








生物技术通报 ›› 2022, Vol. 38 ›› Issue (6): 245-251.doi: 10.13560/j.cnki.biotech.bull.1985.2021-0927
付巧1( ), 林啟兰1, 薛强1, 熊海容1, 王亚伟1,2(
), 林啟兰1, 薛强1, 熊海容1, 王亚伟1,2( )
)
收稿日期:2021-07-17
出版日期:2022-06-26
发布日期:2022-07-11
作者简介:付巧,女,硕士研究生,研究方向:微生物酶工程;E-mail: 基金资助:
FU Qiao1( ), LIN Qi-lan1, XUE Qiang1, XIONG Hai-rong1, WANG Ya-wei1,2(
), LIN Qi-lan1, XUE Qiang1, XIONG Hai-rong1, WANG Ya-wei1,2( )
)
Received:2021-07-17
Published:2022-06-26
Online:2022-07-11
摘要:
采用N端截短方式对Bacillus subtilis 168来源普鲁兰酶进行蛋白结构改造,构建不同形式的截短突变体,考察N端结构对酶学特性的影响。利用基因工程的手段分别删去CBM41结构域N端前2、4和6个氨基酸,获得突变体M1(ΔN2)、M2(ΔN4)和M3(ΔN6)。3种突变体最适反应温度(40-45℃)和最适pH(6.0)均与WT一致,WT、M1、M2和M3的Tm值分别为48.57℃、50.03℃、48.43℃和49.50℃,M1、M2和M3的比活力均高于野生型,是WT的1.18、1.60和2.44倍,WT、M1、M2和M3的Km值分别为23.89、29.01、17.29和19.08 mg/mL。上述结果表明,通过截短普鲁兰酶N端氨基酸可获得性质得到改良的突变体,为提高该酶的热稳定性、比活力和底物结合能力提供新的方法和思路。
付巧, 林啟兰, 薛强, 熊海容, 王亚伟. N端截短CBM41对枯草芽孢杆菌来源普鲁兰酶酶学性质的影响[J]. 生物技术通报, 2022, 38(6): 245-251.
FU Qiao, LIN Qi-lan, XUE Qiang, XIONG Hai-rong, WANG Ya-wei. Effects of CBM41 N-terminal Truncation on the Enzymological Properties of the Pullulanase from Bacillus subtilis 168[J]. Biotechnology Bulletin, 2022, 38(6): 245-251.
| Primer name | Nucleotide sequence(5'-3') | Gene |
|---|---|---|
| WT-F(Nco I) | CATGCCATGGTCAGCATCCGCCGCAG- CTTC | PulB |
| M1-F(Nco I) | CATGCCATGGATAGCATCCGCCGCAG- CTTCGAAG | PulBΔN2 |
| M2-F(Nco I) | CATGCCATGGATCGCCGCAGCTTCGA- AGCGTATG | PulBΔN4 |
| M3-F(Nco I) | CATGCCATGGATAGCTTCGAAGC- GTATGTCGATG | PulBΔN6 |
| WT-R(Xho I) | CCGCTCGAGAGCAAAACTCTTAAGAT- CTGATGC |
表1 野生型及其截短突变体扩增用引物
Table 1 Primers for amplification of wild-type and trun-cated mutants
| Primer name | Nucleotide sequence(5'-3') | Gene |
|---|---|---|
| WT-F(Nco I) | CATGCCATGGTCAGCATCCGCCGCAG- CTTC | PulB |
| M1-F(Nco I) | CATGCCATGGATAGCATCCGCCGCAG- CTTCGAAG | PulBΔN2 |
| M2-F(Nco I) | CATGCCATGGATCGCCGCAGCTTCGA- AGCGTATG | PulBΔN4 |
| M3-F(Nco I) | CATGCCATGGATAGCTTCGAAGC- GTATGTCGATG | PulBΔN6 |
| WT-R(Xho I) | CCGCTCGAGAGCAAAACTCTTAAGAT- CTGATGC |
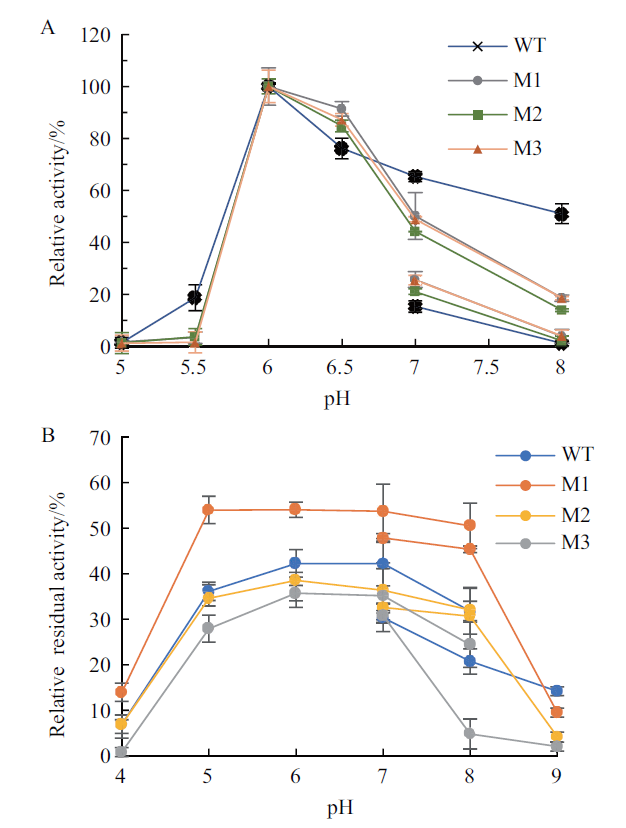
图4 野生型及其截短突变体的最适pH及pH稳定性 A:最适pH,反应温度40℃,反应时间30 min;B:pH稳定性,pH 4.0-9.0处理时间1 h,反应温度40℃。WT为野生型普鲁兰酶;M1,M2和M3为该普鲁兰酶的不同突变体。下同
Fig.4 Optimal pH and pH stability of wild-type pullulan-ase and the truncated mutants A:Optimal pH. The reaction temperature and time:40℃ and 30 min. B:pH stability. pH:4.0-9.0;treatment time:1 h;reaction temperature:40℃. WT:Wild-type pullulanase;M1,M2 and M3:different mutants of pullulanase. The same below
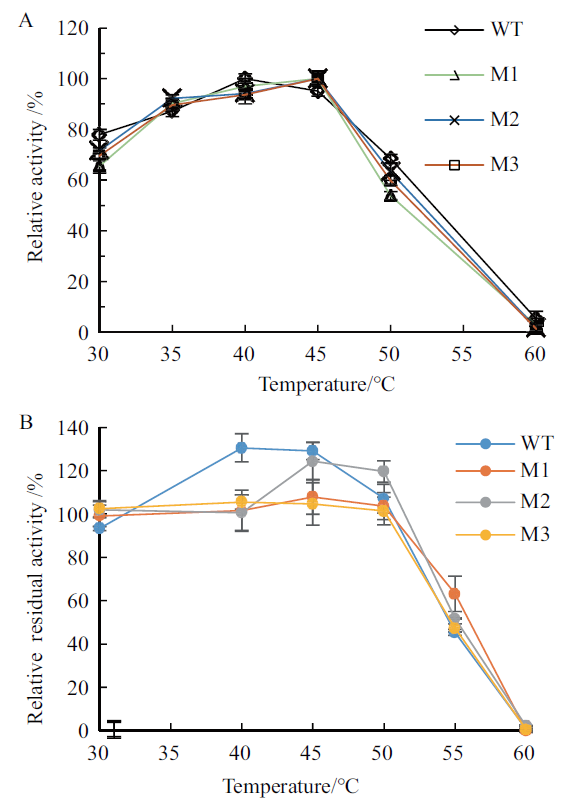
图5 野生型及其截短突变体的最适温度及热稳定性 A:最适温度,反应pH 6.0,反应时间30 min;B:热稳定性,30-60℃处理时间1 h,反应温度40℃
Fig.5 Optimal temperature and thermostability of wild-type pullulanase and the truncated mutants A:Optimal temperature. The reaction pH and time:6.0 and 30 min. B:Thermostability. Temperature:30-60℃;treatment time:1 h;reaction temperature:40℃
| 比酶活Specific enzyme activity/(U·mg-1) | Km/(mg·mL-1) | Vmax/(U·mg-1) | Tm/℃ | |
|---|---|---|---|---|
| WT | 2.30±0.09 | 23.89±0.66 | 4.06±0.09 | 48.57±0.42 |
| M1 | 2.72±0.08 | 29.01±1.21 | 2.35±0.12 | 50.03±0.25 |
| M2 | 3.69±0.07 | 17.29±0.73 | 3.92±0.06 | 48.43±0.22 |
| M3 | 5.62±0.06 | 19.08±0.41 | 7.24±0.11 | 49.50±0.32 |
表2 普鲁兰酶及其截短突变体比酶活、动力学参数及Tm值比较
Table 2 Comparison of specific activity,kinetic parameters and Tm of pullulanase and the truncated mutants
| 比酶活Specific enzyme activity/(U·mg-1) | Km/(mg·mL-1) | Vmax/(U·mg-1) | Tm/℃ | |
|---|---|---|---|---|
| WT | 2.30±0.09 | 23.89±0.66 | 4.06±0.09 | 48.57±0.42 |
| M1 | 2.72±0.08 | 29.01±1.21 | 2.35±0.12 | 50.03±0.25 |
| M2 | 3.69±0.07 | 17.29±0.73 | 3.92±0.06 | 48.43±0.22 |
| M3 | 5.62±0.06 | 19.08±0.41 | 7.24±0.11 | 49.50±0.32 |
| [1] |
Ali G, Rihouey C, Le Cerf D, et al. Effect of carboxymethyl groups on degradation of modified pullulan by pullulanase from Klebsiella pneumoniae[J]. Carbohydr Polym, 2013, 93(1):109-115.
doi: 10.1016/j.carbpol.2012.07.039 URL |
| [2] |
Talekar S, Pandharbale A, Ladole M, et al. Carrier free co-immobilization of alpha amylase, glucoamylase and pullulanase as combined cross-linked enzyme aggregates(combi-CLEAs):a tri-enzyme biocatalyst with one pot starch hydrolytic activity[J]. Bioresour Technol, 2013, 147:269-275.
doi: 10.1016/j.biortech.2013.08.035 URL |
| [3] | Nie Y, Yan W, Xu Y, et al. High-level expression of Bacillus naganoensis pullulanase from recombinant Escherichia coli with auto-induction:effect of lac operator[J]. PLoS One, 2013, 8(10):e78416. |
| [4] |
Ram KA, Venkatasubramanian K. Enhancement of starch conversion efficiency with free and immobilized pullulanase and alpha-1, 4-glucosidase[J]. Biotechnol Bioeng, 1982, 24(2):355-369.
pmid: 18546308 |
| [5] |
Meng F, Zhu X, Nie T, et al. Enhanced expression of pullulanase in Bacillus subtilis by new strong promoters mined from transcriptome data, both alone and in combination[J]. Front Microbiol, 2018, 9:2635.
doi: 10.3389/fmicb.2018.02635 URL |
| [6] | da Silva VM, Cabral AD, Sperança MA, et al. High-resolution structure of a modular hyperthermostable endo-β-1, 4-mannanase from Thermotoga petrophila:The ancillary immunoglobulin-like module is a thermostabilizing domain[J]. Biochim Biophys Acta Proteins Proteom, 2020, 1868(8):140437. |
| [7] |
Malle D, Itoh T, Hashimoto W, et al. Overexpression, purification and preliminary X-ray analysis of pullulanase from Bacillus subtilis strain 168[J]. Acta Crystallogr Sect F Struct Biol Cryst Commun, 2006, 62(pt 4):381-384.
doi: 10.1107/S1744309106007901 URL |
| [8] | Chang MH, Chu XY, Lv J, et al. Improving the thermostability of acidic pullulanase from Bacillus naganoensis by rational design[J]. PLoS One, 2016, 11(10):e0165006. |
| [9] |
Turkenburg JP, Brzozowski AM, Svendsen A, et al. Structure of a pullulanase from Bacillus acidopullulyticus[J]. Proteins, 2009, 76(2):516-519.
doi: 10.1002/prot.22416 URL |
| [10] |
Zeng Y, Xu J, Fu X, et al. Effects of different carbohydrate-binding modules on the enzymatic properties of pullulanase[J]. Int J Biol Macromol, 2019, 137:973-981.
doi: S0141-8130(19)33084-3 pmid: 31295482 |
| [11] | 陈阿娜, 刘秀霞, 戴晓峰, 等. N端截短对嗜酸普鲁兰芽孢杆菌普鲁兰酶酶学特性及功能的影响[J]. 生物工程学报, 2016, 32(3):355-364. |
| Chen AN, Liu XX, Dai XF, et al. Effect of N-terminal truncation of Bacillus acidopullulyticus pullulanase on enzyme properties and functions[J]. Chin J Biotechnol, 2016, 32(3):355-364. | |
| [12] |
Duan X, Wu J. Enhancing the secretion efficiency and thermostability of a Bacillus deramificans pullulanase mutant(D437H/D503Y)by N-terminal domain truncation[J]. Appl Environ Microbiol, 2015, 81(6):1926-1931.
doi: 10.1128/AEM.03714-14 URL |
| [13] |
Jiao Y, Wu Y, Chen H, et al. The impact of N-terminal nonessential domains on the enzymological properties of the pullulanase from a marine Bacillus megaterium[J]. Biotechnol Lett, 2019, 41(6/7):849-857.
doi: 10.1007/s10529-019-02686-2 URL |
| [14] |
Chen A, Sun Y, Zhang W, et al. Downsizing a pullulanase to a small molecule with improved soluble expression and secretion efficiency in Escherichia coli[J]. Microb Cell Fact, 2016, 15:9.
doi: 10.1186/s12934-015-0403-5 pmid: 26762529 |
| [15] | 韩来闯, 马闪闪, 刘亚娟, 等. 构建重组质粒的二步PCR方法[J]. 河南科学, 2015, 33(8):1321-1325. |
| Han LC, Ma SS, Liu YJ, et al. Construction of recombinant plasmid by a simple step-reverse PCR method[J]. Henan Sci, 2015, 33(8):1321-1325. | |
| [16] |
Kang J, Park KM, Choi KH, et al. Molecular cloning and biochemical characterization of a heat-stable type I pullulanase from Ther-motoga neapolitana[J]. Enzym Microb Technol, 2011, 48(3):260-266.
doi: 10.1016/j.enzmictec.2010.11.006 URL |
| [17] | 邢岩, 邱爽, 聂慧慧, 等. Thermotoga petrophila普鲁兰酶基因的异源表达与酶学性质分析[J]. 河南农业大学学报, 2018, 52(3):404-411, 423. |
| Xing Y, Qiu S, Nie HH, et al. Heterologous expression and enzymatic characterization of the pullulanase from Thermotoga petrophila[J]. J Henan Agric Univ, 2018, 52(3):404-411, 423. | |
| [18] |
Ericsson UB, Hallberg BM, Detitta GT, et al. Thermofluor-based high-throughput stability optimization of proteins for structural studies[J]. Anal Biochem, 2006, 357(2):289-298.
pmid: 16962548 |
| [19] |
Janeček Š, Majzlová K, Svensson B, et al. The starch-binding domain family CBM41-An in silico analysis of evolutionary relationships[J]. Proteins, 2017, 85(8):1480-1492.
doi: 10.1002/prot.25309 URL |
| [20] | 王馨叶. 普鲁兰酶催化效率强化的分子改造及其分泌表达调控[D]. 无锡: 江南大学, 2019. |
| Wang XY. Molecular engineering of pullulanase for enhancement of catalytic efficiency and its extracellular expression regulation[D]. Wuxi: Jiangnan University, 2019. | |
| [21] |
Nisha M, Satyanarayana T. The role of N1 domain on the activity, stability, substrate specificity and raw starch binding of amylopullulanase of the extreme thermophile Geobacillus thermoleovorans[J]. Appl Microbiol Biotechnol, 2015, 99(13):5461-5474.
doi: 10.1007/s00253-014-6345-8 pmid: 25573470 |
| [22] | 叶延欣, 闫鹏飞, 胡渝, 等. F/10木聚糖酶二级结构含量对热稳定性的影响[J]. 河南农业大学学报, 2013, 47(4):446-450. |
| Ye YX, Yan PF, Hu Y, et al. Influence of secondary structure content on F/10 xylanase thermostability[J]. J Henan Agric Univ, 2013, 47(4):446-450. | |
| [23] |
Guillén D, Sánchez S, Rodríguez-Sanoja R. Carbohydrate-binding domains:multiplicity of biological roles[J]. Appl Microbiol Biotechnol, 2010, 85(5):1241-1249.
doi: 10.1007/s00253-009-2331-y pmid: 19908036 |
| [24] |
Li WF, Zhou XX, Lu P. Structural features of thermozymes[J]. Biotechnol Adv, 2005, 23(4):271-281.
pmid: 15848038 |
| [1] | 张开平, 刘燕丽, 涂绵亮, 李继伟, 吴文标. 烟曲霉A-16产纤维素酶工艺优化及酶学特性[J]. 生物技术通报, 2022, 38(9): 215-225. |
| [2] | 郭静静, 郭磊磊, 赵云岫, 戴亦军. Ensifer meliloti 1021烟酰胺酶的酶学特性及3-氰基吡啶调控机理的研究[J]. 生物技术通报, 2019, 35(8): 51-58. |
| [3] | 秦日甜, 谢占玲. 镰刀菌Q7-31T果胶酶PGL1的分离纯化、酶学性质鉴定及结构分析[J]. 生物技术通报, 2018, 34(4): 151-160. |
| [4] | 于林港, 宿玲恰, 吴敬. Escherichia coli str. K-12 substr. MG1655海藻糖酶Tre F的重组表达及性质研究[J]. 生物技术通报, 2017, 33(4): 177-184. |
| [5] | 贾博涵,周伟,赵罗迪,杨埔,苟敏. 一株产纤维素酶细菌的分离鉴定及酶学特性研究[J]. 生物技术通报, 2014, 0(11): 187-192. |
| [6] | 张艳;路福平;刘逸寒;李玉;张春峰;. 长野芽孢杆菌普鲁兰酶基因在枯草芽孢杆菌中的表达及酶学性质研究[J]. , 2012, 0(07): 114-118. |
| [7] | 孙劭靖;路福平;姜楠;李丽;徐健勇;曹慕琛;宋诙;. 一株产耐热普鲁兰酶菌株Anoxybacillus sp. LM14-2分离鉴定及酶学性质研究[J]. , 2011, 0(09): 136-141. |
| [8] | 张丽靖;沈江锋;金庆超;杨郁;. 一株酸性淀粉酶产生菌的分离、鉴定及酶学特性初步研究[J]. , 2011, 0(05): 142-145. |
| [9] | 谢振荣;慕跃林;闫丽娟;赵春雷;黄遵锡;. α-葡萄糖苷酶高产菌株HB-9-5的选育及产酶条件的优化[J]. , 2010, 0(06): 206-211. |
| [10] | 梅岩;陈丽梅;. 植物甲酸脱氢酶的研究进展[J]. , 2010, 0(05): 23-26. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||