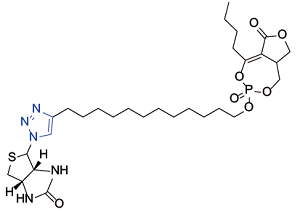 | Lysophospholipase; exported lipase 2; esterase; a/β hydrolase; BEM4 6-like protein |
| Nitro-fatty acids[ | 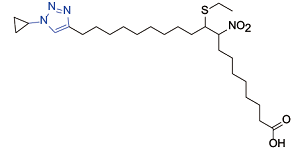 | Extended synaptotagmin 2; signal transducer and activator of transcription 3; toll-like receptor 2; retinoid X receptor alpha; glucocorticoid receptor |
| Natural product: BE-43547A2[ |  | Eukaryotic translation elongation factor 1 |
表2 生物正交化学反应用于小分子化合物的靶点鉴定实例
Table 2 Examples of bioorthogonal chemistry for target identification of small molecular compounds
| 小分子化合物 Small molecule compound | 基于活性的探针结构 ABPs | 靶蛋白 Protein target |
|---|---|---|
| Catechol estrogens[ |  | Cytochrome c; superoxide dismutase |
| Calenduloside E[ |  | Hsp90 |
| Swertiamarin[ |  | AKT-PH |
| Salinipostin A[ |  | Lysophospholipase; exported lipase 2; esterase; a/β hydrolase; BEM4 6-like protein |
| Nitro-fatty acids[ |  | Extended synaptotagmin 2; signal transducer and activator of transcription 3; toll-like receptor 2; retinoid X receptor alpha; glucocorticoid receptor |
| Natural product: BE-43547A2[ |  | Eukaryotic translation elongation factor 1 |
| 小分子化合物Small molecule compound | 探针结构ABPs | 靶蛋白Protein target |
|---|---|---|
| Anticancer pyrroloquinazoline: LBL1[ | Nuclear lamins | |
| Benzoxepane Derivatives[ | PKM2 | |
| 7-oxocallitrisic acid[ | Carnitine palmitoyltransferase 1A | |
| MCC950[ | Carbonic Anhydrase 2 | |
| Protopanaxadiol[ | Retinoblastoma Binding Protein 4 |
表3 光亲和探针用于小分子化合物的靶点鉴定实例
Table 3 Photoaffinity probe for the target identification of small molecular compounds
| 小分子化合物Small molecule compound | 探针结构ABPs | 靶蛋白Protein target |
|---|---|---|
| Anticancer pyrroloquinazoline: LBL1[ | Nuclear lamins | |
| Benzoxepane Derivatives[ | PKM2 | |
| 7-oxocallitrisic acid[ | Carnitine palmitoyltransferase 1A | |
| MCC950[ | Carbonic Anhydrase 2 | |
| Protopanaxadiol[ | Retinoblastoma Binding Protein 4 |

图4 非标记法识别药物靶标蛋白策略 A:药物亲和反应的靶点稳定性;B:蛋白质热稳定性分析;C:热蛋白质组分析;D:氧化蛋白稳定性
Fig. 4 Schematics of label-free target identification methods A: Drug affinity responsive target stability(DARTS). B: Cellular thermal shift assay(CETSA). C: Thermal proteome profiling(TPP). D: Stability of proteins from rates of oxidation(SPROX)
| [1] | Van Emden HF. Crop production and crop protection: estimated losses in major food and cash crops[J]. J Agric Sci, 1996, 127(1): 137. |
| [2] | 吴剑, 宋宝安. 绿色农药创新及靶标研究现状与思考[J]. 中国科学基金, 2020, 34(4): 486-494. |
| Wu J, Song BA. Current situation and thinking for the innovation of green pesticide[J]. Bull Natl Nat Sci Found China, 2020, 34(4): 486-494. | |
| [3] |
Guo ZR. The modification of natural products for medical use[J]. Acta Pharm Sin B, 2017, 7(2): 119-136.
doi: 10.1016/j.apsb.2016.06.003 pmid: 28303218 |
| [4] | 邵旭升, 杜少卿, 李忠, 等. 中国绿色农药的研究和发展[J]. 世界农药, 2020, 42(4)16-24. |
| Shao XS, Du SQ, Li Z, et al. Research and development of green pesticides in China[J]. World Pestic, 2020, 42(4)16-24. | |
| [5] |
Cheng D, Feng MX, Ji YF, et al. Effects of celangulin IV and V from Celastrus angulatus maxim on Na+/K+-ATPase activities of the oriental armyworm(Lepidoptera: Noctuidae)[J]. J Insect Sci, 2016, 16(1): 59.
doi: 10.1093/jisesa/iew051 URL |
| [6] |
Moffat JG, Vincent F, Lee JA, et al. Opportunities and challenges in phenotypic drug discovery: an industry perspective[J]. Nat Rev Drug Discov, 2017, 16(8): 531-543.
doi: 10.1038/nrd.2017.111 pmid: 28685762 |
| [7] |
Heilker R, Lessel U, Bischoff D. The power of combining phenotypic and target-focused drug discovery[J]. Drug Discov Today, 2019, 24(2): 526-532.
doi: S1359-6446(18)30155-7 pmid: 30359770 |
| [8] |
Schirle M, Jenkins JL. Identifying compound efficacy targets in phenotypic drug discovery[J]. Drug Discov Today, 2016, 21(1): 82-89.
doi: S1359-6446(15)00290-1 pmid: 26272035 |
| [9] |
Liu GY, Ju XL, Cheng J, et al. 3D-QSAR studies of insecticidal anthranilic diamides as ryanodine receptor activators using CoMFA, CoMSIA and DISCOtech[J]. Chemosphere, 2010, 78(3): 300-306.
doi: 10.1016/j.chemosphere.2009.10.038 URL |
| [10] |
Meissner F, Geddes-McAlister J, Mann M, et al. The emerging role of mass spectrometry-based proteomics in drug discovery[J]. Nat Rev Drug Discov, 2022, 21(9): 637-654.
doi: 10.1038/s41573-022-00409-3 |
| [11] |
Majumder A, Biswal MR, Prakash MK. One drug multiple targets: an approach to predict drug efficacies on bacterial strains differing in membrane composition[J]. ACS Omega, 2019, 4(3): 4977-4983.
doi: 10.1021/acsomega.8b02862 |
| [12] |
Strassberger V, Fugmann T, Neri D, et al. Chemical proteomic and bioinformatic strategies for the identification and quantification of vascular antigens in cancer[J]. J Proteom, 2010, 73(10): 1954-1973.
doi: 10.1016/j.jprot.2010.05.018 URL |
| [13] |
Fedorov II, Lineva VI, Tarasova IA, et al. Mass spectrometry-based chemical proteomics for drug target discoveries[J]. Biochemistry Moscow, 2022, 87(9): 983-994.
doi: 10.1134/S0006297922090103 |
| [14] |
Humphrey SJ, James DE, Mann M. Protein phosphorylation: a major switch mechanism for metabolic regulation[J]. Trends Endocrinol Metab, 2015, 26(12): 676-687.
doi: 10.1016/j.tem.2015.09.013 URL |
| [15] |
Rix U, Superti-Furga G. Target profiling of small molecules by chemical proteomics[J]. Nat Chem Biol, 2009, 5(9): 616-624.
doi: 10.1038/nchembio.216 pmid: 19690537 |
| [16] |
Harding MW, Galat A, Uehling DE, et al. A receptor for the immuno-suppressant FK506 is a cis-trans peptidyl-prolyl isomerase[J]. Nature, 1989, 341(6244): 758-760.
doi: 10.1038/341758a0 |
| [17] |
Bach S, Knockaert M, Reinhardt J, et al. Roscovitine targets, protein kinases and pyridoxal kinase[J]. J Biol Chem, 2005, 280(35): 31208-31219.
doi: 10.1074/jbc.M500806200 pmid: 15975926 |
| [18] |
Gyenis L, Kuś A, Bretner M, et al. Functional proteomics strategy for validation of protein kinase inhibitors reveals new targets for a TBB-derived inhibitor of protein kinase CK2[J]. J Proteom, 2013, 81: 70-79.
doi: 10.1016/j.jprot.2012.09.017 URL |
| [19] |
Faiella L, Piaz FD, Bisio A, et al. A chemical proteomics approach reveals Hsp27 as a target for proapoptotic clerodane diterpenes[J]. Mol BioSyst, 2012, 8(10): 2637-2644.
doi: 10.1039/c2mb25171j pmid: 22802135 |
| [20] |
Lu LN, Qi ZJ, Zhang JW, et al. Separation of binding protein of celangulin V from the midgut of Mythimna separata walker by affinity chromatography[J]. Toxins, 2015, 7(5): 1738-1748.
doi: 10.3390/toxins7051738 URL |
| [21] |
Hu LH, Iliuk A, Galan J, et al. Identification of drug targets in vitro and in living cells by soluble-nanopolymer-based proteomics[J]. Angewandte Chemie Int Ed, 2011, 50(18): 4133-4136.
doi: 10.1002/anie.v50.18 URL |
| [22] |
Bantscheff M, Scholten A, Heck AJR. Revealing promiscuous drug-target interactions by chemical proteomics[J]. Drug Discov Today, 2009, 14(21/22): 1021-1029.
doi: 10.1016/j.drudis.2009.07.001 URL |
| [23] |
Adam GC, Sorensen EJ, Cravatt BF. Chemical strategies for functional proteomics[J]. Mol Cell Proteom, 2002, 1(10): 781-790.
doi: 10.1074/mcp.R200006-MCP200 URL |
| [24] |
Benns HJ, Tate EW, Child MA. Activity-based protein profiling for the study of parasite biology[J]. Curr Top Microbiol Immunol, 2019, 420:155-174.
doi: 10.1007/82_2018_123 pmid: 30105424 |
| [25] |
Zhou YQ, Xiao YL. Target identification of bioactive natural products[J]. Acta Chim Sinica, 2018, 76(3): 177.
doi: 10.6023/A17110484 |
| [26] | 杨婉琪, 张崇敬. 基于分子原型和分子探针的药用活性分子蛋白作用靶标研究[J]. 药学学报, 2020, 55(7): 1439-1452. |
| Yang WQ, Zhang CJ. Protein targets of medicinally active molecules based on their original structures and molecular probes[J]. Acta Pharm Sin, 2020, 55(7): 1439-1452. | |
| [27] |
Wright MH, Tao Y, Drechsel J, et al. Quantitative chemoproteomic profiling reveals multiple target interactions of spongiolactone derivatives in leukemia cells[J]. Chem Commun, 2017, 53(95): 12818-12821.
doi: 10.1039/C7CC04990K URL |
| [28] |
Wang C, Chen N. Activity-based protein profiling[J]. Acta Chim Sinica, 2015, 73(7): 657.
doi: 10.6023/A15040223 |
| [29] |
Zanon PRA, Lewald L, Hacker SM. Isotopically labeled desthiobiotin azide(isoDTB)tags enable global profiling of the bacterial cysteinome[J]. Angewandte Chemie Int Ed, 2020, 59(7): 2829-2836.
doi: 10.1002/anie.v59.7 URL |
| [30] |
Kong LM, Deng X, Zuo ZL, et al. Identification and validation of p50 as the cellular target of eriocalyxin B[J]. Oncotarget, 2014, 5(22): 11354-11364.
doi: 10.18632/oncotarget.v5i22 URL |
| [31] |
Kwok BHB, Koh B, Ndubuisi MI, et al. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IκB kinase[J]. Chem Biol, 2001, 8(8): 759-766.
doi: 10.1016/S1074-5521(01)00049-7 URL |
| [32] |
Yang CR, Peng B, Cao SL, et al. Synthesis, cytotoxic evaluation and target identification of thieno[2, 3-d]pyrimidine derivatives with a dithiocarbamate side chain at C2 position[J]. Eur J Med Chem, 2018, 154: 324-340.
doi: 10.1016/j.ejmech.2018.05.028 URL |
| [33] |
Chen ZZ, Jiang ZY, Chen N, et al. Target discovery of ebselen with a biotinylated probe[J]. Chem Commun, 2018, 54(68): 9506-9509.
doi: 10.1039/C8CC04258F URL |
| [34] |
Martín-Serrano Á, Gonzalez-Morena JM, Barbero N, et al. Biotin-labelled clavulanic acid to identify proteins target for haptenation in serum: implications in allergy studies[J]. Front Pharmacol, 2020, 11: 594755.
doi: 10.3389/fphar.2020.594755 URL |
| [35] |
Zhou W, Chen MM, Liu HL, et al. Dihydroartemisinin suppresses renal fibrosis in mice by inhibiting DNA-methyltransferase 1 and increasing Klotho[J]. Acta Pharmacol Sin, 2022, 43(10): 2609-2623.
doi: 10.1038/s41401-022-00898-3 pmid: 35347248 |
| [36] |
Dai Z, An LY, Chen XY, et al. Target fishing reveals a novel mechanism of 1,2,4-oxadiazole derivatives targeting Rpn6, a subunit of 26S proteasome[J]. J Med Chem, 2022, 65(6): 5029-5043.
doi: 10.1021/acs.jmedchem.1c02210 pmid: 35253427 |
| [37] |
Orahoske CM, Afrin M, Li YX, et al. Identification of prazosin as a potential flagellum attachment zone 1(FAZ1)inhibitor for the treatment of human African trypanosomiasis[J]. ACS Infect Dis, 2022, 8(8): 1711-1726.
doi: 10.1021/acsinfecdis.2c00331 pmid: 35894227 |
| [38] |
Speers AE, Cravatt BF. Profiling enzyme activities in vivo using click chemistry methods[J]. Chem Biol, 2004, 11(4): 535-546.
doi: 10.1016/j.chembiol.2004.03.012 URL |
| [39] |
Wang JG, Tan XF, Nguyen VS, et al. A quantitative chemical proteomics approach to profile the specific cellular targets of andrographolide, a promising anticancer agent that suppresses tumor metastasis[J]. Mol Cell Proteom, 2014, 13(3): 876-886.
doi: 10.1074/mcp.M113.029793 URL |
| [40] |
Ciepla P, Konitsiotis AD, Serwa RA, et al. New chemical probes targeting cholesterylation of Sonic Hedgehog in human cells and zebrafish[J]. Chem Sci, 2014, 5(11): 4249-4259.
pmid: 25574372 |
| [41] |
Wang JG, Zhang JB, Zhang CJ, et al. In situ proteomic profiling of curcumin targets in HCT116 colon cancer cell line[J]. Sci Rep, 2016, 6(1): 1-8.
doi: 10.1038/s41598-016-0001-8 |
| [42] |
Chen B, Long QS, Zhao YL, et al. Sulfone-based probes unraveled dihydrolipoamide S-succinyltransferase as an unprecedented target in phytopathogens[J]. J Agric Food Chem, 2019, 67(25): 6962-6969.
doi: 10.1021/acs.jafc.9b02059 URL |
| [43] |
Liang HC, Liu YC, Chen H, et al. In situ click reaction coupled with quantitative proteomics for identifying protein targets of catechol estrogens[J]. J Proteome Res, 2018, 17(8): 2590-2599.
doi: 10.1021/acs.jproteome.8b00021 URL |
| [44] |
Tian Y, Wang S, Shang H, et al. The clickable activity-based probe of anti-apoptotic calenduloside E[J]. Pharm Biol, 2019, 57(1): 133-139.
doi: 10.1080/13880209.2018.1557699 pmid: 30843752 |
| [45] |
Zhang M, Ma XY, Xu HL, et al. A natural AKT inhibitor swertiamarin targets AKT-PH domain, inhibits downstream signaling, and alleviates inflammation[J]. FEBS J, 2020, 287(9): 1816-1829.
doi: 10.1111/febs.15112 pmid: 31665825 |
| [46] |
Yoo E, Schulze CJ, Stokes BH, et al. The antimalarial natural product salinipostin A identifies essential α/β serine hydrolases involved in lipid metabolism in P.falciparum parasites[J]. Cell Chem Biol, 2020, 27(2): 143-157.e5.
doi: 10.1016/j.chembiol.2020.01.001 URL |
| [47] |
Fang MY, Huang KH, Tu WJ, et al. Chemoproteomic profiling reveals cellular targets of nitro-fatty acids[J]. Redox Biol, 2021, 46: 102126.
doi: 10.1016/j.redox.2021.102126 URL |
| [48] | Liu C, Wang L, Sun YJ, et al. Probe synthesis reveals eukaryotic translation elongation Factor 1 Alpha 1 as the anti-pancreatic cancer target of BE-43547A2[J]. Angewandte Chemie, 2022, 134(34): e202206953. |
| [49] | Cai Q, Li ZQ, Wei JJ, et al. Assembly of indole-2-carboxylic acid esters through a ligand-free copper-catalysed cascade process[J]. Chem Commun, 2009(48): 7581-7583. |
| [50] |
Yan JB, Yao RF, Chen L, et al. Dynamic perception of jasmonates by the F-box protein COI1[J]. Mol Plant, 2018, 11(10): 1237-1247.
doi: S1674-2052(18)30241-7 pmid: 30092285 |
| [51] |
Eirich J, Orth R, Sieber SA. Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells[J]. J Am Chem Soc, 2011, 133(31): 12144-12153.
doi: 10.1021/ja2039979 pmid: 21736328 |
| [52] |
Lamos SM, Krusemark CJ, McGee CJ, et al. Mixed isotope photoaffinity reagents for identification of small-molecule targets by mass spectrometry[J]. Angewandte Chemie Int Ed, 2006, 45(26): 4329-4333.
doi: 10.1002/anie.v45:26 URL |
| [53] |
Li BX, Chen JJ, Chao B, et al. Anticancer pyrroloquinazoline LBL1 targets nuclear lamins[J]. ACS Chem Biol, 2018, 13(5): 1380-1387.
doi: 10.1021/acschembio.8b00266 pmid: 29648791 |
| [54] |
Gao CL, Hou GG, Liu J, et al. Synthesis and target identification of benzoxepane derivatives as potential anti-neuroinflammatory agents for ischemic stroke[J]. Angewandte Chemie Int Ed, 2020, 59(6): 2429-2439.
doi: 10.1002/anie.v59.6 URL |
| [55] |
Kennedy CR, Goya Grocin A, Kovačič T, et al. A probe for NLRP3 inflammasome inhibitor MCC950 identifies carbonic anhydrase 2 as a novel target[J]. ACS Chem Biol, 2021, 16(6): 982-990.
doi: 10.1021/acschembio.1c00218 pmid: 34003636 |
| [56] | Zhuo FF, Guo Q, Zheng YZ, et al. Photoaffinity labeling-based chemoproteomic strategy reveals RBBP4 as a cellular target of protopanaxadiol against colorectal cancer cells[J]. ChemBioChem, 2022, 23(13): e202200038. |
| [57] |
Corson TW, Cavga H, Aberle N, et al. Triptolide directly inhibits dCTP pyrophosphatase[J]. ChemBioChem, 2011, 12(11): 1767-1773.
doi: 10.1002/cbic.201100007 pmid: 21671327 |
| [58] |
Klaić L, Morimoto RI, Silverman RB. Celastrol analogues as inducers of the heat shock response. design and synthesis of affinity probes for the identification of protein targets[J]. ACS Chem Biol, 2012, 7(5): 928-937.
doi: 10.1021/cb200539u pmid: 22380712 |
| [59] |
Zhao Q, Ding Y, Deng ZS, et al. Natural products triptolide, celastrol, and withaferin A inhibit the chaperone activity of peroxiredoxin I[J]. Chem Sci, 2015, 6(7): 4124-4130.
doi: 10.1039/c5sc00633c pmid: 28717468 |
| [60] |
Lomenick B, Jung G, Wohlschlegel JA, et al. Target identification using drug affinity responsive target stability(DARTS)[J]. Curr Protoc Chem Biol, 2011, 3(4): 163-180.
doi: 10.1002/9780470559277.ch110180 pmid: 22229126 |
| [61] |
Zhang X, Xu H, Bi XY, et al. Src acts as the target of matrine to inhibit the proliferation of cancer cells by regulating phosphorylation signaling pathways[J]. Cell Death Dis, 2021, 12(10): 931.
doi: 10.1038/s41419-021-04221-6 pmid: 34642304 |
| [62] |
Kim Y, Sugihara Y, Kim TY, et al. Identification and validation of VEGFR2 kinase as a target of voacangine by a systematic combination of DARTS and MSI[J]. Biomolecules, 2020, 10(4): 508.
doi: 10.3390/biom10040508 URL |
| [63] |
Zhu Z, Li RM, Qin W, et al. Target engagement of ginsenosides in mild cognitive impairment using mass spectrometry-based drug affinity responsive target stability[J]. J Ginseng Res, 2022, 46(6): 750-758.
doi: 10.1016/j.jgr.2021.12.003 pmid: 36312734 |
| [64] |
Zhao B, Fan SJ, Fan ZJ, et al. Discovery of pyruvate kinase as a novel target of new fungicide candidate 3-(4-methyl-1, 2, 3-thiadiazolyl)-6-trichloromethyl-[1, 2, 4]-triazolo-[3, 4-b][1, 3, 4]-thiadizole[J]. J Agric Food Chem, 2018, 66(46): 12439-12452.
doi: 10.1021/acs.jafc.8b03797 URL |
| [65] |
Geng J, Liu W, Gao J, et al. Andrographolide alleviates Parkinsonism in MPTP-PD mice via targeting mitochondrial fission mediated by dynamin-related protein 1[J]. Br J Pharmacol, 2019, 176(23): 4574-4591.
doi: 10.1111/bph.v176.23 URL |
| [66] |
Hu HF, Xu WW, Li YJ, et al. Anti-allergic drug azelastine suppresses colon tumorigenesis by directly targeting ARF1 to inhibit IQGAP1-ERK-Drp1-mediated mitochondrial fission[J]. Theranostics, 2021, 11(4): 1828-1844.
doi: 10.7150/thno.48698 URL |
| [67] |
Ren YS, Li HL, Piao XH, et al. Drug affinity responsive target stability(DARTS)accelerated small molecules target discovery: principles and application[J]. Biochem Pharmacol, 2021, 194: 114798.
doi: 10.1016/j.bcp.2021.114798 URL |
| [68] |
West GM, Tucker CL, Xu T, et al. Quantitative proteomics approach for identifying protein-drug interactions in complex mixtures using protein stability measurements[J]. Proc Natl Acad Sci USA, 2010, 107(20): 9078-9082.
doi: 10.1073/pnas.1000148107 pmid: 20439767 |
| [69] |
Molina DM, Jafari R, Ignatushchenko M, et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay[J]. Science, 2013, 341(6141): 84-87.
doi: 10.1126/science.1233606 pmid: 23828940 |
| [70] |
Savitski MM, Reinhard FBM, Franken H, et al. Tracking cancer drugs in living cells by thermal profiling of the proteome[J]. Science, 2014, 346(6205): 1255784.
doi: 10.1126/science.1255784 URL |
| [71] |
Islam A, Su AJ, Zeng ZM, et al. Capsaicin targets tNOX(ENOX2)to inhibit G1 cyclin/CDK complex, as assessed by the cellular thermal shift assay(CETSA)[J]. Cells, 2019, 8(10): 1275.
doi: 10.3390/cells8101275 URL |
| [72] |
Dziekan JM, Yu H, Chen D, et al. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay[J]. Sci Transl Med, 2019, 11(473): eaau3174.
doi: 10.1126/scitranslmed.aau3174 URL |
| [73] |
Wang JJ, Weng QF, Yin F, et al. Interactions of destruxin A with silkworms'arginine tRNA synthetase and lamin-C proteins[J]. Toxins, 2020, 12(2): 137.
doi: 10.3390/toxins12020137 URL |
| [74] | 金金, 梁旭俊, 毕武, 等. 基于液相色谱-串联质谱技术鉴定药物靶标的研究进展[J]. 生命科学研究, 2022. DOI: 10.16605/j.cnki.1007-7847.2022.08.0187. |
| Jin J, Liang XJ, Bi W, et al. Advances in the identification of drug targets based on liquid chromatography-tandem mass spectrometry[J]. Life Sci Res, 2022. DOI: 10.16605/j.cnki.1007-7847.2022.08.0187. | |
| [75] |
Li CH, Zhou Y, Tu PF, et al. Natural carbazole alkaloid murrayafoline A displays potent anti-neuroinflammatory effect by directly targeting transcription factor Sp1 in LPS-induced microglial cells[J]. Bioorg Chem, 2022, 129: 106178.
doi: 10.1016/j.bioorg.2022.106178 URL |
| [76] |
Hatstat AK, Quan BY, Bailey MA, et al. Chemoproteomic-enabled characterization of small GTPase Rab1a as a target of an N-arylbenzimidazole ligand's rescue of Parkinson's-associated cell toxicity[J]. RSC Chem Biol, 2022, 3(1): 96-111.
doi: 10.1039/D1CB00103E URL |
| [77] |
Ogburn RN, Jin L, Meng H, et al. Discovery of tamoxifen and N-desmethyl tamoxifen protein targets in MCF-7 cells using large-scale protein folding and stability measurements[J]. J Proteome Res, 2017, 16(11): 4073-4085.
doi: 10.1021/acs.jproteome.7b00442 URL |
| [78] |
Geer Wallace MA, Kwon DY, Weitzel DH, et al. Discovery of manassantin A protein targets using large-scale protein folding and stability measurements[J]. J Proteome Res, 2016, 15(8): 2688-2696.
doi: 10.1021/acs.jproteome.6b00237 pmid: 27322910 |
| [79] |
Peng H, Guo HB, Pogoutse O, et al. An unbiased chemical proteomics method identifies FabI as the primary target of 6-OH-BDE-47[J]. Environ Sci Technol, 2016, 50(20): 11329-11336.
doi: 10.1021/acs.est.6b03541 URL |
| [80] | 郝海平, 叶慧, 皖宁, 等. 一种基于化学蛋白质组学的小分子靶标筛选方法及其应用:CN112485442A[P]. 2021-03-12. |
| Hao HP, Ye H, Wan N. A Chemical proteomics Based Small Mole-cular Target Screening Method and Its Application:CN112485442A[P]. 2021-03-12. | |
| [81] |
Zhu YY, Wan N, Shan XN, et al. Celastrol targets adenylyl cyclase-associated protein 1 to reduce macrophages-mediated inflammation and ameliorates high fat diet-induced metabolic syndrome in mice[J]. Acta Pharm Sin B, 2021, 11(5): 1200-1212.
doi: 10.1016/j.apsb.2020.12.008 pmid: 34094828 |
| [82] |
Deng GL, Zhou LS, Wang BL, et al. Targeting cathepsin B by cycloastragenol enhances antitumor immunity of CD8 T cells via inhibiting MHC-I degradation[J]. J Immunother Cancer, 2022, 10(10): e004874.
doi: 10.1136/jitc-2022-004874 URL |
| [83] | Chan JNY, Vuckovic D, Sleno L, et al. Target identification by chromatographic co-elution: monitoring of drug-protein interactions without immobilization or chemical derivatization[J]. Mol Cell Proteomics, 2012, 11(7): M111.016642. |
| [84] |
Schäkermann S, Wüllner D, Yayci A, et al. Applicability of chromatographic Co-elution for antibiotic target identification[J]. Proteomics, 2021, 21(1): 2000038.
doi: 10.1002/pmic.v21.1 URL |
| [85] |
Ramsay RR, Popovic-Nikolic MR, Nikolic K, et al. A perspective on multi-target drug discovery and design for complex diseases[J]. Clin Transl Med, 2018, 7(1): e3.
doi: 10.1186/s40169-017-0181-2 URL |
| [86] |
Chen JX, Song BA. Natural nematicidal active compounds: recent research progress and outlook[J]. J Integr Agric, 2021, 20(8): 2015-2031.
doi: 10.1016/S2095-3119(21)63617-1 URL |
| [87] |
Croston GE. The utility of target-based discovery[J]. Expert Opin Drug Discov, 2017, 12(5): 427-429.
doi: 10.1080/17460441.2017.1308351 URL |
| [1] | 周闪闪, 黄远龙, 黄建忠, 李善仁. 溶杆菌中活性天然产物的研究进展[J]. 生物技术通报, 2023, 39(10): 41-49. |
| [2] | 刘晓黎, 童真艺, 赵亮, 尹丽, 刘晨光. 非抗生素类活性物质抗幽门螺杆菌研究进展[J]. 生物技术通报, 2022, 38(9): 96-105. |
| [3] | 张国宁, 冯婧娴, 杨颖博, 陈万生, 肖莹. 环糊精葡萄糖基转移酶在天然产物糖基化修饰中的应用[J]. 生物技术通报, 2022, 38(3): 246-255. |
| [4] | 成温玉, 张博昕, 赵鸿远, 陈艳, 谢娟平. 天然产物抗猪流行性腹泻病毒研究进展[J]. 生物技术通报, 2022, 38(12): 127-136. |
| [5] | 周正, 李卿, 陈万生, 张磊. 药用植物天然产物生物合成途径及关键催化酶的研究策略[J]. 生物技术通报, 2021, 37(8): 25-34. |
| [6] | 赵鸿远, 王朝, 成温玉, 马宁宁, 李曼, 魏小丽. 抗非洲猪瘟病毒制剂的研究进展[J]. 生物技术通报, 2021, 37(5): 174-181. |
| [7] | 陈鹏. 活性天然产物蛋白靶点的快速筛选策略[J]. 生物技术通报, 2020, 36(11): 180-187. |
| [8] | 申晓林, 袁其朋. 生物合成芳香族氨基酸及其衍生物的研究进展[J]. 生物技术通报, 2017, 33(1): 24-34. |
| [9] | 王丽苹, 罗云孜. 合成生物学在天然产物研究中的应用[J]. 生物技术通报, 2017, 33(1): 35-47. |
| [10] | 匡雪君, 邹丽秋, 孙超, 陈士林. 天然产物合成生物学体系的优化策略[J]. 生物技术通报, 2017, 33(1): 48-57. |
| [11] | 邓会群;王惠利;杨红;洪华珠;李爱英;. 天然产物的C-糖基化研究进展[J]. , 2009, 0(05): 27-30. |
| [12] | 李思经;. 生物技术对发展中国家出口带来威胁[J]. , 1988, 0(08): 8-8. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||